Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2023
Peer-review started: November 23, 2016
First decision: December 19, 2016
Revised: January 6, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 21, 2017
Processing time: 118 Days and 14.8 Hours
To investigate management of patients who develop ipilimumab-mediated enterocolitis, including association of endoscopic findings with steroid-refractory symptoms and utility of infliximab as second-line therapy.
We retrospectively reviewed all patients at our center with metastatic melanoma who were treated with ipilimumab between March 2011 and May 2014. All patients received a standard regimen of intravenous ipilimumab 3 mg/kg every 3 wk for four doses or until therapy was stopped due to toxicity or disease progression. Basic demographic and clinical data were collected on all patients. For patients who developed grade 2 or worse diarrhea (increase of 4 bowel movements per day), additional data were collected regarding details of gastrointestinal symptoms, endoscopic findings and treatment course. Descriptive statistics were used.
A total of 114 patients were treated with ipilimumab during the study period and all were included. Sixteen patients (14%) developed ≥ grade 2 diarrhea. All patients were treated with high-dose corticosteroids (1-2 mg/kg prednisone daily or equivalent). Nine of 16 patients (56%) had ongoing diarrhea despite high-dose steroids. Steroid-refractory patients received one dose of intravenous infliximab at 5 mg/kg, and all but one had brisk resolution of diarrhea. Fourteen of the patients underwent either colonoscopy or sigmoidoscopy with variable endoscopic findings, ranging from mild erythema to colonic ulcers. Among 8 patients with ulcers demonstrated by sigmoidoscopy or colonoscopy, 7 patients (88%) developed steroid-refractory symptoms requiring infliximab. With a median follow-up of 264 d, no major adverse events associated with prednisone or infliximab were reported.
In patients with ipilimumab-mediated enterocolitis, the presence of colonic ulcers on endoscopy was associated with a steroid-refractory course.
Core tip: Immune-mediated enterocolitis is a common toxicity of ipilimumab therapy for melanoma. Infliximab is often needed as a second line therapy in steroid refractory cases. Our findings suggest that colonic ulcers seen on lower gastrointestinal endoscopy may predict a steroid refractory disease course. This would support a role for endoscopy in select cases, and suggest that early initiation of infliximab therapy may be appropriate in patients with colonic ulceration. These results require further exploration in larger patient cohorts.
- Citation: Jain A, Lipson EJ, Sharfman WH, Brant SR, Lazarev MG. Colonic ulcerations may predict steroid-refractory course in patients with ipilimumab-mediated enterocolitis. World J Gastroenterol 2017; 23(11): 2023-2028
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2023.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2023
Ipilimumab (YervoyTM, Bristol-Myers Squibb, Princeton, NJ, United States) is a first-in-class monoclonal antibody that blocks the immune regulatory molecule cytotoxic T-lymphocyte antigen-4 (CTLA-4), resulting in T-cell activation. It was approved by the United States Food and Drug Administration (FDA) in 2011 after two phase 3 studies demonstrated a survival benefit in patients with advanced melanoma[1-5]. It is now being tested in clinical trials in patients with a variety of tumor types.
Ipilimumab administration is associated with numerous immune-related adverse effects (irAE) including enterocolitis, hepatitis, hypophysitis, dermatitis, as well as several others[1,4,6]. Enterocolitis is among the most common toxicities, manifesting as diarrhea, abdominal pain, nausea or hematochezia. The overall incidence of any diarrhea (grade 1 or higher) has been reported as 31%-43% in various studies[1,3-5,7].
Immune-mediated diarrhea is typically treated based on severity. Anti-diarrheal medications such as loperamide are often used for patients with mild symptoms. Corticosteroids are generally administered in cases of moderate to severe diarrhea. In some steroid-refractory cases, the tumor necrosis factor alpha inhibitor infliximab has demonstrated efficacy in alleviating symptoms[8-10]. As more patients receive ipilimumab and similar immune therapies (such as anti-PD-1 therapies), gastroenterologists are increasingly being asked to assist in the management of patients immune-mediated enterocolitis. Questions remain regarding the role of endoscopy, and the optimal timing of infliximab administration.
To better understand the clinical features and treatment approaches to ipilimumab-mediated enterocolitis, we examined our single-center experience with post-FDA approval ipilimumab therapy in a large cohort of patients with metastatic melanoma.
We conducted a retrospective study of all patients treated with ipilimumab for advanced melanoma from March 2011 to May 2014 at our institution. All patients were treated with a standard regimen of 3 mg/kg of intravenous ipilimumab administered every 3 wk for 4 total doses or until development of drug toxicity, disease progression or death. Diarrhea was graded based on the National Cancer Institute’s (NCI’s) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0: (1) Grade 1 - increase of < 4 stools per day over baseline; (2) Grade 2 - increase of 4-6 stools per day; (3) Grade 3 - increase of > 6 stools per day, or incontinence or hospitalization; (4) Grade 4 - life-threatening consequences; and (5) Grade 5 - death.
Using the electronic medical record, demographic and clinical data were collected on all patients, including age, sex, peripheral white blood cell count, lymphocyte count, neutrophil count, platelet count, information on melanoma treatment response, and mortality. Charts for all patients were reviewed to determine which patients developed grade 2 or worse diarrhea. For patients who developed at least grade 2 diarrhea, additional data were collected, including details of diarrhea, other gastrointestinal symptoms, endoscopy results, pathology results, therapy for diarrhea, and response to therapy. Descriptive statistics were used to evaluate the data. Missing data variables were omitted from the analysis (pairwise deletion).
The study was approved by the Institutional Review Board of the Johns Hopkins University.
A total of 114 patients were treated with ipilimumab for metastatic melanoma during the study period; all were included in the study. Baseline demographic and laboratory characteristics were similar between patients who developed grade 2 (increase of 4 bowel movements per day) or worse diarrhea and those who did not (Table 1). A total of 16 patients (14%) developed grade 2 or worse diarrhea.
| Ipilimumab induced enterocolitis | Ipilimumab without enterocolitis | |
| Demographics | ||
| Total number of patients | 16 | 98 |
| Mean age in years | 63 | 61 |
| Female sex, n (%) | 8 (50) | 28 (29) |
| Laboratory characteristics prior to ipilimumab | ||
| Mean white blood cell count, cells/cu. mm (range) | 6720 (3510-17100) | 7530 (1080-25800) |
| Reference range 4500-11000 cells/cu. mm | ||
| Mean lymphocyte count, cells per cu. mm (range) | 1570 (592-4610) | 1440 (97-4420) |
| Reference range 1150-4800 cells/cu. mm | ||
| Mean neutrophil count, cells per cu. mm (range) | 4350 (2040-11610) | 5200 (668-23500) |
| Reference range 1800-7000 cells/cu. mm |
Out of 114 total patients, baseline CBC and differential data were missing for 2 patients. One patient moved to another city only 21 d after starting ipilimumab therapy and no additional follow-up data were available regarding her condition. All other data were available for analysis.
Sixteen patients developed ipilimumab-mediated enterocolitis. Clinical features and treatment outcomes are shown in Table 2. Onset of diarrhea occurred after a median of 2 doses of ipilimumab and after a median of 33 d from the first dose of ipilimumab (range 5-94 d). Patients had a median of 6 bowel movements per day with stool being described as watery and non-bloody in most patients; one patient reported trace amounts of blood in the stool initially. Most patients (63%) reported abdominal pain with a cramping character, while a minority of patients had fever, anorexia, or nausea.
| Onset of diarrhea | |
| After 1 dose of ipilimumab | 3 (19) |
| After 2 doses of ipilimumab | 7 (43) |
| After 3 doses of ipilimumab | 3 (19) |
| After 4 doses of ipilimumab | 3 (19) |
| Diarrhea details | |
| Number of bowel movements/day, median (range) | 6 (5-12) |
| Grade 2 diarrhea | 9 (56) |
| Grade 3 diarrhea | 7 (44) |
| Grade 4/5 diarrhea | 0 |
| Associated symptoms | |
| Abdominal pain | 10 (63) |
| Nausea or vomiting | 3 (19) |
| Fever | 2 (13) |
| Anorexia | 2 (13) |
| Endoscopic findings | |
| Mucosal erythema, edema, or erosions only | 6 (43) |
| Ulcers | 8 (57) |
| Treatment of diarrhea | |
| High dose corticosteroids | 16 (100) |
| Infliximab | 9 (56) |
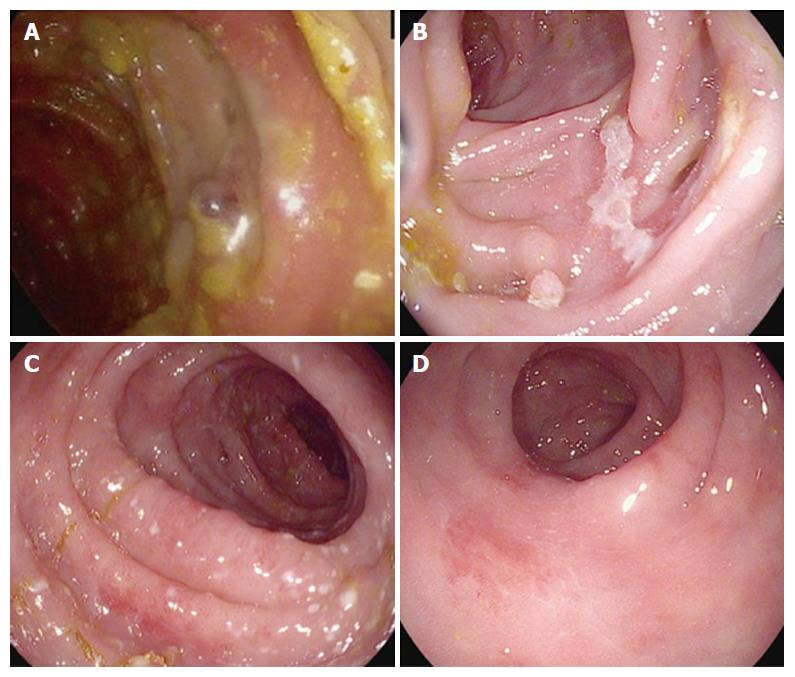
Standard medical workup included polymerase chain reaction stool test for Clostridium difficile toxin and stool culture for routine enteric pathogens (including Salmonella, Campylobacter, Shigella, and Escherichia coli), which were negative in all patients. Testing for celiac disease was not routinely performed. All but two patients underwent endoscopic evaluation with either flexible sigmoidoscopy (4 patients) or full colonoscopy (10 patients). Endoscopic appearance was variable: some patients had only mild edema and erythema of the mucosa (6 patients), while others had ulcers in the colon (8 patients). All 10 patients who underwent a full colonoscopy had at least patches of abnormal mucosa in the right and left colon. Histologic analysis revealed crypt apoptosis, crypt abscesses, and/or cryptitis in 12 of 14 patients (86%).
Patients with grade 2 diarrhea were treated with high-dose corticosteroids (1-2 mg/kg prednisone per day or equivalent). Most patients were also treated with loperamide at the onset of symptoms. Ipilimumab therapy was suspended at the onset of grade ≥ 2 diarrhea.
In 7 patients (44%), gastrointestinal symptoms resolved after administration of high-dose corticosteroids. Nine patients (56%) experienced ongoing diarrhea despite steroids and were treated with a single dose of 5 mg/kg of intravenous infliximab. Eight patients (89%) reported improvement of gastrointestinal symptoms within 1-2 wk of infliximab therapy. One patient had ongoing diarrheal symptoms after one dose of infliximab. Prednisone was weaned off, but he continued to have symptoms. He was treated with a second dose of infliximab 9 wk after the first dose with improvement in diarrhea.
Seven of 8 patients (88%) who had mucosal ulceration on sigmoidoscopy or colonoscopy developed steroid refractory diarrhea requiring infliximab, whereas only 2 of 6 patients without colonic ulcerations required infliximab (positive likelihood ratio = 3.89, 95%CI: 0.65-23.2; negative likelihood ratio = 0.28, 95%CI: 0.08-1.02). Also of note, the one patient who required 2 doses of infliximab had multiple long ulcers (approximately 1 centimeter ulcers) on colonoscopy. See Figure 1 for summary of endoscopic findings and treatment outcomes. Grade of diarrhea did not appear to correlate with steroid refractory symptoms.
One patient developed severe diarrhea with 12 bowel movements per day only 8 d after her first dose of ipilimumab. She presented to the hospital with diarrhea and fevers 4 d after the onset of symptoms. A CT scan of the abdomen and pelvis revealed moderate diffuse thickening of the small bowel, which was not present on an abdominal CT scan 1 wk prior to receiving ipilimumab. Sigmoidoscopy performed the following day showed normal colonic mucosa and no evidence of colitis on histology. She was diagnosed with ipilimumab-mediated enteritis but did not improve with prednisone 80mg daily. After 2.5 wk of prednisone therapy, she was given a dose of infliximab with resolution of her diarrhea within 7 d.
Three patients had already received all four doses of ipilimumab prior to developing enterocolitis. Four other patients were ultimately able to resume ipilimumab and complete the full 4 doses of therapy (2 patients who only received steroids, and 2 patients who received steroids and infliximab). Hence a total of 7 of 16 (44%) patients ultimately completed all 4 doses of ipilimumab therapy.
Follow-up was available on patients for a median of 264 d (range 17-1055) from the date of first ipilimumab infusion to last oncology follow-up or death. One patient suffered labile mood while on high-dose prednisone and another patient reported hyperglycemia and vaginal candidiasis while on high-dose prednisone. No other adverse events, including other infections or bowel perforation, were reported through the follow-up period.
Diarrhea and enterocolitis are among the most common immune-related adverse events associated with ipilimumab therapy. With the increasing use of immune-based therapies for patients with various cancer types, gastroenterologists are likely to see a growing burden of immune-mediated gastrointestinal toxicities. In the present analysis, we report a single center experience of 114 patients with metastatic melanoma who presented with ipilimumab-mediated enterocolitis. Overall, 14% of patients developed grade ≥ 2 diarrhea. More than half of those patients had steroid-refractory symptoms requiring treatment with infliximab. Interestingly, 8 of 9 patients (88%) with ulcers found on colonoscopy/sigmoidoscopy experienced a steroid-refractory course requiring infliximab (Figure 1).
While the cohort size in our study is relatively small, this potential association deserves further investigation. If validated, this finding could have important implications in the management of some patients with ipilimumab-mediated enterocolitis. Prompt evaluation with colonoscopy/sigmoidoscopy may provide prognostic information, allowing clinicians to consider initiation of infliximab therapy in patients whose symptoms do not rapidly respond to corticosteroids and who are found to have mucosal ulceration.
Prior studies have estimated incidence of ipilimumab-mediated diarrhea (any grade) to be in the range of 14%-29%[1,3,10,11]. In our cohort, we observed ≥ grade 2 and grade 3 diarrhea in 14% and 6% of patients, respectively, which is consistent with previous reports[1,4,5,10,12].
Initial experience with ipilimumab in the clinical trial setting seemed to indicate that steroid-refractory colitis was rare. For example, in a landmark phase 3 trial, only 4 of 179 (2%) patients with diarrhea or colitis required treatment with infliximab[1]. An earlier phase 2 study by Beck and colleagues reported refractory enterocolitis requiring infliximab in only 4 of 41 (10%) patients with enterocolitis[11].
By contrast, more contemporary experience with ipilimumab in the post-FDA approval era has provided evidence that steroid-refractory enterocolitis may be more common than initially thought. A large, retrospective, single-center review recently reported steroid-refractory symptoms in approximately 30% of patients with a serious immune-related adverse event[10]. Another recent study reported steroid-refractory symptoms in 26% of patients with ipilimumab-induced colitis. In our study, 9 of 16 (56%) patients had steroid-refractory symptoms requiring infliximab rescue therapy.
Of those, 8 patients experienced symptom improvement after a single dose of infliximab. One patient experienced lingering symptoms requiring a second dose of infliximab. This is consistent with previously published series, which have reported efficacy rates of infliximab in treating steroid-refractory ipilimumab-induced diarrhea between 72%-100%[8-10]. Administration of steroids and infliximab does not seem to adversely affect the anti-tumor response of immune based therapy. A recent report from the Memorial Sloan Kettering group indicates that the use of immune suppressive medications for the treatment of immune-related adverse events did not adversely impact overall survival or time to change in oncologic therapy in patients with melanoma[10].
The mechanism of ipilimumab-mediated enterocolitis has not been precisely elucidated. Indeed, with the increase availability of novel biologic agents, there is a growing recognition of the spectrum biologic drug-mediated enterocolitis. Each of these drugs may affect different lymphocyte targets of the gut mucosal immune system leading to enterocolitis. Ipilimumab specifically targets the inhibitory T-cell surface protein CTLA-4. Prior investigation has shown that T-cell depletion does not appear to be a feature in the pathogenesis of ipilimumab-mediated enterocolitis[7]. Berman and colleagues posit that ipilimumab results in dysregulation of mucosal immunity, evidenced by alterations in antibody levels to various microbial antigens and elevated fecal calprotectin levels[13]. Further understanding of ipilimumab and other biologic-mediated enterocolitides may have broader implications for understanding other inflammatory gastrointestinal disease states.
In conclusion, although our study was limited by its retrospective design, small cohort size and the fact that all patients were treated at a single tertiary center, our findings raise the possibility that the presence of colonic ulcers in patients with ipilimumab-mediated enterocolitis may predict a steroid refractory course. These results should be explored in larger patient cohorts in order to elucidate the utility of endoscopic evaluation and early administration of infliximab.
Ipilimumab is a novel T-cell activating therapy used in the treatment of melanoma and potentially other malignancies. Immune-mediated enterocolitis is one of the well-known toxicities of this drug. Treatment paradigms for the management of ipiliumumab-mediated enterocolitis are evolving. In particular, the management of corticosteroid refractory cases is still being understood. As immune activating therapies are being increasingly used in the oncology realm, management of patients with immune-mediated enterocolitis will continue to be a clinical challenge.
First line therapy for ipilimumab-mediated enterocolitis often includes corticosteroids, with growing evidence to support the use of infliximab in refractory cases. There are no well-known predictors of steroid refractory disease course and the role of endoscopy in such cases is unclear. In this report, the authors report on a large cohort of patients treated with ipilimumab with particular focus on the role of endoscopic findings.
In the present manuscript, the authors present the novel finding that severity of endoscopic findings, namely colonic ulcers, could be a predictor of steroid refractory disease course in patients with ipilimumab-mediated enterocolitis.
This manuscript would support a role for colonoscopy or sigmoidoscopy in patients with ipilimumab-mediated enterocolitis. Patients with worse endoscopic findings, such as colonic ulcers, may be more likely to have refractory disease course and this may be a reason to offer earlier rescue therapy, such as infliximab, in such patients.
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is an immune regulatory molecule found on the surface of T-lymphocytes. CTLA-4 is the target of ipilimumab and activation of CTLA-4 results in T-cell activation.
This is an interesting and well-written paper.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ahluwalia N, Pellicano R S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11751] [Article Influence: 783.4] [Reference Citation Analysis (0)] |
| 2. | Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3331] [Cited by in RCA: 3402] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 3. | Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 910] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 4. | O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 6. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 7. | Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, McDonald GB. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010;55:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Johnston RL, Lutzky J, Chodhry A, Barkin JS. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci. 2009;54:2538-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 813] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 11. | Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 647] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 12. | Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S, Targan SR, Wang HL. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PubMed] |