Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2168
Peer-review started: December 1, 2016
First decision: January 10, 2017
Revised: January 17, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: March 28, 2017
Processing time: 119 Days and 22.9 Hours
To design a miniature magnetically anchored and controlled camera system to reduce the number of trocars which are required for laparoscopy.
The system consists of a miniature magnetically anchored camera with a 30° downward angle, an external magnetically anchored unit, and a vision output device. The camera weighs 12 g, measures Φ10.5 mm × 55 mm and has two magnets, a vision model, a light source, and a metal hexagonal nut. To test the prototype, the camera was inserted through a 12-mm conventional trocar in an ex vivo real liver laparoscopic training system. A trocar-less laparoscopic cholecystectomy was performed 6 times using a 12-mm and a 5-mm conventional trocar. In addition, the same procedure was performed in four canine models.
Both procedures were successfully performed using only two conventional laparoscopic trocars. The cholecystectomy was completed without any major complication in 42 min (38-45 min) in vitro and in 50 min (45-53 min) using an animal model. This camera was anchored and controlled by an external unit magnetically anchored on the abdominal wall. The camera could generate excellent image. with no instrument collisions.
The camera system we designed provides excellent optics and can be easily maneuvered. The number of conventional trocars is reduced without adding technical difficulties.
Core tip: This study introduced a miniature magnetically anchored and controlled camera system. The miniature magnetically anchored camera is among the smallest size, and it can pass through a conventional 12-mm trocar. Magnetically anchored instruments are positioned intra-abdominally and stabilized through a coupling force to external magnets on the abdominal skin. In this way, the instruments do not share space with the trocar during surgery. By using this camera system, the number of trocars required for conventional laparoscopy could be reduced without adding technical difficulties.
- Citation: Dong DH, Zhu HY, Luo Y, Zhang HK, Xiang JX, Xue F, Wu RQ, Lv Y. Miniature magnetically anchored and controlled camera system for trocar-less laparoscopy. World J Gastroenterol 2017; 23(12): 2168-2174
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2168
Laparoscopic surgery has significantly evolved as a conventional surgical procedure for smaller incisions and faster recovery since its emergence in the late 1980s[1,2]. It is a standard alternative practice to the traditional open operation in cholecystectomy, nephrectomy, and other procedures[3-5]. Conventional laparoscopic surgery requires 3 or more 5-10 mm trocars. The number of trocars is associated with postoperative pain, cosmesis, and the risk of bleeding or organ damage[6,7]. Minimizing the invasiveness of surgery is a fundamental driving force for surgeons and patients seeking new procedures with fewer trocars.
Single-site laparoscopy (SSL), represented by laparoendoscopic single-site (LESS) surgery and natural orifice transluminal endoscopic surgery, has recently gained more interest among minimally invasive laparoscopic surgeons[8-10]. SSL is superior to conventional multiport laparoscopy for cosmesis because the new procedure relies on a single port site that is limited to an inconspicuous position. In theory, the number of trocars is reduced in SSL. However, in reality, the incision length of the single port in SSL is much greater than that used in conventional multiport laparoscopy (approximately 25-30 mm vs 5-12 mm), which increases the risk of post-operative inflammation[11,12]. Moreover, because all of the instruments are restricted to a single trocar, SSL is also technically demanding, and the technical challenges are intrinsically linked to loss of triangulation and instrument conflicts[13-15]. As a result, the widespread adoption of SSL is limited.
In the hands of laparoscopic surgeons, laparoscopic surgery has relied on fewer conventional trocars and multiple instruments constrained in a single trocar to overcome the challenges faced by SSL. Therefore, our team attempted to perform trocar-less laparoscopy by developing a miniature magnetically anchored camera that can pass through a conventional laparoscopic trocar. The magnetically anchored and controlled instruments were first introduced by Caddedu in 2007 and were called magnetically anchored and guided systems[16]. Such instruments are positioned intra-abdominally and stabilized through a coupling force to external magnets on the abdominal skin. In this way, the instruments do not share space with the trocar during surgery.
In the present study, we present the initial development of an MMAC weighing 12 g and measuring Φ10.5 mm × 55 mm. It has a 30° downward angle and can be inserted into the abdomen through a conventional 12 mm trocar. By using this miniature camera, the number of conventional trocars is reduced without adding more demanding technics. This camera provides excellent optics of the surgical space and can easily be maneuvered in the abdomen. With this camera, a trocar-less laparoscopic cholecystectomy using two conventional trocars was performed in an animal model.
Similar to our previous work, the miniature magnetically anchored and controlled camera system consists of an MMAC with a 30° downward angle, an external magnetically anchored unit, and a vision output device. The camera is the internal component and is inserted into the abdominal cavity through a conventional trocar. Its position in the abdomen is controlled by the external magnetically anchored unit via a magnetic force through the abdominal wall. On the basis of previous work, the external magnetically anchored unit is composed of 15 magnets (60 mm × 10 mm × 4 mm, NdFeB N50 permanent magnet). The external magnetically anchored unit regulates the magnetic force according to abdominal wall thickness. The vision output device displays an image captured by the miniature camera and supports power for the system.
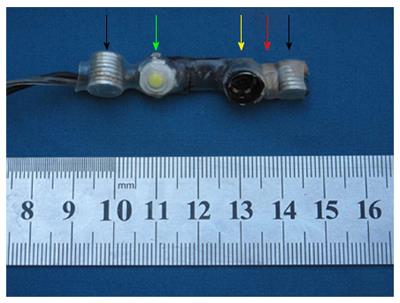
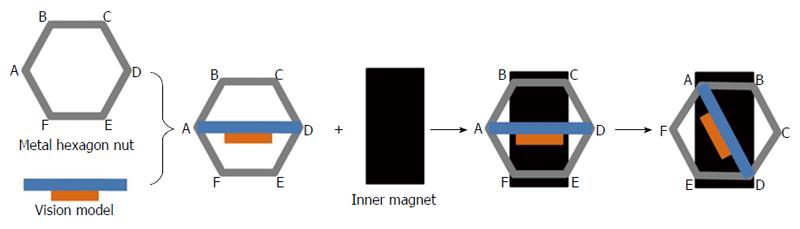
The size of the camera is the most important part for trocar-less laparoscopy surgery. The MMAC measures Φ10.5 mm × 55 mm and is composed of two magnets (Φ8 mm × 7 mm, NdFeB N50 permanent magnet), a vision model, a metal hexagonal nut, and a light source (Figure 1). The vision model is produced by Audenson Technology Corporation (Shenzhen, China) and consists of a 1/5″ 1024 × 768 pixel color CMOS camera and a 720-line TV contained in an 8 mm × 6 mm × 2.5 mm cuboid. The focal length of the camera lens is 6-8 cm. The metal hexagonal nut is fixed on the lateral surface of the vision model. The nut is used to achieve the 30° downward angle of the vision model. A 3 W hemispherical light-emitting diode (LED) (Juli Industrial Development Corporation, Shenzhen, China) is the light source. It provides high-color temperature (6000-6500 K) and luminous flux (200-220 LM) equal to that of xenon lamps used in conventional laparoscopy. The MMAC weighs approximately 12 g.
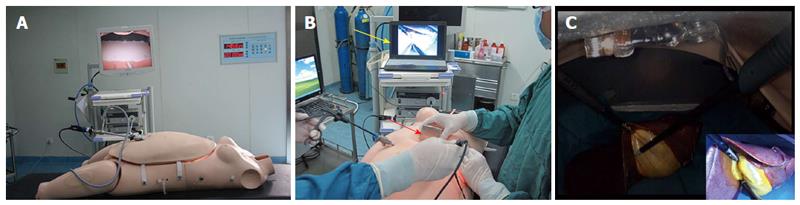
A laparoscopy training platform, called the ex vivo real-liver laparoscopic training system, was used as the bench test in this study (Figure 2A). It was composed of a special dummy and a laparoscope. The abdominal wall of the dummy was made of a material and a nonmagnetic metal support that mimics the elasticity and shape of a human pneumoperitoneum. A partial porcine liver was placed in the dummy for the laparoscopic cholecystectomy model.
In the bench test, a laparoscope was inserted through an umbilical trocar when necessary to visualize the camera performance. The trocar-less laparoscopic cholecystectomy was performed using a 12-mm trocar and a 5-mm conventional trocar. The 12-mm trocar was placed below the xiphoid, and the 5-mm trocar was placed at the right mid-clavicular line. The MMAC was inserted into the abdomen through the 12-mm conventional trocar and then coupled to the external magnetically anchored unit by gently depressing it. The camera was maneuvered into position using the external magnet. The conventional laparoscopic instruments (Hangzhou Kangji Medical Instrument Co., Ltd., Zhejiang, China) were inserted through the 12- and 5-mm trocars as needed during the cholecystectomy. The gallbladder was freed from its hepatic attachments, and the cystic duct was transected. After completing the procedure, the gallbladder was extracted from the xiphoid defect. The camera was removed by pulling cables after decoupling the external magnet. The procedure was performed 6 times. The performance of the MMAC was evaluated by manipulation, operative time, and the achievement of critical views.
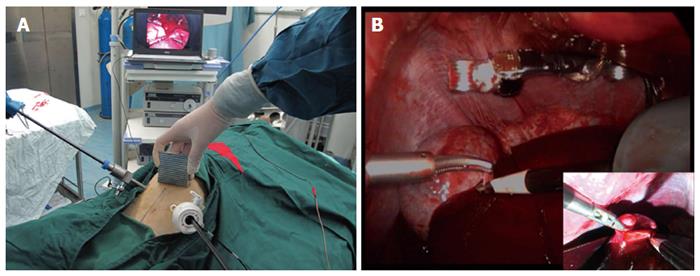
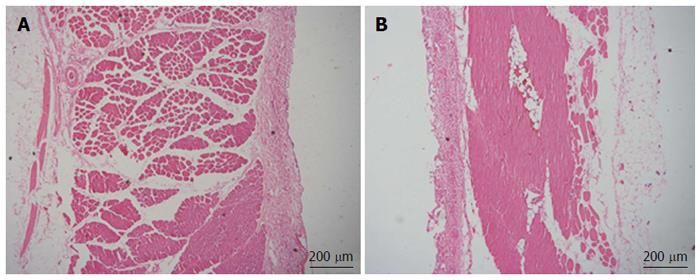
After obtaining approval from the institutional animal care and use committee of our institution, the trocar-less laparoscopic cholecystectomy was performed in 4 male canines weighing a mean of 15 kg. The surgical instruments and techniques used were the same as for the in vitro test. Additionally, the abdominal cavity was insufflated with carbon dioxide to a pressure of 15 mm Hg. The surgeon manipulated the external magnet during the procedure to adjust the view of the MMAC. After the operation, the surgeon closed the incision with an interrupted absorbable suture. In addition to the indicators mentioned above, estimated blood loss, adverse events, and biosafety were used to assess the camera. Biosafety was evaluated by peritoneal specimens in the active area of the magnetic camera. They were harvested after the operation and examined using HE staining.
The trocar-less laparoscopic cholecystectomy using 12- and 5-mm conventional trocars was successfully performed in vitro in all 6 cases. The MMAC easily passed through the 12-mm conventional trocar, and only thin wires for powering and imaging were left in the trocar, which provided sufficient space for conventional laparoscopic instruments and did not cause instrument conflicts. The camera was anchored and controlled well by the external magnetically anchored unit on the dummy skin surface. Decoupling did not occur during the operation, even when the wires were used to pull out the camera. The camera’s 30° downward angle enabled critical views of the procedure. The image generated by the camera was excellent because it was equipped with the high-color temperature LED and the focal length of the camera lens was appropriate for the height of the pneumoperitoneum. The mean operative time was 42 min (38-45 min) (Figure 2B and C).
After confirming the camera’s feasibility for trocar-less laparoscopic cholecystectomy in vitro, similar procedures were also completed in 4 canines without adverse events, such as bile spillage. The mean estimated blood loss was 6 mL (3-10 mL). In the canine model, the camera was also inserted into the abdomen through a 12-mm conventional trocar and was easily “pulled up” to the thin abdominal wall (approximately 1 cm according to the preoperative ultrasound). The camera was manipulated into position by smoothly sliding the external magnetically anchored unit. Because of the 30° downward angle, the critical views were achieved well, and the image quality was as excellent as it was in vitro, with high resolution and sufficient lighting (Figure 3). Approximately once per operation, the camera had to be pulled out by the wires to clean fog off of the lens. There were no instrument collisions. The mean operative time was 50 min (45-53 min) in vivo. Hematoxylin-eosin staining showed no significant tissue damage at the muscle layer in the camera’s active area, thus supporting its biosafety in vivo (Figure 4).
SSL was once considered a promising alternative approach to conventional laparoscopic surgery[9]. Unfortunately, all instruments were constrained in a single port, which was technically demanding, and the incision length impeded the widespread adoption of SSL for laparoscopic surgery[11-15]. The new approach must maintain the ergonomics of traditional laparoscopy and reduce the incision length. We have therefore investigated trocar-less laparoscopy, which relies on conventional laparoscopic trocars. For example, a laparoscopic cholecystectomy was performed using 2 conventional trocars in this study. Because trocar-less laparoscopy is predicated on conventional trocars, the surgical instruments for the new approach, such as the retractor and cautery device, are the same as those used for conventional laparoscopy. Trocar-less laparoscopy addresses the dilemma of SSL while offering additional benefits. It saves money and training time compared with SSL, which encourages its clinical adoption.
The new instrument’s special positioning technology is the key to trocar-less laparoscopy and should address the following requirements: First, the new instrument should not occupy space in the trocar during surgery to avoid instrument conflicts. Second, the new instrument should use the entire insufflated abdomen to accommodate the required changes in position. Many positioning technologies have been attempted in trocar-less surgery, such as vacuum positioning, needle anchoring, and magnetic anchoring[16-18]. Magnetically anchoring is the most promising positioning technology because the coupling force is generated by internal and outer components without direct contact, thus allowing the instrument to be free from the trocar and enabling the full use of the insufflated abdomen. Other positioning technologies have deficiencies in reliable anchoring and convenient guidance.
Surgeons who perform minimally invasive laparoscopic procedures have significant interests in developing magnetically anchored and controlled instruments. Numerous instruments have been developed, such as cameras, retractors, dissectors, and even surgical robots[19-25]. Most of these instruments are above Φ20 mm because they aim to address instrument collisions in SSL, which are not suitable for trocar-less surgery that relies on conventional trocars. Our team has strongly advocated this promising technology in the hope of further advancing minimally invasive surgery. Our team previously developed a deployable magnetically anchored retractor that could pass through a conventional 12 mm trocar[26]. We also developed an MMAC measuring Φ11 mm × 50 mm aimed at reducing the incision of SSL ( unpublished data). However, the view angle of this camera is 90° downward, whereas conventional laparoscopy has a 30° downward angle. In our current work, we refined the camera’s internal magnets and vision model to minimize its size. More significantly, the latest generation of the magnetically anchored camera achieves a 30° downward angle using a metal hexagonal nut, thus making the new camera suitable for trocar-less surgery. The 30° downward angle was achieved as follows: (1) the bottom of the vision model was fixed to point A and point D of the metal hexagonal nut; (2) the metal nut was attracted by an inner magnet (the inner magnet was vertical to the long axis of the camera) so the vision model would achieve a different downward angle by turning the nut; and (3) when LAE was parallel to the long axis of the inner magnet, the vision model had a 30° downward angle (Figure 5). This is a simple but reliable method to achieve the 30° downward angle because the nut is hexagonal.
This is a small pilot study limited to a canine model. The new generation of cameras will be a vital advance only if the following problems are addressed: First, if the new camera can cooperate with other magnetically anchored and controlled instruments (such as the magnetically anchored retractor that we developed previously), the trocar-less laparoscopy performed with a single conventional trocar will be possible. Unfortunately, in our experience, multiple outer magnets cause magnet-to-magnet interference and operator hand-to-magnet collisions. The minimum separation distance of outer magnets is considered to be 3 cm[16]. Future studies should focus on developing an external magnet platform to reconcile the collisions by keeping the outer magnets at an appropriate separation distance. Second, kinematic safety is still a blind area in magnetically anchored and controlled instruments. Although decoupling rarely occurs in current research because the latest camera is light and the outer magnet is sufficiently large, it is necessary to resolve the cause of decoupling to operate magnetically anchored and controlled instruments safely. Abdominal wall thickness has been regarded as a relative factor[27]. However, more issues need to be explored for the further development of magnetically anchored and controlled instruments.
In conclusion, we have successfully designed, manufactured, and implemented a new magnetically anchored and controlled camera system to perform trocar-less laparoscopy. The system consists of an MMAC with a 30° downward angle, a vision output device, and an external magnetically anchored unit. The miniature camera measures Φ10.5 mm × 55 mm and weighs 12 g. It can be inserted into a 12-mm conventional trocar and easily maneuvered in the abdomen. The image generated by the camera is excellent and sufficient to perform cholecystectomy. Pilot studies in canine models have demonstrated the feasibility of canine laparoscopic cholecystectomy using the MMAC with a 30° downward angle and only 2 conventional trocars. Future studies will aim to modify the current device and develop new magnetically anchored and controlled instruments to minimize the invasiveness of laparoscopic surgery further.
Laparoscopy is a promising minimally invasive method that is based on multiple trocars. Decreasing the number of trocars necessary for laparoscopy could further reduce surgical trauma and achieve a better cosmetic outcome. Caddedu first introduced magnetically anchored and controlled instruments in 2007. Such instruments could not share space with the trocar during surgery. However, previous magnetically anchored instruments were greater than Φ20 mm to address instrument collisions in single-site laparoscopy (SSL). This would not be suitable for conventional laparoscopy. Therefore, the authors designed a miniature magnetically anchored and controlled camera system that could pass through the conventional laparoscopic trocar. By using this camera system, the number of trocars required for conventional laparoscopy could be reduced without adding technical difficulties.
A magnetically anchored instrument is the most promising positioning method for trocar-less laparoscopy because the coupling force is generated by internal and outer components without direct contact, thus allowing the instrument to be free from the trocar and enabling full use of the insufflated abdomen. However, previous magnetically anchored instruments are too large to pass through a conventional laparoscopic trocar.
In this study, the authors provided a miniature magnetically anchored camera measuring Φ10.5 mm × 55 mm, which was the first able to pass through the conventional 12 mm trocar. A trocar-less laparoscopic cholecystectomy using 2 conventional trocars was performed using this camera system. The authors developed an artful method to achieve the 30° downward angle of the camera by simply using a metal hexagon, which is crucial to obtain excellent intraoperative optics.
The miniature magnetically anchored and controlled camera system provided in this study could realize trocar-less laparoscopy by replacing the trocar used for the laparoscopy. Combined with other instruments, a laparoscopic cholecystectomy based on only one conventional trocar could be achieved in the future by using the camera system, which would be much less invasive than current SSL.
Magnetically anchored instruments are positioned intra-abdominally and stabilized through a coupling force to external magnets on the abdominal skin. In this way, the instruments do not share space with the trocar during surgery. The miniature magnetically anchored camera is a type of magnetically anchored instrument used for providing intraoperative optics.
The research group presented an interesting concept. The miniature magnetically anchored and controlled camera system has drawn the interest of surgeons to conduct further research and make a judgment about it. It may be a promising method for trocar-less laparoscopy. This is a very interesting work.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Azodi M S- Editor: Yu J L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Cooperman AM. Laparoscopic cholecystectomy for severe acute, embedded, and gangrenous cholecystitis. J Laparoendosc Surg. 1990;1:37-40. [PubMed] |
| 2. | Cuschieri A, Dubois F, Mouiel J, Mouret P, Becker H, Buess G, Trede M, Troidl H. The European experience with laparoscopic cholecystectomy. Am J Surg. 1991;161:385-387. [PubMed] |
| 3. | Sajid MS, Khawaja AH, Sains P, Singh KK, Baig MK. A systematic review comparing laparoscopic vs open adhesiolysis in patients with adhesional small bowel obstruction. Am J Surg. 2016;212:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Taghavi S, Ambur V, Jayarajan SN, Gaughan J, Toyoda Y, Dauer E, Sjoholm LO, Pathak A, Santora T, Goldberg AJ. Postoperative outcomes with cholecystectomy in lung transplant recipients. Surgery. 2015;158:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187:1392-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Lowry PS, Moon TD, D’Alessandro A, Nakada SY. Symptomatic port-site hernia associated with a non-bladed trocar after laparoscopic live-donor nephrectomy. J Endourol. 2003;17:493-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Salö M, Järbur E, Hambraeus M, Ohlsson B, Stenström P, Arnbjörnsson E. Two-trocar appendectomy in children - description of technique and comparison with conventional laparoscopic appendectomy. BMC Surg. 2016;16:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Cai HH, Liu MB, He YL. Treatment of Early Stage Endometrial Cancer by Transumbilical Laparoendoscopic Single-Site Surgery Versus Traditional Laparoscopic Surgery: A Comparison Study. Medicine (Baltimore). 2016;95:e3211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Hernandez J, Ross S, Morton C, McFarlin K, Dahal S, Golkar F, Albrink M, Rosemurgy A. The learning curve of laparoendoscopic single-site (LESS) cholecystectomy: definable, short, and safe. J Am Coll Surg. 2010;211:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Hungness ES, Sternbach JM, Teitelbaum EN, Kahrilas PJ, Pandolfino JE, Soper NJ. Per-oral Endoscopic Myotomy (POEM) After the Learning Curve: Durable Long-term Results With a Low Complication Rate. Ann Surg. 2016;264:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Ishibashi S, Takeuchi H, Fujii K, Shiraishi N, Adachi Y, Kitano S. Length of laparotomy incision and surgical stress assessed by serum IL-6 level. Injury. 2006;37:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Watanabe J, Ota M, Fujii S, Suwa H, Ishibe A, Endo I. Randomized clinical trial of single-incision versus multiport laparoscopic colectomy. Br J Surg. 2016;103:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Milas M, Deveđija S, Trkulja V. Single incision versus standard multiport laparoscopic cholecystectomy: up-dated systematic review and meta-analysis of randomized trials. Surgeon. 2014;12:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Rivas H, Varela E, Scott D. Single-incision laparoscopic cholecystectomy: initial evaluation of a large series of patients. Surg Endosc. 2010;24:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Wang GJ, Afaneh C, Aull M, Charlton M, Ramasamy R, Leeser DB, Kapur S, Del Pizzo JJ. Laparoendoscopic single site live donor nephrectomy: single institution report of initial 100 cases. J Urol. 2011;186:2333-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Park S, Bergs RA, Eberhart R, Baker L, Fernandez R, Cadeddu JA. Trocar-less instrumentation for laparoscopy: magnetic positioning of intra-abdominal camera and retractor. Ann Surg. 2007;245:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Hiroki C, YI S, Yu WW. A Study on Wire-Wire Driven Abdominal Cavity Mobile Micro Robot. The 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems. 2010;2816-2821. |
| 18. | Ohdaira T, Endo K, Abe N, Yasuda Y. Usefulness in NOTES of an intra-abdominal antifogging wireless charge-coupled device (CCD) camera with pantograph-type needle unit for placement to the intra-abdominal wall. Surg Endosc. 2010;24:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Arain NA, Cadeddu JA, Hogg DC, Bergs R, Fernandez R, Scott DJ. Magnetically anchored cautery dissector improves triangulation, depth perception, and workload during single-site laparoscopic cholecystectomy. J Gastrointest Surg. 2012;16:1807-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Arain NA, Rondon L, Hogg DC, Cadeddu JA, Bergs R, Fernandez R, Scott DJ. Magnetically anchored camera and percutaneous instruments maintain triangulation and improve cosmesis compared with single-site and conventional laparoscopic cholecystectomy. Surg Endosc. 2012;26:3457-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Terry BS, Mills ZC, Schoen JA, Rentschler ME. Single-Port-Access Surgery with a Novel Magnet Camera System. 2012;1187-1193. |
| 22. | Best SL, Bergs R, Scott DJ, Fernandez R, Mashaud LB, Cadeddu JA. Solo surgeon laparo-endoscopic single site nephrectomy facilitated by new generation magnetically anchored and guided systems camera. J Endourol. 2012;26:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Lehman AC, Dumpert J, Wood NA, Redden L, Visty AQ, Farritor S, Varnell B, Oleynikov D. Natural orifice cholecystectomy using a miniature robot. Surg Endosc. 2009;23:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Raman JD, Bergs RA, Fernandez R, Bagrodia A, Scott DJ, Tang SJ, Pearle MS, Cadeddu JA. Complete transvaginal NOTES nephrectomy using magnetically anchored instrumentation. J Endourol. 2009;23:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Simi M, Ciuti G, Tognarelli S, Valdastri P, Menciassi A, Dario P. Magnetic link design for a robotic laparoscopic camera. J Appl Phys. 2010;107:09B302. [DOI] [Full Text] |
| 26. | Shang Y, Guo H, Zhang D, Xue F, Yan X, Shi A, Dong D, Wang S, Ma F, Wang H. An application research on a novel internal grasper platform and magnetic anchoring guide system (MAGS) in laparoscopic surgery. Surg Endosc. 2017;31:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Best SL, Bergs R, Gedeon M, Paramo J, Fernandez R, Cadeddu JA, Scott DJ. Maximizing coupling strength of magnetically anchored surgical instruments: how thick can we go? Surg Endosc. 2011;25:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |