Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2060
Peer-review started: December 12, 2016
First decision: December 29, 2016
Revised: January 24, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: March 21, 2017
Processing time: 98 Days and 0.9 Hours
To elucidate the factors associated with residual gastroesophageal reflux disease (GERD) symptoms in patients receiving proton pump inhibitor (PPI) maintenance therapy in clinical practice.
The study included 39 GERD patients receiving maintenance PPI therapy. Residual symptoms were assessed using the Frequency Scale for Symptoms of GERD (FSSG) questionnaire and the Gastrointestinal Symptom Rating Scale (GSRS). The relationships between the FSSG score and patient background factors, including the CYP2C19 genotype, were analyzed.
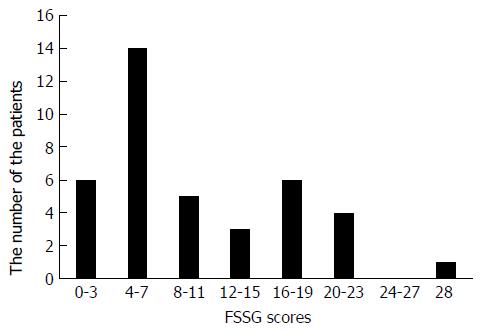
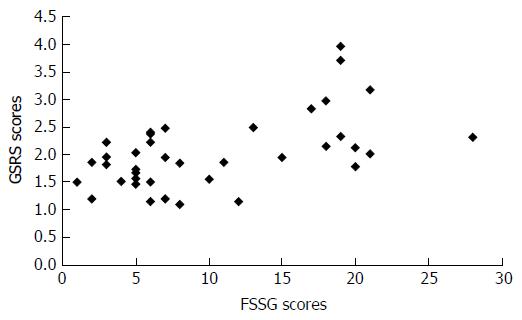
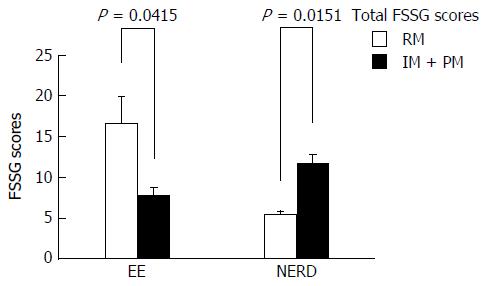
The FSSG scores ranged from 1 to 28 points (median score: 7.5 points), and 19 patients (48.7%) had a score of 8 points or more. The patients’ GSRS scores were significantly correlated with their FSSG scores (correlation coefficient = 0.47, P < 0.005). In erosive esophagitis patients, the FSSG scores of the CYP2C19 rapid metabolizers (RMs) were significantly higher than the scores of the poor metabolizers and intermediate metabolizers (total scores: 16.7 ± 8.6 vs 7.8 ± 5.4, P < 0.05; acid reflux-related symptom scores: 12 ± 1.9 vs 2.5 ± 0.8, P < 0.005). In contrast, the FSSG scores of the CYP2C19 RMs in the non-erosive reflux disease patients were significantly lower than those of the other patients (total scores: 5.5 ± 1.0 vs 11.8 ± 6.3, P < 0.05; dysmotility symptom-related scores: 1.0 ± 0.4 vs 6.0 ± 0.8, P < 0.01).
Approximately half of the GERD patients receiving maintenance PPI therapy had residual symptoms associated with a lower quality of life, and the CYP2C19 genotype appeared to be associated with these residual symptoms.
Core tip: The relationships between residual gastroesophageal reflux disease (GERD) symptoms in patients receiving proton pump inhibitor (PPI) maintenance therapy and patient background factors, including the CYP2C19 genotype, were evaluated. Approximately half of the GERD patients receiving maintenance PPI therapy had residual symptoms associated with a lower quality of life. Although the CYP2C19 genotype appeared to be associated with these residual symptoms, the impact in the erosive esophagitis patients was distinct from the impact in the non-erosive reflux disease patients.
- Citation: Kawara F, Fujita T, Morita Y, Uda A, Masuda A, Saito M, Ooi M, Ishida T, Kondo Y, Yoshida S, Okuno T, Yano Y, Yoshida M, Kutsumi H, Hayakumo T, Yamashita K, Hirano T, Hirai M, Azuma T. Factors associated with residual gastroesophageal reflux disease symptoms in patients receiving proton pump inhibitor maintenance therapy. World J Gastroenterol 2017; 23(11): 2060-2067
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2060
Gastroesophageal reflux disease (GERD) encompasses disorders in which gastric reflux leads to various symptoms and complications[1,2]. In GERD patients, reflux of gastric juice and other fluid causes not only mucosal injuries but also esophageal dysmotility associated with endogenous cytokines, which in turn leads to the appearance of GERD symptoms[3-6]. Proton pump inhibitors (PPIs) are used as first-choice drugs for GERD patients[7-9]. However, the efficacy of PPIs differs among patients depending on background factors, including genetic polymorphisms of cytochrome P450 (CYP) 2C19, which participates in the metabolic clearance and effectiveness of PPIs[10-15]. Moreover, non-erosive reflux disease (NERD) is more difficult to treat using PPIs than erosive esophagitis (EE)[16-18]. In clinical practice, GERD patients are not necessarily treated using strategies that take into account their individual backgrounds. Therefore, some cases might be treated for a long time without sufficient effects[19]. However, few reports have evaluated residual GERD symptoms in patients receiving maintenance therapy tailored to CYP2C19 genetic polymorphisms and endoscopic findings.
In this study, we investigated the residual symptoms of GERD patients receiving PPI maintenance therapy in clinical practice using the Frequency Scale for Symptoms of GERD (FSSG) questionnaire and the Gastrointestinal Symptom Rating Scale (GSRS). Additionally, we evaluated the relationships between the FSSG score and patient background factors, including the CYP2C19 genotype.
This study was conducted between February 2011 and March 2012. The study protocol was approved by the ethics committee of Kobe University Hospital, and all patients provided written informed consent before enrollment. Thirty-nine GERD patients receiving maintenance PPI therapy at Kobe University Hospital (Kobe-shi, Hyogo, Japan) were enrolled in this study. The FSSG questionnaire[20,21], which consists of 12 questions related to 7 acid reflux symptoms (RS) and 5 dysmotility-like symptoms (DS), was used to assess the GERD symptoms. The total FSSG score, FSSG-RS score, and FSSG-DS score were defined as the scores obtained by adding the scores from the 12 questions, the 7 acid reflux symptom-related questions, and the 5 dyspeptic symptom-related questions, respectively.
Additionally, the GSRS questionnaire[22,23] was used to assess the patients’ health-related quality of life (QOL). The CYP2C19 genotypes were determined using the polymerase chain reaction-restriction fragment length polymorphism technique with allele-specific primers using a DNA sample extracted from each patient’s peripheral blood leukocytes. Based on the finding of the wild-type allele or the two mutated alleles (*2 and *3), the patients were classified as rapid metabolizers (RM: homozygous for the wild-type allele), intermediate metabolizers (IM: carrier of only one mutated allele), or poor metabolizers (PM: homozygous for the mutated allele)[24-27]. We examined the patients’ medical records and investigated their clinical characteristics, including gender, age, BMI, alcohol consumption habits, smoking habits, PPI doses, use of concomitant drugs against GERD, use of Ca antagonists, acetylsalicylic acid (ASA) and non-steroidal anti-inflammatory drugs (NSAIDs), and endoscopic findings (atrophic gastritis and hiatal hernia). Hiatal hernia was diagnosed based on a proximal translocation of the esophagogastric junction of more than 2 cm above the diaphragmatic hiatus. Patients with an atrophic mucosa endoscopically graded as C-2, C-3, O-1, O-2, and O-3 according to the Kimura-Takemoto classification[28] were defined as positive for atrophic gastritis. Patients with esophageal mucosal breaks were classified into the EE group [Modified Los Angeles (LA) classification grades A, B, C and D], and the remaining patients were classified into the NERD group (grades N and M)[3,29,30].
Spearman’s rank correlation coefficient was used to assess the correlation between the FSSG and GSRS scores in the GERD patients. A bivariate analysis (Mann-Whitney U test, Pearson’s correlation coefficient, or Kruskal-Wallis test) was performed to assess differences in the FSSG scores (total score, RS score or DS score) and background factors in the EE and NERD patients. All statistical analyses were performed using JMP 10 (SAS Institute, Cary, NC, United States). P < 0.05 indicated statistical significance.
The clinical characteristics of the GERD patients are shown in Table 1. The patients were divided into 19 EE patients and 20 NERD patients according to the endoscopic findings. The mean age of the patients was 68.5 ± 11.9 years, with a range from 40 to 84 years. Prokinetic agents were the most common concomitant drugs used together with PPIs for the GERD patients (25.6%, 10/39). Other common concomitant drugs were histamine H2-receptor antagonists (H2RAs), gastro-protective agents and kampo medicine (herbal medicine).
| Background factors | The number of applicable subjects for the factor |
| Erosive esophagitis/NERD | 19/20 |
| Gender (male/female) | 17/22 |
| Atrophic gastritis (+/-) | 20/19 |
| Hiatal hernia (+/-) | 24/15 |
| Alcohol consumption habit (+/-/unknown) | 16/21/2 |
| Smoking habit (+/-/unknown) | 4/33/2 |
| PPI types (omeprazole/lansoprazole/rabeprazole/unknown) | 5/11/22/1 |
| PPI dose (half/full/double/unknown) | 8/26/4/1 |
| Concomitant drug against GERD (+/-) | 14/25 |
| Ca antagonist (+/-) | 15/24 |
| ASA (+/-) | 7/32 |
| NSAIDs (+/-) | 2/37 |
| CYP2C19 genotype (RM/IM/PM/unknown) | 13/20/5/1 |
| Maintenance PPI therapy period (< 6 mo/6-12 mo/> 12 mo) | 3/1/35 |
| Age (mean ± SD) | 68.5 ± 11.9 |
| BMI (mean ± SD) | 22.7 ± 3.1 |
The total FSSG scores of the patients ranged between 1 and 28 points. The average total FSSG score was 10.6 ± 7.3 points, and the median was 7.5 points. Nineteen (11 EE and 8 NERD) patients (48.7%) had a total FSSG score of more than 8 points, and 11 (6 EE and 5 NERD) patients (28.2%) had a score of more than 16 points (Figure 1). As shown in Figure 2, the total FSSG scores significantly correlated with the total GSRS scores (P < 0.005). Similarly, significant correlations were observed in both the EE and NERD patients (P < 0.01 and P < 0.05).
Tables 2 and 3 show bivariate analyses of the factors associated with the FSSG scores in the EE and NERD patients, respectively. The CYP2C19 genotype was a significant factor associated with the FSSG score in both the EE and NERD patients. Subjects with the CYP2C19 RM genotype in the EE patient group had significantly higher FSSG scores than the EE subjects with the other CYP2C19 genotypes (16.7 ± 8.6 vs 7.8 ± 5.4, P = 0.0415). In contrast, the subjects with the CYP2C19 RM genotype in the NERD patient group had significantly lower FSSG scores than the NERD subjects with the other CYP2C19 genotypes (5.5 ± 1.0 vs 11.8 ± 6.3, P = 0.0151) (Figure 3).
| Background factors | FSSG scores of the applicable subjects for the factor [mean ± SD (the number)] | FSSG scores of the inapplicable subjects for the factor [mean ± SD (the number)] | P value |
| Gender (male) | 11.4 ± 8.0 (9) | 10.8 ± 8.1 (10) | 0.9673 |
| Atrophic gastritis | 9.0 ± 9.0 (7) | 12.3 ± 7.2 (12) | 0.2696 |
| Hiatal hernia | 9.1 ± 7.2 (12) | 14.6 ± 8.2 (7) | 0.0817 |
| Alcohol consumption habit | 10.8 ± 8.1 (10) | 11.4 ± 8.0 (9) | 0.6225 |
| Smoking habit | 10.3 ± 8.7 (3) | 11.3 ± 8.0 (16) | 0.9552 |
| Use of half dose of PPI | 5.5 ± 0.7 (2) | 11.3 ± 8.1 (16) | 0.5251 |
| Use of rabeprazole | 11.2 ± 8.8 (12) | 9.7 ± 6.1 (6) | 0.9625 |
| Concomitant drug against GERD | 15.6 ± 8.5 (8) | 7.8 ± 5.6 (11) | 0.0512 |
| Use of Ca antagonist | 10.2 ± 5.7 (6) | 11.5 ± 8.8 (13) | 1.0000 |
| Use of ASA | 4.0 ± 2.8 (2) | 11.9 ± 7.9 (17) | 0.1610 |
| Use of NSAIDs | 8.0 (1) | 11.3 ± 8.0 (18) | 1.0000 |
| CYP2C19 RM genotype | 16.7 ± 8.6 (7) | 7.8 ± 5.4 (12) | 0.0415 |
| Age | ρ = -0.4083 | 0.0826 | |
| BMI | ρ = -0.1490 | 0.5428 |
| Background factors | FSSG scores of the applicable subjects for the factor [mean ± SD (the number)] | FSSG scores of the inapplicable subjects for the factor [mean ± SD (the number)] | P value |
| Gender (male) | 6.5 ± 5.2 (8) | 11.3 ± 6.2 (12) | 0.0807 |
| Atrophic gastritis | 9.6 ± 6.7 (13) | 9.0 ± 5.4 (7) | 0.9365 |
| Hiatal hernia | 7.1 ± 5.0 (12) | 12.9 ± 6.4 (8) | 0.1510 |
| Alcohol consumption habit | 9.0 ± 6.8 (6) | 10.2 ± 6.4 (12) | 0.4221 |
| Smoking habit | 6.0 (1) | 10.0 ± 6.5 (17) | 0.6270 |
| Use of half dose of PPI | 7.2 ± 5.7 (6) | 10.4 ± 6.3 (14) | 0.2454 |
| Use of rabeprazole | 11.8 ± 7.0 (10) | 7.0 ± 4.3 (10) | 0.2095 |
| Concomitant drug against GERD | 10.5 ± 6.7 (6) | 8.9 ± 6.1 (14) | 0.5896 |
| Use of Ca antagonist | 9.0 ± 6.2 (9) | 9.7 ± 6.4 (11) | 0.5159 |
| Use of ASA | 9.6 ± 6.0 (5) | 9.3 ± 6.4 (15) | 0.8262 |
| Use of NSAIDs | 18.0 (1) | 8.9 ± 6.0 (19) | 0.2568 |
| CYP2C19 RM genotype | 5.5 ± 1.0 (6) | 11.8 ± 6.3 (13) | 0.0151 |
| Age | ρ = 0.0963 | 0.6863 | |
| BMI | ρ = -0.3714 | 0.1069 |
We also examined the correlation between the CYP2C19 genotypes and the FSSG-RS or FSSG-DS scores in the EE and NERD patients. In the EE patients, the FSSG-RS scores of the subjects with the CYP2C19 RM genotype were significantly higher than those of the subjects with the other CYP2C19 genotypes (11 ± 1.9 vs 3.8 ± 0.8, P = 0.0044). In contrast, the FSSG-DS scores of the NERD patients with the CYP2C19 RM genotype were significantly lower than the scores of the NERD subjects with the other CYP2C19 genotypes (1.3 ± 0.4 vs 5.2 ± 0.8, P = 0.0069).
Significant differences in the FSSG-RS scores in the EE patients (RM: 11.0 ± 1.9, IM: 3.6 ± 0.9, PM: 4.5 ± 1.5, P = 0.0147) and the FSSG-DS scores in the NERD patients (RM: 1.3 ± 0.4, IM: 4.7 ± 0.8, PM: 7.0 ± 2.3, P = 0.0177) were also observed in the bivariate analyses among the three CYP2C19 genotypes.
This study clarified the actual state of residual GERD symptoms in patients receiving PPI maintenance therapy in clinical practice and assessed the relationships between background factors, including the CYP2C19 genotype, and residual GERD symptoms.
The FSSG questionnaire was used to assess residual GERD symptoms in this study because this questionnaire has been reported to be useful for the evaluation of these symptoms and has been used in clinical practice[20,21]. The investigation using the FSSG questionnaire revealed that approximately half of the GERD patients receiving PPI maintenance therapy had residual GERD symptoms that led to a lower health-related QOL. McColl et al[31] reported that the agreement between clinicians and patients in their assessments of the severity of reflux symptoms was poor and that clinicians tended to underestimate the severity of patients’ GERD symptoms.
In the analyses that divided the GERD patients into EE and NERD patients, the CYP2C19 genotype was the only significant factor associated with the total FSSG score in both groups of patients. CYP2C19 genetic polymorphisms have been reported to influence the effects of therapy on reflux symptoms and the healing of mucosal injuries[10,27,32]. However, few studies have evaluated the relationship between the CYP2C19 genotype and GERD symptoms in GERD patients (including NERD patients) receiving PPI maintenance therapy. In the present study, the impact of CYP2C19 genetic polymorphisms in EE patients was quite different from the impact in NERD patients. The total FSSG and FSSG-RS scores of the EE subjects with the CYP2C19 RM genotype were significantly higher than the scores of the EE subjects with the other CYP2C19 genotypes. These results are consistent with previous reports that insufficient acid inhibition is achieved by PPI in patients with the CYP2C19 RM genotype, which lowers the healing rate of EE[33,34]. Increasing the PPI dose in EE patients with the CYP2C19 RM genotype may improve their GERD symptoms.
In contrast, the total FSSG and FSSG-DS scores of the NERD subjects with the CYP2C19 RM genotype were significantly lower than the scores of the subjects with the other CYP2C19 genotypes in this study, suggesting that subjects with the CYP2C19 PM or CYP2C19 IM genotype receiving PPI maintenance therapy might suffer more frequently from GERD-related dyspeptic symptoms. However, this finding is difficult to explain because the CYP2C19 genotype has not been reported to be associated with the therapeutic outcomes in NERD patients[17]. Certain biological phenomena, such as reflux of digestive juices except gastric acid[4,35], mucosal hypersensitivity[36], esophageal dysmotility and psychological factors[37], are thought to cause symptoms in patients with NERD who are refractory to PPI therapy[38]. CYP2C19 gene polymorphisms might be a risk factor for PPI-refractory NERD through the biological phenomena mentioned above. An increase in the PPI dose in NERD patients with the CYP2C19 PM or CYP2C19 IM genotype is unlikely to improve their GERD symptoms. Regarding therapy for NERD patients, multifaceted approaches that include not only gastric acid suppression but also lifestyle improvement, clinical management of psychogenic factors and the use of concomitant drugs, such as kampo medicine, are desirable[39-42].
In Japan, the number of patients with GERD is expected to increase in the future due to the reduction of Helicobacter pylori infection, the spread of a Westernized diet, the increase in the aging population and the increase in gastric acid secretion in the younger generation[43-45]. Treatment of GERD symptoms is a clinically important issue because the health-related QOL of GERD patients is reduced in proportion to the extent of GERD symptoms, and ideally, tailored therapy based on the characteristics of each patient should be provided. To this end, quantifying GERD symptoms using a tool such as the FSSG questionnaire and making a differential diagnosis of EE and NERD are useful approaches. Furthermore, testing for the CYP2C19 genotype may be useful for treatment strategy decisions for PPI-refractory GERD patients. Other treatments that are not gastric acid inhibitory drugs may be required for PPI-refractory NERD patient with the CYP2C19 IM or PM genotype.
The present study has several limitations. The number of subjects was not large, which may have influenced the statistical analyses. The diagnosis of NERD was confirmed only by the medical history and the endoscopic findings. Further studies with a larger number of subjects are needed to clarify the relationship between the CYP2C19 genotype and the residual symptoms of GERD patients receiving maintenance PPI therapy.
In conclusion, approximately half of the GERD patients receiving maintenance PPI therapy had residual symptoms associated with a lower quality of life. Although CYP2C19 genetic polymorphisms appeared to be associated with these residual symptoms, the impact of the genetic polymorphisms differed significantly between the EE and NERD patients. NERD patients with the CYP2C19 IM or PM genotype might require additional treatment other than PPIs. Further studies on the usefulness of the treatment strategy tailored to the CYP2C19 genotype are required for PPI-refractory GERD patients.
In the clinical practice of maintenance proton pump inhibitor (PPI) therapy for gastroesophageal reflux disease (GERD) patients, some cases might be treated for a long time without sufficient effects.
Few reports have evaluated the relationship between the cytochrome P450 2C19 (CYP2C19) genotype and GERD symptoms in patients, including non-erosive reflux disease (NERD) patients, receiving PPI maintenance therapy.
In this study, the impact of CYP2C19 genetic polymorphisms differed significantly between the erosive esophagitis (EE) and NERD patients. In the EE patients, the Frequency Scale for Symptoms of GERD (FSSG) scores of the CYP2C19 rapid metabolizers (RMs) were significantly higher than the scores of the poor and intermediate metabolizers. In contrast, in the NERD patients, the FSSG scores of the CYP2C19 RMs were significantly lower than the scores of the other groups.
Testing of the CYP2C19 genotype may be useful when determining a treatment strategy for PPI-refractory GERD patients.
Genetic polymorphisms of CYP2C19 participate in the metabolic clearance and effectiveness of PPIs.
This is a well-designed study that investigates a common problem facing gastroenterologists and other treating physicians of GERD. The manuscript is well written; the results are well explained, the discussion is exhaustive, and the limitations of the study are clearly stated.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jadallah A, Keyashian A, Zerem E S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; the Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-120; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2449] [Article Influence: 128.9] [Reference Citation Analysis (2)] |
| 2. | Fock KM, Talley NJ, Fass R, Goh KL, Katelaris P, Hunt R, Hongo M, Ang TL, Holtmann G, Nandurkar S. Asia-Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol. 2008;23:8-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1652] [Article Influence: 63.5] [Reference Citation Analysis (1)] |
| 4. | Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 423] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 6. | Rieder F, Cheng L, Harnett KM, Chak A, Cooper GS, Isenberg G, Ray M, Katz JA, Catanzaro A, O’Shea R. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007;132:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 547] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Caro JJ, Salas M, Ward A. Healing and relapse rates in gastroesophageal reflux disease treated with the newer proton-pump inhibitors lansoprazole, rabeprazole, and pantoprazole compared with omeprazole, ranitidine, and placebo: evidence from randomized clinical trials. Clin Ther. 2001;23:998-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Habu Y, Maeda K, Kusuda T, Yoshino T, Shio S, Yamazaki M, Hayakumo T, Hayashi K, Watanabe Y, Kawai K. “Proton-pump inhibitor-first” strategy versus “step-up” strategy for the acute treatment of reflux esophagitis: a cost-effectiveness analysis in Japan. J Gastroenterol. 2005;40:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Furuta T, Sugimoto M, Shirai N, Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics. 2007;8:1199-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Furuta T, Shimatani T, Sugimoto M, Ishihara S, Fujiwara Y, Kusano M, Koike T, Hongo M, Chiba T, Kinoshita Y; Acid-Related Symptom Research Group. Investigation of pretreatment prediction of proton pump inhibitor (PPI)-resistant patients with gastroesophageal reflux disease and the dose escalation challenge of PPIs-TORNADO study: a multicenter prospective study by the Acid-Related Symptom Research Group in Japan. J Gastroenterol. 2011;46:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Watanabe A, Iwakiri R, Yamaguchi D, Higuchi T, Tsuruoka N, Miyahara K, Akutagawa K, Sakata Y, Fujise T, Oda Y. Risk factors for resistance to proton pump inhibitor maintenance therapy for reflux esophagitis in Japanese women over 60 years. Digestion. 2012;86:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K, Hanai H. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Kawamura M, Ohara S, Koike T, Iijima K, Suzuki J, Kayaba S, Noguchi K, Hamada S, Noguchi M, Shimosegawa T; Study Group of GERD. The effects of lansoprazole on erosive reflux oesophagitis are influenced by CYP2C19 polymorphism. Aliment Pharmacol Ther. 2003;17:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Hagymási K, Müllner K, Herszényi L, Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2011;12:873-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Uemura N, Inokuchi H, Serizawa H, Chikama T, Yamauchi M, Tsuru T, Umezu T, Urata T, Yurino N, Tanabe S. Efficacy and safety of omeprazole in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Scarpignato C. Poor effectiveness of proton pump inhibitors in non-erosive reflux disease: the truth in the end! Neurogastroenterol Motil. 2012;24:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Yamamichi N, Mochizuki S, Asada-Hirayama I, Mikami-Matsuda R, Shimamoto T, Konno-Shimizu M, Takahashi Y, Takeuchi C, Niimi K, Ono S. Lifestyle factors affecting gastroesophageal reflux disease symptoms: a cross-sectional study of healthy 19864 adults using FSSG scores. BMC Med. 2012;10:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Kusano M, Shimoyama Y, Sugimoto S, Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H, Ino K. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Danjo A, Yamaguchi K, Fujimoto K, Saitoh T, Inamori M, Ando T, Shimatani T, Adachi K, Kinjo F, Kuribayashi S. Comparison of endoscopic findings with symptom assessment systems (FSSG and QUEST) for gastroesophageal reflux disease in Japanese centres. J Gastroenterol Hepatol. 2009;24:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1034] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 23. | Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 447] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 24. | Fukuen S, Fukuda T, Maune H, Ikenaga Y, Yamamoto I, Inaba T, Azuma J. Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese population. Pharmacogenetics. 2002;12:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Sugimoto K, Uno T, Tateishi T. Effects of the CYP3A5 genotype on omeprazole sulfoxidation in CYP2C19 PMs. Eur J Clin Pharmacol. 2008;64:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Li Y, Zhang W, Guo D, Zhou G, Zhou H, Xiao Z. Pharmacokinetics of the new proton pump inhibitor ilaprazole in Chinese healthy subjects in relation to CYP3A5 and CYP2C19 genotypes. Clin Chim Acta. 2008;391:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Furuta T, Sugimoto M, Kodaira C, Nishino M, Yamade M, Ikuma M, Shirai N, Watanabe H, Umemura K, Kimura M. CYP2C19 genotype is associated with symptomatic recurrence of GERD during maintenance therapy with low-dose lansoprazole. Eur J Clin Pharmacol. 2009;65:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (3)] |
| 29. | Hoshihara Y. [Endoscopic findings of GERD]. Nihon Rinsho. 2004;62:1459-1464. [PubMed] |
| 30. | Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006;41:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | McColl E, Junghard O, Wiklund I, Revicki DA. Assessing symptoms in gastroesophageal reflux disease: how well do clinicians’ assessments agree with those of their patients? Am J Gastroenterol. 2005;100:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther. 2006;44:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Furuta T, Shirai N, Sugimoto M, Ohashi K, Ishizaki T. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2004;5:181-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Furuta T, Sugimoto M, Shirai N. Individualized therapy for gastroesophageal reflux disease: potential impact of pharmacogenetic testing based on CYP2C19. Mol Diagn Ther. 2012;16:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Iwakiri K, Kawami N, Sano H, Tanaka Y, Umezawa M, Kotoyori M, Hoshihara Y, Sakamoto C. Acid and non-acid reflux in Japanese patients with non-erosive reflux disease with persistent reflux symptoms, despite taking a double-dose of proton pump inhibitor: a study using combined pH-impedance monitoring. J Gastroenterol. 2009;44:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Nagahara A, Miwa H, Minoo T, Hojo M, Kawabe M, Osada T, Kurosawa A, Asaoka D, Terai T, Ohkusa T. Increased esophageal sensitivity to acid and saline in patients with nonerosive gastro-esophageal reflux disease. J Clin Gastroenterol. 2006;40:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Kovács Z, Kerékgyártó O. Psychological factors, quality of life, and gastrointestinal symptoms in patients with erosive and non-erosive reflux disorder. Int J Psychiatry Med. 2007;37:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Fass R. Persistent heartburn in a patient on proton-pump inhibitor. Clin Gastroenterol Hepatol. 2008;6:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Fass R. Proton pump inhibitor failure--what are the therapeutic options? Am J Gastroenterol. 2009;104 Suppl 2:S33-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Tominaga K, Iwakiri R, Fujimoto K, Fujiwara Y, Tanaka M, Shimoyama Y, Umegaki E, Higuchi K, Kusano M, Arakawa T. Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J Gastroenterol. 2012;47:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Miyamoto M, Manabe N, Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med. 2010;49:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Yoshida N, Kamada K, Tomatsuri N, Suzuki T, Takagi T, Ichikawa H, Yoshikawa T. Management of recurrence of symptoms of gastroesophageal reflux disease: synergistic effect of rebamipide with 15 mg lansoprazole. Dig Dis Sci. 2010;55:3393-3398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 44. | Kinoshita Y, Kawanami C, Kishi K, Nakata H, Seino Y, Chiba T. Helicobacter pylori independent chronological change in gastric acid secretion in the Japanese. Gut. 1997;41:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Haruma K, Kamada T, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, Inoue M, Kishimoto S, Kajiyama G, Miyoshi A. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |