Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2201
Peer-review started: December 24, 2016
First decision: January 19, 2017
Revised: February 2, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 28, 2017
Processing time: 94 Days and 17.3 Hours
To assessed the effect of liver cirrhosis (LC) on the poorly understood long-term mortality risk after first-ever mechanical ventilation (1-MV) for acute respiratory failure.
All patients in Taiwan given a 1-MV between 1997 and 2013 were identified in Taiwan’s Longitudinal Health Insurance Database 2000. Each patient with LC was individually matched, using a propensity-score method, to two patients without LC. The primary outcome was death after a 1-MV.
A total of 16653 patients were enrolled: 5551 LC-positive (LC[Pos]) patients, including 1732 with cryptogenic LCs and 11102 LC-negative (LC[Neg]) controls. LC[Pos] patients had more organ failures and were more likely to be admitted to medical department than were LC[Neg] controls. LC[Pos] patients had a significantly lower survival rate (AHR = 1.38, 95%CI: 1.32-1.44). Moreover, the mortality risk was significantly higher for patients with non-cryptogenic LC than for patients with cryptogenic LC (AHR = 1.43, 95%CI: 1.32-1.54) and patients without LC (AHR = 1.56, 95%CI: 1.32-1.54). However, there was no significant difference between patients with cryptogenic and without LC (HR = 1.05, 95%CI: 0.98-1.12).
LC, especially non-cryptogenic LC, significantly increases the risk of death after a 1-MV.
Core tip: Liver cirrhosis, especially non-cryptogenic liver cirrhosis, significantly increases the risk of death after acute respiratory failure.
- Citation: Lai CC, Ho CH, Cheng KC, Chao CM, Chen CM, Chou W. Effect of liver cirrhosis on long-term outcomes after acute respiratory failure: A population-based study. World J Gastroenterol 2017; 23(12): 2201-2208
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2201
The burden of liver cirrhosis (LC) is increasing worldwide because of increases in alcohol abuse and in hepatitis B and C virus infections[1,2]. In France, the prevalence of LC was estimated to be 0.3%, and in the United Kingdom and Sweden, the annual incidence was 14.55-15.3 per 100000 population[3]. Furthermore, its associated morbidity and mortality are also gradually increasing. LC has become the 14th most common cause of death in adults worldwide: it caused 1.03 million deaths per year[4]. In Europe, LC is the fourth most common cause of death: 170000 deaths per year[3]. Chronic liver disease and cirrhosis is the ninth most common cause of death in Taiwan and, the overall incidence rate of death was 30.2 per 100000 per-years (42526 deaths per 140814448 person-years) from chronic liver disease and cirrhosis between 2000 and 2011[5].
Several major complications, such as variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatopulmonary syndrome, can develop in patients with decompensated LC. Because acute organ failure occurs in cirrhotic patients, they might require admission to an Intensive Care Unit (ICU). Several studies[6-12] have investigated the outcome of patients with LC in the ICU; three found that the mortality rate of this group ranged from 36% to 86%[6,7,11]. Other studies[9,13,14] reported that organ failures in critical cirrhotic patients were associated with poor outcomes. One recent study[9] said that using mechanical ventilation (MV) when admitting a patient with advanced cirrhosis was an independent risk factor of mortality. In fact, acute respiratory failure that requires invasive MV is one of the most common clinical causes of ICU admission. However, only one study[12] has assessed the prognosis of critical cirrhotic patients who require MV. Moreover, no study has specifically analyzed the effect of LC on the outcome of patients who require MV. Therefore, we investigated the long-term outcomes of patients with LC who underwent their first-ever MV (1-MV).
In addition to viral hepatitis- and alcohol-related LC, cryptogenic cirrhosis, which is defined as LC that cannot be explained by conventional clinical, laboratory, or histological findings[15,16], is becoming increasingly prevalent in Asia[17-19]. The clinical manifestations and outcomes of LC and cryptogenic LC are different[20]. Thus, we also investigated whether the effects of non-cryptogenic LC and cryptogenic LC on the patients requiring 1-MV are different.
This study used Taiwan’s National Health Insurance Research Database (NHIRD). Taiwan’s NHI is a single-payer compulsory system that enrolls more than 23 million of the country’s legal residents; more than 99.7% of the population is covered. The NHIRD uses the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes to provide detailed healthcare services information on the clinical visits for each insured beneficiary. We used the Longitudinal Health Insurance Database 2000 (LHID2000) which contains 1 million subjects who randomly selected NHI beneficiaries (about 4.34% of the total population) from the year 2000 Registry of Beneficiaries of the NHIR. The LHID2000 are representative of the demographic distribution of Taiwanese population and provides data on outpatient and inpatient medical care, diagnoses, surgical procedures, and prescribed medications on a longitudinal cohort from 1996 to 2013. The study was approved by the Institutional Review Board (IRB 10409-E04) at Chi Mei Medical Center. Because the data used in this study have been deidentified and released to the public for research purposes, the need for informed consent from enrolled patients was waived.
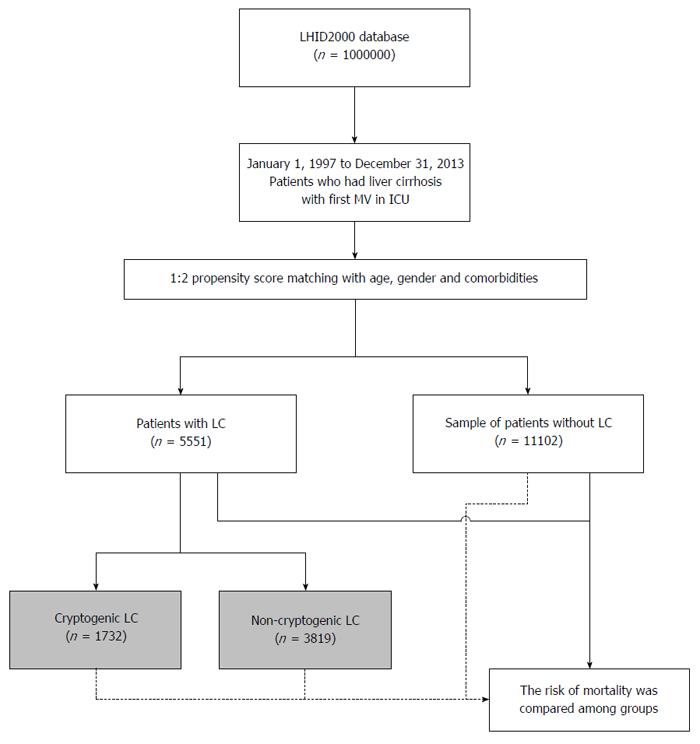
We enrolled all inpatients with a 1-MV for acute respiratory failure (ARF) during their first hospitalization between 1997 and 2013 (n = 58383). Based on a recent study that used the LHID2000[21], our inclusion criteria for patients with LC (LC[Pos]) (ICD-9-CM codes 571.2, 571.5, and 571.6) were three outpatient visits in one year in which LC was diagnosed, or one inpatient admission for LC. Patients who were diagnosed with LC after a 1-MV were excluded (n = 1013). Each enrolled LC[Pos] patient (n = 5551, including 1732 with cryptogenic LC) was then, using propensity score matching, individually matched to two controls without LC (LC[Neg]) (Figure 1). The propensity score, i.e., the probability of having LC, was estimated using a logistic regression model conditional on the covariates of age at times of 1-MV, gender, and individual comorbidities: diabetes mellitus (DM), hypertension (HTN), coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), cancer, stroke, and congestive heart failure (CHF)[21]. In addition, we recorded other liver diseases: hepatitis B virus (HBV) (ICD-9-CM codes 070.2, 070.3, and V02.61), hepatitis C virus (HCV) (070.41, 070.44, 070.51, 070.54, V02.62, and 070.7), and cryptogenic LC, which was defined as LC without a history of HBV, HCV, alcohol drinking, autoimmune disease, hemachromatosis, Wilson’s disease, and alpha-1 antitrypsin deficiency. All of the cryptogenic LC patients had received prior examinations of abdominal echography, and associated laboratory examinations, such as hepatitis B and hepatitis C markers, autoimmune tests. The characteristics of the two groups (LC[Pos] and LC[Neg]) were balanced after the propensity score matching.
The primary endpoint of the study was mortality after 1-MV. Patients were followed from the index admission date until death or the end of 2013. The secondary aim was to identify the risk factors for all-cause mortality after a 1-MV. We hypothesized that mortality is higher in LC[Pos] patients than in LC[Neg] patients who require MV. The demographic and clinical characteristics of age, gender, department to which admitted, number of organ failures, and comorbidities were used to estimate the mortality risk.
Differences in baseline characteristics between groups were evaluated using Pearson’s χ2 test for categorical variables. The actuarial survival rate of the two groups was determined using the Kaplan-Meier method, and a log-rank test was used to compare the difference between the two survival curves. The effect of LC on the mortality risk after 1-MV was assessed using a Cox proportional hazards regression model. Covariates included in the Cox model were age, gender, department to which admitted, number of organ failures, and comorbidities. The proportional hazards assumption was verified using plots of natural log transformed (ln) (survival function) vs ln (time). Significance was set at P < 0.05. SAS 9.4 for Windows (SAS Institute, Cary, NC, United States) was used for all analyses.
We enrolled 16653 patients: 5551 LC[Pos] patients and 11102 LC[Neg] controls (Table 1). LC[Pos] patients had more organ failures, were more likely to be admitted to a medical department, and had a higher mortality rate than did LC[Neg] controls.
| Variables | LC[Pos] patients(n = 5551) | LC[Neg] patients(n = 11102) | P value |
| Gender | 0.99 | ||
| Male | 3655 (65.84) | 7311 (65.85) | |
| Female | 1896 (34.16) | 3791 (34.15) | |
| Age group (yr) | 0.59 | ||
| < 50 | 1259 (22.68) | 2417 (21.77) | |
| 50-64 | 1441 (25.96) | 2899 (26.11) | |
| 65-79 | 1914 (34.48) | 3902 (35.15) | |
| ≥ 80 | 937 (16.88) | 1884 (16.97) | |
| Department | < 0.01a | ||
| Surgical | 542 (9.76) | 1684 (15.17) | |
| Medical | 5009 (90.24) | 9418 (84.83) | |
| Number of organ failures | < 0.01a | ||
| 0 | 3404 (61.32) | 8433 (75.96) | |
| 1 | 1791 (32.26) | 2415 (21.75) | |
| ≥ 2 | 356 (6.41) | 254 (2.29) | |
| Comorbidity | |||
| DM | 2048 (36.89) | 4082 (36.77) | 0.87 |
| HTN | 2454 (44.21) | 4895 (44.09) | 0.89 |
| CAD | 1077 (19.40) | 2144 (19.31) | 0.89 |
| ESRD | 627 (11.30) | 1287 (11.59) | 0.57 |
| COPD | 1133 (20.41) | 2277 (20.51) | 0.88 |
| Cancer | 1362 (24.54) | 2739 (24.67) | 0.85 |
| Stroke | 980 (17.65) | 1944 (17.51) | 0.82 |
| CHF | 784 (14.12) | 1547 (13.93) | 0.74 |
| HBV | 14 (0.25) | 28 (0.25) | 1.00 |
| HCV | 21 (0.38) | 35 (0.32) | 0.51 |
| Cryptogenic LC[Pos] | 1732 (31.20) |
Overall, LC[Pos] patients had a higher risk of death than did LC[Neg] patients (adjusted hazard ratio (AHR) = 1.38; 95%CI: 1.32-1.44). The AHR was higher (1.96; 95%CI: 1.76-2.18) for patients < 50 years old than for patients in other age groups. In addition, both men and women admitted by medical and surgical departments, patients with ≤ 1 organ failure, and patients with comorbid DM, HTN, CAD, ESRD, COPD, cancer, stroke, or CHF had significantly (P < 0.05) higher AHRs (Table 2). In contrast, there were no significant differences for patients with ≥ two organ failures, HBV, or HCV.
| No. of deaths | LC[Pos] patients(n = 5551) | LC[Neg] patients(n = 11102) | Adjusted hazard ratio(95%CI) |
| Overall | 3747 (67.50) | 5902 (53.16) | 1.38 (1.32-1.44)a |
| Age (yr) | |||
| < 50 | 763 (13.75) | 744 (6.70) | 1.96 (1.76-2.18)a |
| 50-64 | 911 (16.41) | 1369 (12.33) | 1.40 (1.29-1.53)a |
| 65-79 | 1368 (24.64) | 2458 (22.14) | 1.24 (1.16-1.32)a |
| ≥ 80 | 705 (12.70) | 1331 (11.99) | 1.41 (1.04-1.25)a |
| Gender | |||
| Male | 2464 (44.39) | 3770 (33.96) | 1.42 (1.35-1.49)a |
| Female | 1283 (23.11) | 2132 (19.20) | 1.30 (1.21-1.39)a |
| Department | |||
| Surgical | 255 (4.59) | 595 (5.36) | 1.32 (1.14-1.54)a |
| Medical | 3492 (62.91) | 5307 (47.80) | 1.37 (1.31-1.43)a |
| Number of organ failures | |||
| 0 | 2074 (37.36) | 4015 (36.16) | 1.40 (1.33-1.47)a |
| 1 | 1379 (24.84) | 1691 (15.23) | 1.26 (1.17-1.35)a |
| ≥ 2 | 294 (5.30) | 196 (1.77) | 1.16 (0.96-1.41) |
| Comorbidity | |||
| DM | 1374 (24.75) | 2432 (21.91) | 1.19 (1.12-1.28)a |
| HTN | 1583 (28.52) | 2851 (25.68) | 1.15 (1.08-1.22)a |
| CAD | 716 (12.90) | 1276 (11.49) | 1.17 (1.07-1.28)a |
| ESRD | 488 (8.79) | 920 (8.29) | 1.19 (1.06-1.33)a |
| COPD | 789 (14.21) | 1459 (13.14) | 1.15 (1.05-1.26)a |
| Cancer | 972 (17.51) | 1668 (15.02) | 1.31 (1.21-1.42)a |
| Stroke | 680 (12.25) | 1261 (11.36) | 1.16 (1.06-1.28)a |
| CHF | 563 (10.14) | 1002 (9.03) | 1.21 (1.09-1.34)a |
| HBV | 7 (0.13) | 12 (0.11) | 0.54 (0.11-2.57) |
| HCV | 11 (0.20) | 20 (0.18) | 0.43 (0.14-1.28) |
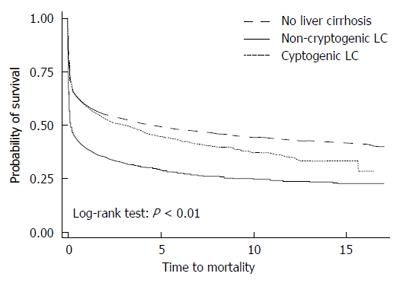
Kaplan-Meier survival curves showed that mortality in patients with non-cryptogenic LC after 1-MV precipitously declined early on and ran parallel thereafter (Figure 2); although the starting point was lower, the trajectory had not changed. In addition, the patients with cryptogenic LC had a higher mortality rate than did LC[Neg] patients, but lower than did patients with non-cryptogenic LC. The absolute survival rate also showed that LC[Neg] patients had the highest 1-, 3-, 5-, and 10-year survival rates, followed by the patients with cryptogenic LC.
Overall, the risk of mortality was significantly higher for patients with non-cryptogenic LC than for patients with cryptogenic LC and for LC[Neg] patients (Table 3). The risk differences in mortality between the patients with non-cryptogenic LC and LC[Neg] patients were significant across the subgroups for men and for women as well as across age groups. The mortality risk was higher (AHR = 2.17, 95%CI: 1.94-2.43) for patients < 50 years old than for patients ≥ 50 years old.
| LC[Neg] | Cryptogenic LC[Pos] | Non-cryptogenic LC[pos] | |
| Overall | 1.00 (ref.) | 1.05 (0.98-1.12) | 1.56 (1.49-1.63)a |
| Patients with LC only | 1.00 (ref.) | 1.43 (1.32-1.54)a | |
| Males only | |||
| Overall | 1.00 (ref.) | 1.06 (0.97-1.16) | 1.58 (1.49-1.67)a |
| Patients with LC only | 1.00 (ref.) | 1.40 (1.27-1.55)a | |
| Females only | |||
| Overall | 1.00 (ref.) | 1.02 (0.92-1.13) | 1.52 (1.40-1.64)a |
| Patients with LC only | 1.00 (ref.) | 1.48 (1.31-1.67)a | |
| Age group: < 50 | |||
| Overall | 1.00 (ref.) | 1.31 (1.07-1.60)a | 2.17 (1.94-2.43)a |
| Patients with LC only | 1.00 (ref.) | 1.68 (1.36-2.08)a | |
| Age group: 50-64 | |||
| Overall | 1.00 (ref.) | 0.96 (0.82-1.13) | 1.59 (1.44-1.74)a |
| Patients with LC only | 1.00 (ref.) | 1.70 (1.42-2.03)a | |
| Age group: 65-79 | |||
| Overall | 1.00 (ref.) | 1.07 (0.96-1.18) | 1.35 (1.25-1.46)a |
| Patients with LC only | 1.00 (ref.) | 1.27 (1.13-1.43)a | |
| Age group: ≥ 80 | |||
| Overall | 1.00 (ref.) | 0.92 (0.80-1.06) | 1.31 (1.17-1.45)a |
| Patients with LC only | 1.00 (ref.) | 1.40 (1.19-1.64)a |
This is the first study that investigates (1) the effect of LC on the outcomes of the patients after 1-MV; and (2) the different effects of non-cryptogenic LC and cryptogenic LC on this specific group. We have several significant findings.
First, after adjusting for possible confounding factors, we found that LC itself was significantly associated with poor patient outcomes after a 1-MV (AHR = 1.38, 95%CI: 1.32-1.44). Although other studies have shown the grave outcomes of patients critically ill with LC[9,12,22,23] and one[24] reported that the overall in-hospital mortality rate of patients with LC in their Acute Physiology and Chronic Health Evaluation III (APACHE III)-matched group was higher than that in the LC[Neg] group (73.6% vs 57.5%, P = 0.026), the present study is the first one to show the negative effects of LC on the outcomes of critically ill patients who require MV. Moreover, we found that this kind of significant association was apparent only for patients with non-cryptogenic LC (AHR = 1.56, 95%CI: 1.49-1.63), but not for cryptogenic patients (AHR = 1.05, 95%CI: 0.98-1.12). All of these findings indicate that LC, especially non-cryptogenic LC, is associated with poor outcomes for critically ill patients who require MV.
Second, we found that non-cryptogenic LC was significantly associated with worse outcomes in patients after a 1-MV than cryptogenic LC (AHR = 1.43, 95%CI: 1.32-1.54). In contrast, one retrospective Malaysian cohort study[20] reported, after comparing the clinical outcomes in 94 cases cryptogenic LC and 207 cases of non-cryptogenic LC, cases that there was no significant difference in mortality between these two groups; however, the sample in that study was relatively small. A Japanese study[25], which compared 68 patients with cirrhotic non-alcoholic steatohepatitis (NASH) and 69 with HCV-induced LC, found that the 5-year survival rates and liver-related mortality were not significantly different in the two groups. A Sri Lankan study[26] of 306 alcoholic LC[Pos] and 243 cryptogenic LC[Pos] patients also found that survival rates were not significantly different between the two groups. The difference between the present study and these three Asian studies can be explained by different study designs and patient populations. Our study focused only on the mortality of patients after a 1-MV, and we used all-cause mortality for outcome analysis. However, additional large-scale studies are warranted to determine whether the effects of LC and cryptogenic LC on different specific groups are different.
Third, we also investigated the negative effects of LC on the outcomes of patients (stratified by age and gender) after a 1-MV. We found that all LC[Pos] patients had higher mortality risks, but that only non-cryptogenic LC[Pos] patients had significantly higher AHRs regardless of age group and gender. The < 50 years old group had the highest AHR for mortality of all age groups. Thus, our findings suggest that we should pay more attention to developing methods to reduce the negative effects of LC for these younger high-risk patients. However, additional case-control studies are needed to confirm such a relationship. We also found that AHRs for mortality were not significantly different between male and female LC[Pos] patients after a 1-MV. Two recent studies[27,28] in Taiwan reported that in-hospital mortality was significantly more highly associated with men than with women, but an American study[29] reported the opposite. Differences in our findings might be attributable to our having enrolled only LC[Pos] patients, unlike the study populations of these other studies.
Our study has some strengths. It is a large population-based analysis of the effect of LC on patients given a 1-MV. NHIRD includes data on over 99% of all residents in Taiwan, therefore, it allows large-scale and longitudinal follow-up epidemiological studies and health services research. In addition, this kind of nationwide study design largely reduces the effect of referral bias, which is often seen in critical care studies. This investigation should provide robust data on the characteristics and effects of critical cirrhotic patients requiring MV in Taiwan.
Our study also has some limitations. First, because our study relies on administrative databases rather than on actual patient charts for all diagnoses, including comorbidities, and on the claims data and ICD-9-CM diagnosis codes, some of the diagnoses might be incorrect. Alcoholic and NASH were the two major cause of LC. However, this study is using the NHIRD database, which cannot provide history of alcoholic using and the diagnosis of NASH. Therefore, we cannot make sure the diagnosis of alcoholic LC and analysis the effect of alcoholic LC. Besides, there are no images or lab data to support the diagnoses, our conclusions cannot be totally convincing. Nonetheless, the Taiwan NHI Bureau randomly reviews patient charts and interviews patients to verify the accuracy of the coding. Hospitals with outlier charges or practices might be audited and subsequently heavily penalized for malpractice or discrepancies. Therefore, the potential risk for bias based on coding practices can be minimized. Second, because the NHIRD does not contain data that differentiate disease severities, we were unable to take into account the illness severity scores of cirrhotic patients who required MV; thus, we included the number of organ failures as a proxy for severity. Although we found LC[Pos] with MOF had higher risk of death than without MOF, the difference did not reach statistical significance. It may be due to the limited case number. Further larger scale study may be warranted to investigate this issue. Third, as in all observational studies, our study might contain some residual confounding, which prevents us from arriving at conclusions about causality but only correlations between risk factors and mortality. Moreover, the primary reasons for admitting these LC[Pos] patients with a 1-MV are unknown, as are additional details about the severity of their LC. Finally, the enrolled patients were selected from a heterogeneous general population, which more than likely makes generalizing our conclusions too arbitrary. However, given the large magnitude of the observed effects in this study, these limitations are unlikely to have compromised the results. Further investigation about the cause of death using other databank is required.
In conclusion, LC, especially non-cryptogenic LC, significantly increases the risk of mortality after a 1-MV. The greatest negative effect of LC was on patients < 50 years old.
In addition to viral hepatitis- and alcohol-related liver cirrhosis (LC), cryptogenic cirrhosis, which is defined as LC that cannot be explained by conventional clinical, laboratory, or histological findings, is becoming increasingly prevalent in Asia. The clinical manifestations and outcomes of LC and cryptogenic LC are different, especially for patients using mechanical ventilation (MV). Thus, the authors investigated the long-term outcomes of patients with LC who underwent their first-ever MV (1-MV), and also compared the different impact of 1-MV on the patients with non-cryptogenic LC or cryptogenic LC.
Multiple organ failures in critical cirrhotic patients were associated with poor outcomes. The use of MV for a patient with advanced cirrhosis was an independent risk factor of mortality. However, no large study has specifically analyzed the effect of LC on the long-term outcome of patients who underwent their 1-MV.
A total of 16653 patients were enrolled. LC patients had a significantly lower survival rate (AHR = 1.38) after their 1-MV. Moreover, the mortality risk was significantly higher for patients with non-cryptogenic LC than for patients with cryptogenic LC (AHR = 1.43) and patients without LC (AHR = 1.56). However, there was no significant difference between patients with cryptogenic and without LC (AHR = 1.05, 95%CI: 0.98-1.12). The risk differences in mortality between the patients with non-cryptogenic LC and patients without LC were significant across the subgroups for men and for women as well as across age groups. The mortality risk was higher (AHR = 2.17) for patients < 50 years old than for patients ≥ 50 years old.
After adjusting for possible confounding factors, we found that LC itself was significantly associated with poor patient outcomes after a 1-MV. Non-cryptogenic LC was also significantly associated with worse outcomes in patients after a 1-MV than cryptogenic LC and patients without LC. The < 50 years old group had the highest AHR for mortality of all age groups. Thus, our findings suggest that we should pay more attention to developing methods to reduce the negative effects of LC for these younger high-risk patients.
LC, especially non-cryptogenic LC, significantly increases the risk of death after a 1-MV.
Very good work has been performed by Lai CC et al comparing the effect of LC on the poorly understood long-term mortality risk after a 1-MV for acute respiratory failure. Congratulation to the authors for adding valuable data for LC, especially non-cryptogenic LC, significantly increases the risk of death after a 1-MV.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Harada K, Isik A S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 2. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 3. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 908] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 4. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9574] [Article Influence: 736.5] [Reference Citation Analysis (0)] |
| 5. | Chiang CJ, Yang YW, Chen JD, You SL, Yang HI, Lee MH, Lai MS, Chen CJ. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology. 2015;61:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Aggarwal A, Ong JP, Younossi ZM, Nelson DR, Hoffman-Hogg L, Arroliga AC. Predictors of mortality and resource utilization in cirrhotic patients admitted to the medical ICU. Chest. 2001;119:1489-1497. [PubMed] |
| 7. | Chen YC, Tsai MH, Ho YP, Hsu CW, Lin HH, Fang JT, Huang CC, Chen PC. Comparison of the severity of illness scoring systems for critically ill cirrhotic patients with renal failure. Clin Nephrol. 2004;61:111-118. [PubMed] |
| 8. | Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care. 2012;16:R227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Levesque E, Hoti E, Azoulay D, Ichaï P, Habouchi H, Castaing D, Samuel D, Saliba F. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Shawcross DL, Austin MJ, Abeles RD, McPhail MJ, Yeoman AD, Taylor NJ, Portal AJ, Jamil K, Auzinger G, Sizer E. The impact of organ dysfunction in cirrhosis: survival at a cost? J Hepatol. 2012;56:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Shellman RG, Fulkerson WJ, DeLong E, Piantadosi CA. Prognosis of patients with cirrhosis and chronic liver disease admitted to the medical intensive care unit. Crit Care Med. 1988;16:671-678. [PubMed] |
| 12. | Levesque E, Saliba F, Ichaï P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Saliba F, Ichai P, Levesque E, Samuel D. Cirrhotic patients in the ICU: prognostic markers and outcome. Curr Opin Crit Care. 2013;19:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 14. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 733] [Article Influence: 66.6] [Reference Citation Analysis (1)] |
| 15. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3717] [Article Influence: 161.6] [Reference Citation Analysis (2)] |
| 16. | Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19:3375-3384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Chitturi S, Farrell GC, George J. Non-alcoholic steatohepatitis in the Asia-Pacific region: future shock? J Gastroenterol Hepatol. 2004;19:368-374. [PubMed] |
| 19. | Dassanayake AS, Kasturiratne A, Rajindrajith S, Kalubowila U, Chakrawarthi S, De Silva AP, Makaya M, Mizoue T, Kato N, Wickremasinghe AR. Prevalence and risk factors for non-alcoholic fatty liver disease among adults in an urban Sri Lankan population. J Gastroenterol Hepatol. 2009;24:1284-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Mohammed OK, Mahadeva S. Clinical outcomes of cryptogenic compared with non-cryptogenic cirrhosis: A retrospective cohort study. J Gastroenterol Hepatol. 2015;30:1423-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Cheng CY, Ho CH, Wang CC, Liang FW, Wang JJ, Chio CC, Chang CH, Kuo JR. One-Year Mortality after Traumatic Brain Injury in Liver Cirrhosis Patients--A Ten-Year Population-Based Study. Medicine (Baltimore). 2015;94:e1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Cholongitas E, Senzolo M, Patch D, Shaw S, Hui C, Burroughs AK. Review article: scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther. 2006;24:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Tsai MH, Chen YC, Ho YP, Fang JT, Lien JM, Chiu CT, Liu NJ, Chen PC. Organ system failure scoring system can predict hospital mortality in critically ill cirrhotic patients. J Clin Gastroenterol. 2003;37:251-257. [PubMed] |
| 24. | Fu CM, Chang CH, Fan PC, Tsai MH, Lin SM, Kao KC, Tian YC, Hung CC, Fang JT, Yang CW. Prognosis of critically ill cirrhotic versus non-cirrhotic patients: a comprehensive score-matched study. BMC Anesthesiol. 2014;14:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Senanayake SM, Niriella MA, Weerasinghe SK, Kasturiratne A, de Alwis JP, de Silva AP, Dassanayake AS, de Silva HJ. Survival of patients with alcoholic and cryptogenic cirrhosis without liver transplantation: a single center retrospective study. BMC Res Notes. 2012;5:663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Chen CJ, Shi HY, Lee KT, Huang TY. In-hospital mortality prediction in patients receiving mechanical ventilation in Taiwan. Am J Crit Care. 2013;22:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Chen CM, Lai CC, Cheng KC, Weng SF, Liu WL, Shen HN. Effect of end-stage renal disease on long-term survival after a first-ever mechanical ventilation: a population-based study. Crit Care. 2015;19:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |