Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2246
Peer-review started: January 12, 2017
First decision: February 9, 2017
Revised: February 24, 2017
Accepted: March 4, 2017
Article in press: March 4, 2017
Published online: March 28, 2017
Processing time: 76 Days and 20.7 Hours
Focal dermal hypoplasia (FDH) is a rare disorder of the mesodermal and ectodermal tissues. Here we present an eight-year-old female known to have FDH who presents with poor weight gain and dysphagia. She was diagnosed with multiple esophageal papillomas and eosinophilic esophagitis. She was successfully treated with argon plasma coagulation and ingested fluticasone propionate, which has not been described previously in a child.
Core tip: Focal dermal hypoplasia (FDH) is a rare connective tissue disorder associated with squamous papillomas of the esophagus in older individuals. This case discusses an 8-year-old female with FDH who presented with dysphagia. She was found to have esophageal papillomas and eosinophilic esophagitis. Treatment of eosinophilic esophagitis is highlighted. Argon plasma coagulation has been shown to be safe for use in the small diameter of the esophagus of children but not specifically for destruction of esophageal papillomas. A successful approach to debulking esophageal papillomas in a child using argon plasma coagulation is described in this case.
- Citation: Pasman EA, Heifert TA, Nylund CM. Esophageal squamous papillomas with focal dermal hypoplasia and eosinophilic esophagitis. World J Gastroenterol 2017; 23(12): 2246-2250
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2246.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2246
Focal dermal hypoplasia (FDH) or Goltz syndrome is a rare disorder of defective ectodermal and mesodermal tissue development[1]. There are only about 300 reported cases of FDH. The disorder is inherited in an X-linked dominant manner with a female predominance of 9:1. Females are heterozygous or mosaic for mutation in the PORCN gene; affected males are typically mosaic[2,3]. The primary clinical manifestations of FDH occur due to dysplasia of the connective tissue of the skin and skeletal tissue. The dermal connective tissue is attenuated, with thin-appearing collagen fibers leading to hypoplastic and atrophic areas of skin that often follow the lines of Blaschko. Skeletal malformations include syndactyly, oligodactyly, and split-hand/foot malformation[4,5]. Cleft lip can be present leading to feeding difficulty[6]. Mucocutaneous squamous papillomas have been reported on the mouth, nose, larynx, anus, and genitals[3,7,8]. There have been reports of multiple esophageal squamous papillomas in individuals over the age of 30[9,10]. These patients were described as having chronic dysphagia. We report the presence of distal esophageal papillomas in an eight-year-old child who had no such projections seen previously on barium swallow two years prior to endoscopy.
An eight-year-old female with focal dermal hypoplasia presented to the pediatric gastroenterology clinic for poor weight gain, dysphagia, and early satiety. She previously had a gastrostomy tube placed at three weeks of age due to cleft lip and palate, which were later repaired. The gastrostomy tube remained in place and was used sporadically as the patient and the parents were motivated to transition to oral intake of formula and foods. She had a history of difficulty with oral intake in the past; however, a tonsillectomy for tonsillar hypertrophy had led to improved feeding. Two years prior to establishing care with the pediatric gastroenterology clinic, she had a swallow study and a fluoroscopic upper gastrointestinal series that were normal. She denied odynophagia, although she endorsed the sensation of food getting stuck in her throat and chest. She reported having to clear her throat and drink water frequently during meals. She had no cough or respiratory complaints.
On exam she was very thin and emaciated with dysmorphic facial features and thin hair. She had multiple scars on her face, which appeared as an asymmetric, vascular, excoriated rash. There was a scar under her nose from the upper lip to the right of midline from previous cleft lip repair. She had a grade II/VI systolic murmur. Her lungs were clear. She had normoactive bowel sounds; her abdomen was soft, non-tender with no organomegaly. She had a low profile balloon gastrostomy tube in place.
An esophogram demonstrated multiple filling defects in the distal esophagus. The patient’s history of cleft lip and facial dysmorphism precluded esophageal manometric evaluation. The medical team elected to evaluate motility with an esophageal transit study using esophageal scintigraphy, which was remarkable for delayed clearance of contrast from the esophagus, especially in the supine position. She had gastric emptying scintigraphy, which showed mild delayed emptying (39% at two hours and 85% at four hours).
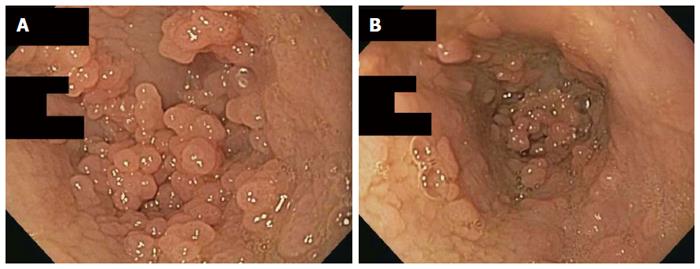
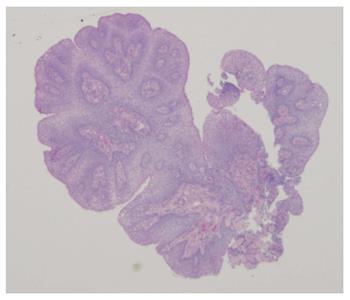
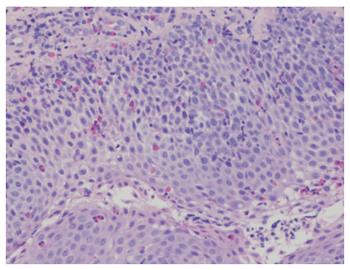
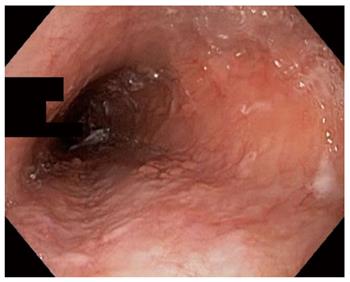
An esophagogastroduodenoscopy (EGD) was performed with pancreatic stimulation; she had normal pancreatic enzyme levels. On endoscopy, however, she was noted to have multiple papillomas in her esophagus (Figure 1). Pathology of the specimen was consistent with a squamous papilloma (Figure 2). Human papilloma virus polymerase chain reaction testing was negative. She had esophageal eosinophilia on biopsies with > 80 eosinophils per high power field (hpf) in her distal esophagus and > 20 eosinophils per hpf in her proximal esophagus (Figure 3). Subsequent EGD after being on a proton pump inhibitor for over 6 weeks showed a similar number of papillomas, with biopsies revealing up to 20 eosinophils per hpf in the distal esophagus and 15 eosinophils per hpf in the proximal esophagus. At the time of the second EGD, debulking of her papillomas was completed using argon plasma coagulation. This procedure was performed using ERBE VIO APC system (ERBE USA Inc., Marietta, GA) and the PRECISE setting with an effect of 5. On re-evaluation three months after initial debulking, there was significantly less of a papilloma burden with only a small cluster of papillomas remaining. This small cluster was ablated again, using the argon plasma coagulation. Follow-up EGD demonstrated successful elimination of papillomas (Figure 4).
Options for the treatment of eosinophilic esophagitis were discussed with the patient and parents. Allergy testing directed diet was not pursued. Given her skin disorder the implementation and interpretation of skin prick testing or skin atopy patch testing would have been technically difficult. Empiric food elimination diet or elemental diets were also presented but declined by the family as they felt any progress made in her transition from gastrostomy feeds to oral feeds would be lost with the initiation of restrictive diets or unpalatable formula. To treat the eosinophilic esophagitis, the patient was instructed to take fluticasone propionate metered dose inhaler 440 mcg directly into the mouth, swallowed rather than inhaled, twice a day. Her biopsy at three-month follow-up EGD demonstrated 15 eosinophils per hpf. The patient reported resolution of her dysphagia but continued early satiety with solid food. A combination of treatment with the promotility stimulant erythromycin ethylsuccinate 3 mg/kg per dose prior to each meal, along with introduction of overnight formula feedings via gastrostomy tube, facilitated adequate weight gain.
This case presents a child with FDH, a connective tissue disorder known to be associated with the formation of squamous papillomas in the nose, mouth, pharynx, airway, rectum, vagina and esophagus. The esophageal papillomas are hypothesized to be related to the high incidence of severe gastroesophageal reflux starting in infancy in FDH[9]. In two previous case reports, esophageal papillomas were noted at 30 and 56 years old; both patients were female with oral papillomas. The older patient had complained of dysphagia since she was a teen but did not definitely have esophageal papillomas identified until age 56[9,10]. Based on radiographic studies performed two years prior to our patient’s presentation, it is likely that she developed her papillomas between ages six and eight. What is unique in this scenario is that our patient is much younger than what has previously been reported in the literature about the development of esophageal squamous papillomas. Her complaint of dysphagia was supported by poor esophageal transit on nuclear medicine study.
Further complicating this patient’s presentation was eosinophilic esophagitis. There is evidence that connective tissue disorders are a risk factor for eosinophilic esophagitis. Ehler’s Danlos, Marfan, and Loeys-Dietz syndromes all have significantly higher rates of eosinophilic esophagitis than expected rates in the general population[11]. Although the exact cause of this association is unknown, it is speculated that it is likely related to poor esophageal connective tissue repair as well as to increases in immune modulating molecules in the esophageal tissue. To our knowledge this is the only report of a patient with FDH and eosinophilic esophagitis.
Although the delayed esophageal clearance was most likely related to the distal esophageal papillomas, it is known that motility deficits can lead to food impaction in eosinophilic esophagitis[12]. This can lead to serious complications such as perforation. Treatment of eosinophilic esophagitis has been shown to decrease the risk of impaction[13]. The patient in this case showed a partial histological response to ingested steroid therapy with a decrease in her mucosal eosinophil count. Her dysphagia improved with simultaneous treatment of her esophageal papillomas and eosinophilic esophagitis. We can only speculate, but suspect the largest symptomatic response as far as improved dysphagia was from debulking the papillomas.
Argon plasma coagulation has been shown to be an effective treatment for esophageal pathology with mucosal overgrowth such as Barrett’s esophagus[14]. Furthermore, it has been shown to be a safe treatment in children. Di Nardo et al[15] recently described a group of children with esophageal inlet patch who were unresponsive to proton pump inhibitor alone but responded well to argon plasma coagulation. Papillomatosis disease of the airway has been treated effectively with argon plasma coagulation; however, the technique does not appear to have been applied to esophageal papillomas[16]. We utilized the PRECISE setting, which is an ERBE proprietary setting. This setting creates superficial coagulation and tissue destruction using a low-energy output per unit time, which allows for cautery in temperature-sensitive or thin-walled structures[17]. The use of argon plasma coagulation in the small diameter esophagus of a child allowed the safe and controlled destruction of the papillomas while lowering the concern for unintended thermal damage. The desired endoscopic and symptomatic result was obtained using this technique.
There are multiple unique aspects to this single case description. We demonstrate that esophageal papillomas can be safely debulked using argon plasma coagulation. We also demonstrate a patient with focal dermal hypoplasia presenting with esophageal papillomas at an age much younger than previously shown in the literature. Finally, we identified a patient with both focal dermal hypoplasia and eosinophilic esophagitis. This is a potential association that has not yet been described, but has biologic plausibility given the association between eosinophilic esophagitis and connective tissue disorders. The young age of this patient and her comorbid eosinophilic esophagitis and esophageal papillomas present an argument for endoscopic evaluation of patients with focal dermal hypoplasia for pathological causes of feeding disorders or dysphagia.
An 8-year-old girl with focal dermal hypoplasia presented with dysphagia.
Multiple esophageal papillomas noted on esophagogastroduodenoscopy.
Human papilloma virus, squamous papillomas associated with focal dermal hypoplasia.
Human papilloma virus polymerase chain reaction negative.
Esophageal and gastric scintigraphy demonstrated a delayed esophageal clearance and mild dealyed gastric emptying.
Squamous cell papillomas with eosinophilic esophagitis.
Endoscopic application of argon plasma coagulation for debulking of esophageal papillomas.Swallowed fluticasone proprionate metered dose inhaler 440 mcg twice a day for eosinophilic esophagitis.
Focal dermal hypoplasia is a rare entity that is associated with esophageal squamous papillomas; however, these have only previously been identified in adults. Argon plasma coagulation has been used safely in children for the destruction of esophageal pathology but not specifically for papilloma removal.
Focal dermal hypoplasia (FDH) is a very rare connective tissue disorder.
The authors found our patient to have both esophageal papillomas and eosinophilic esophagitis. Her papillomas were part of her rare underlying condition of focal dermal hypoplasia. Her eosinophilic esophagitis was treated using swallowed corticosteroids following accepted guidelines. They described a novel approach to treating esophageal papillomas in children using argon plasma coagulation.
The manuscript is an interesting case report of a rare disease (FDH) combined with eosinophilic esophagitis. It is a well written, referenced and illustrated manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
The views expressed in this article are those of the authors and do not reflect the official policies of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the authors, Department of Defense, or any component agency. While we generally excise references to products, companies, manufactures, organizations, etc. in government-produced works, this report presents a special circumstance when such product inclusions become an integral part of the scientific endeavor.
P- Reviewer: Brecelj J, Garcia-Compean D S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Gorlin RJ, Cohen MMJ, Hennekam RCM. In Oxford Monographs on Medical Genetics. Oxford: Oxford Univ. Press 2001; 571-576. |
| 2. | Grzeschik KH, Bornholdt D, Oeffner F, König A, del Carmen Boente M, Enders H, Fritz B, Hertl M, Grasshoff U, Höfling K. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39:833-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Sutton VR, Van den Veyver IB. Focal Dermal Hypoplasia. 2008 May 15 [Updated 2016 Jul 21]. In: Pagon RA, Adam MP, Bird TD, editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle, 1993-2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1543/. |
| 4. | Goltz RW. Focal dermal hypoplasia syndrome. An update. Arch Dermatol. 1992;128:1108-1111. [PubMed] |
| 5. | Goltz RW, Henderson RR, Hitch JM, Ott JE. Focal dermal hypoplasia syndrome. A review of the literature and report of two cases. Arch Dermatol. 1970;101:1-11. [PubMed] |
| 6. | Ascherman JA, Knowles SL, Troutman KC. Extensive facial clefting in a patient with Goltz syndrome: multidisciplinary treatment of a previously unreported association. Cleft Palate Craniofac J. 2002;39:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Rhee KY, Baek RM, Ahn KJ. Airway management in a patient with focal dermal hypoplasia. Anesth Analg. 2006;103:1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Gordjani N, Herdeg S, Ross UH, Grimme H, Kleinschmidt M, Brandis M. Focal dermal hypoplasia (Goltz-Gorlin syndrome) associated with obstructive papillomatosis of the larynx and hypopharynx. Eur J Dermatol. 1999;9:618-620. [PubMed] |
| 9. | Brinson RR, Schuman BM, Mills LR, Thigpen S, Freedman S. Multiple squamous papillomas of the esophagus associated with Goltz syndrome. Am J Gastroenterol. 1987;82:1177-1179. [PubMed] |
| 10. | Kashyap P, Sweetser S, Farrugia G. Esophageal papillomas and skin abnormalities. Focal dermal hypoplasia (Goltz syndrome) manifesting with esophageal papillomatosis. Gastroenterology. 2011;140:784, 1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, Collins MH, Putnam PE, Franciosi JP, von Tiehl KF. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Santander C, Chavarría-Herbozo CM, Becerro-González I, Burgos-Santamaría D. Impaired esophageal motor function in eosinophilic esophagitis. Rev Esp Enferm Dig. 2015;107:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kuchen T, Straumann A, Safroneeva E, Romero Y, Bussmann C, Vavricka S, Netzer P, Reinhard A, Portmann S, Schoepfer AM. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy. 2014;69:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Vance RB, Dunbar KB. Endoscopic options for treatment of dysplasia in Barrett’s esophagus. World J Gastrointest Endosc. 2015;7:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Di Nardo G, Cremon C, Bertelli L, Oliva S, De Giorgio R, Pagano N. Esophageal Inlet Patch: An Under-Recognized Cause of Symptoms in Children. J Pediatr. 2016;176:99-104.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Miller SM, Bellinger CR, Chatterjee A. Argon plasma coagulation and electrosurgery for benign endobronchial tumors. J Bronchology Interv Pulmonol. 2013;20:38-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kähler GF, Szyrach MN, Hieronymus A, Grobholz R, Enderle MD. Investigation of the thermal tissue effects of the argon plasma coagulation modes “pulsed” and “precise” on the porcine esophagus, ex vivo and in vivo. Gastrointest Endosc. 2009;70:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |