Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.1964
Peer-review started: November 17, 2016
First decision: December 2, 2016
Revised: December 23, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: March 21, 2017
Processing time: 124 Days and 22.2 Hours
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver diseases around the world due to the modern sedentary and food-abundant lifestyle, which is characterized by excessive fat accumulation in the liver related with causes other than alcohol abuse. It is widely acknowledged that insulin resistance, dysfunctional lipid metabolism, endoplasmic reticulum stress, oxidative stress, inflammation, and apoptosis/necrosis may all contribute to NAFLD. Autophagy is a protective self-digestion of intracellular organelles, including lipid droplets (lipophagy), in response to stress to maintain homeostasis. Lipophagy is another pathway for lipid degradation besides lipolysis. It is reported that impaired autophagy also contributes to NAFLD. Some studies have suggested that the histological characteristics of NAFLD (steatosis, lobular inflammation, and peri-sinusoid fibrosis) might be improved by treatment with traditional Chinese herbal extracts, while autophagy may be induced. This review will provide insights into the characteristics of autophagy in NAFLD and the related role/mechanisms of autophagy induced by traditional Chinese herbal extracts such as resveratrol, Lycium barbarum polysaccharides, dioscin, bergamot polyphenol fraction, capsaicin, and garlic-derived S-allylmercaptocysteine, which may inhibit the progression of NAFLD. Regulation of autophagy/lipophagy with traditional Chinese herbal extracts may be a novel approach for treating NAFLD, and the molecular mechanisms should be elucidated further in the near future.
Core tip: Due to the modern sedentary and food-abundant lifestyle, the incidence of non-alcoholic fatty liver disease (NAFLD) has doubled during the past years, and its prevalence ranges from 20% in China and 27% in Hong Kong to 30% in Western countries. Although NAFLD is a major cause of chronic liver diseases, a satisfactory treatment targeting one or several pathological mechanisms of NAFLD has yet to be identified. Recent studies have suggested that Chinese herbal extracts (resveratrol, Lycium barbarum polysaccharides, dioscin, bergamot polyphenol fraction, capsaicin, garlic-derived S-allylmercaptocysteine) may inhibit NAFLD progression by inducing autophagy, the role and mechanisms of which are summarized in this review.
- Citation: Liu C, Liao JZ, Li PY. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J Gastroenterol 2017; 23(11): 1964-1973
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/1964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.1964
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver diseases around the world, and is characterized by an excessively high accumulation of fat deposits in the liver resulting from causes other than chronic alcohol abuse[1,2]. The incidence of NAFLD has doubled during the past years, and its prevalence ranges from 20% in China and 27% in Hong Kong to 30% in Western countries, primarily due to the modern sedentary and food-abundant lifestyle in those regions[3,4]. The spectrum of NAFLD extends from non-alcoholic simple steatosis (NAS) to non-alcoholic steatohepatitis (NASH) and liver cirrhosis. Furthermore, NAFLD can progress to liver cancer without fibrosis[5-7].
NAFLD is often accompanied by obesity, diabetes and hyperlipidemia, and therefore closely associated with insulin resistance and lipid metabolism dysfunction, both of which can lead to the excessive accumulation of lipid droplets in hepatocytes (the first hit). Such lipid accumulation makes hepatocytes particularly vulnerable to internal and external stimuli during the first hit. As a result, lipid peroxidation, oxidative stress, cytokines, endoplasmic reticulum (ER) stress and endotoxins can all further aggravate any pre-existing liver injury, induce inflammation, impair autophagic flux and activate Kupffer cells. These types of cellular responses lead to lobular inflammation, Mallory-Denk bodies, NASH, fibrosis, and finally liver cirrhosis[8-10]. Moreover, a small percentage of such patients develop hepatic carcinoma[11]. Although NAFLD is a major cause of chronic liver diseases, a satisfactory treatment targeting one or several pathological mechanisms of NAFLD has yet to be identified.
Autophagy is a self-digestion process that occurs in all cells. Basal autophagy in eukaryotic cells is a protective response to stress resulting from internal and external stimuli such as injury, infection, etc. Double membrane fragments derived from intracellular organelles, such as mitochondria, pieces of ER and Golgi apparatus, can enfold damaged organelles and misfolded or unfolded proteins, which are then transported to lysosomes for degradation. The degradation products are finally recycled as substrates to be used for new cell formation[12,13].
Three main types of cellular autophagy have been identified: macroautophagy, chaperone-mediated autophagy, and microautophagy. Autophagy is a multi-step process including initiation, elongation, enclosure, maturation and degradation. It is widely acknowledged that about 30 mammalian homologs of yeast autophagy-related proteins (Atg) have been identified which are involved in initiation and elongation of the isolation membrane[14]. The initiation step requires the ULK1-Atg13-Atg101-FIP200 complex and Beclin1-Vps34-Vps15-Atg14L complex[14,15]. Under starvation stress, mTOR is inactivated, resulting in ULK1 activation and phosphorylation of Atg13, Atg101 and FIP200. The above two complexes recruit two conjugation systems including Atg12 conjugation system (including Atg5, Atg12, Atg7, Atg10 and Atg16L1) and LC3 conjugation system (including LC3, Atg4, Atg7 and Atg3), which are essential for elongation and enclosure steps[15].
Basal level of autophagy in a cell helps to maintain its homeostatic state and normal function, and promote its survival under stressful conditions. However, constant stimulation can still lead to autophagic cell death[16,17]. It is well documented that aging, neurodegeneration, tumors, immunological diseases, diabetes and NAFLD have an intertwined relationship with autophagic disorders[18-20]. Thus, maintenance of autophagy balance is important for good health.
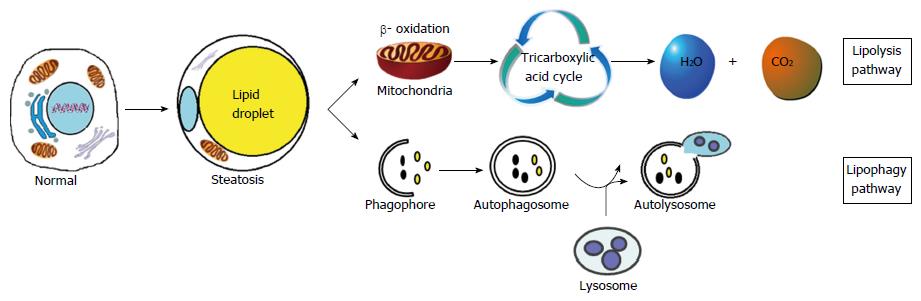
NAFLD is always accompanied by the combined comorbidities of obesity, diabetes and dyslipidemia, otherwise described as metabolic syndrome[21]. The basic pathogenesis of NAFLD is an excessive accumulation of lipid droplets in hepatocytes, resulting from dysfunctional lipid metabolism combined with insulin resistance[22]. The lipid droplets are accumulations of triglyceride that can be easily identified by staining with hematoxylin and eosin or Oil Red O. A therapeutic approach that induces lipid degradation and simultaneously inhibits fat synthesis while maintaining a normal level of lipid metabolism may represent the proper strategy for treating NAFLD.
Two major lipid metabolism pathways have been identified in human: the lipolysis pathway and the lipophagy pathway[23-25] (Figure 1). Lipolysis refers to the gradual degradation of intracellular lipid droplets into free fatty acids and glycerol by the activity of cytoplasmic lipases. These newly released free fatty acids are then transported into mitochondria, where they undergo β-oxidation to form acetyl-CoA, which in turn, is finally converted to carbon dioxide and water via the Krebs cycle. As the major pathway of lipid degradation in eukaryotic cells, lipolysis is a complex multi-step process that also plays a significant role in maintaining energy balance[26].
Another method used by cells to degrade lipids is the lipophagy pathway, by which the double membrane wraps lipid droplets and sends them to lysosomes as autolysosomes for degradation[27]. Lipophagy ensures the degradation of excessive lipid droplets deposited in cells, and the maintenance of cellular “steady state”. Lipolysis and lipophagy both play important roles in the degradation of lipid droplets.
It is widely accepted that autophagy is up-regulated during the early stage of NAFLD as an attempt to prevent lipid accumulation[28]. However, as NAFLD progresses, the autophagy process is blocked[29]. Singh and his research team[30] were the first to identify a relationship between autophagy and lipolysis. They found that mice fed with a high-fat diet or methionine choline-deficient (MCD) diet had significantly decreased levels of autophagy. Treating the mice with 3-maleimidopropionic acid or silencing the ATG5 gene with siRNA sharply increased the accumulation of lipid droplets in liver cells. Furthermore, rapamycin (mTOR inhibitor) was found to promote autophagy and also to alleviate lipid deposition both in vivo and in vitro[31]. Pretreatment with rapamycin (25 ng/mL) resulted in increased autophagy, while the levels of ER stress, apoptosis and lipid droplets decreased in palmitic acid-induced fatty hepatocytes[32]. These findings indicated that autophagy might negatively regulate lipid deposition and ER stress.
In cultured cells and mouse models, knockdown of Atg5 or Atg7 led to increased levels of both ER stress and insulin resistance[33]. Double immunofluorescence studies confirmed that lipid breakdown occurred partially via the autophagy-lysosome pathway, and inhibitors of autophagosome formation or autophagosome-lysosome fusion could markedly reduce such degradation[30]. Furthermore, autophagy was also found to help regulate the inflammatory response. Knockout of Atg5 in mouse macrophages blocked autophagy and increased IL-1β levels following administration of D-galactosamine/lipopolysaccharide[34]. Moreover, several studies conducted with animal models of NAFLD and actual NAFLD patients have reported that autophagy flux was suppressed, and that restoring autophagy balance could alleviate the histologic signs of fatty liver disease[32,33].
Briefly, short-term inhibition of autophagy in NAFLD could be induced through the mTOR complex, while long-term inhibition could be regulated via the transcription factors FoxO and TFEB, which control the transcription of autophagic genes and are inhibited by insulin-induced activation of Akt/PKB and mTOR, respectively. mTOR could be over-activated in the liver, presumably as a result of over-nutrition and/or hyperinsulinemia. Calcium-dependent protease calpain-2 induced by obesity could also lead to the degradation of Atg7, with impaired autophagy. A reduction in expression of cathepsins B, D and L and a defect in lysosomal acidification could impair substrate degradation in autolysosomes. Finally, high-fat diet could lead to impaired autophagosome-lysosome fusion. In turn, decreasing hepatic autophagy and the associated lysosomal degradation could increase the ER stress in NAFLD[15].
In conclusion, the progression of NAFLD is closely associated with impaired autophagy flux, and restoring autophagy balance improves NAFLD.
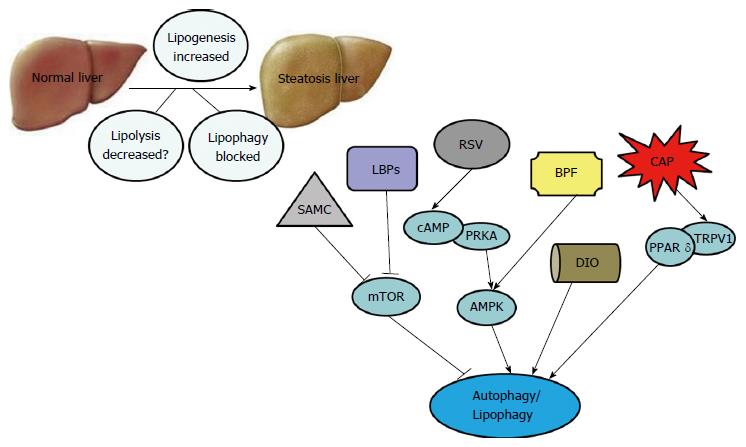
Traditional Chinese herbal extracts, usually extracted from native plants, have been used in various clinics for thousands of years. In the past, due to the lack of advanced analytical technologies, people had little understanding of the mechanisms by which certain Chinese herbal extracts might treat or cure certain diseases. However, due to recent advances in technologies used in biochemistry and pharmacology, more and more people have become aware of the strong and lasting efficacy of the Chinese herbal extracts used to maintain health. Moreover, several such medicines are now used in the clinical treatment of NAFLD[35,36]. Recent studies have suggested that Chinese herbal extracts function by inducing autophagy, which may inhibit NAFLD progression (Table 1).
| Chinese herbs | Model | Treatment | Pharmacological mechanisms | Ref. | ||
| Animal model | Cell model | Animal model | Cell model | |||
| Resveratrol (RSV) | ULK1 heterozygous knockout mice were fed with high-fat diet for 12 wk | - | Oral feeding with 50 mg/kg per day RSV from week 9 to week 12 | - | Improved NAS score, insulin resistance, oxidative stress, inflammation, glucose tolerance and modulated autophagy | [45] |
| 4-wk induction of NAFLD with high-fat diet (60% fat) in 129/SvJ mice | Steatosis was induced by incubating HepG2 cells with palmitate acid (0.2 mmol/L) for 24 h | Diet containing RSV (0.4%) for 4 wk | Treated with RSV at various concentrations (10, 20, 40, 80 μmol/L) for a further 24 h | Reduced lipid accumulation, stimulated β-oxidation and induced autophagy through cAMP-PRKA-AMPK-SIRT1 | [46] | |
| Lycium barbarum polysaccharides (LBPs) | NASH induced by high-fat diet for 12 wk in adult female Sprague-Dawley rats | Steatosis was induced by incubating BRL-3A cells with sodium palmitate acid | Oral gavage feeding with 1 mg/kg per day LBPs once a day from week 9 to week 12 | Treated with LBPs for 24 h | Reduced insulin resistance, serum aminotransferases, inflammatory responses, apoptosis and induced autophagy | [51] |
| Dioscin | NAFLD induced by high-fat diet (45% kcal fat) for 10 wk in C57BL/6J mice and ob/ob mice | - | Oral feeding with different dioscin concentrations (20, 40, 80 mg/kg per day) | - | Reduced body weight, lipid accumulation, inflammation oxidative damage and induced β-oxidation, autophagy, energy expenditure | [55] |
| Bergamot polyphenol fraction (BPF) | NAFLD induced by cafeteria diet (15% fat) every other day in addition to standard chow diet ad libitum for 14 wk in male Rcc:Han WIST rats | - | Drinking water containing 50 mg/kg per day BPF for 3 mo | - | Reduced serum triglyceride, blood glucose, hepatic steatosis and induced autophagy | [59] |
| Capsaicin | NAFLD induced by high-fat diet (49% fat) for 24 wk in TRPV1-/- and C57BL/6 wild-type mice | Steatosis was induced by incubating HepG2 cells with 1 mmol/l oleate/palmitate (2:1) | Diet containing 0.01% capsaicin for 24 wk | Treated by various capsaicin concentrations (0.1-10 μmol/L) | Reduced lipogenesis (FAS, SREBP-1, LXR, PPARα) and induced lipolysis (phospho-HSL, CPT1), autophagy through PPARδ-dependent manner | [65] |
| Garlic-derived S-allylmercaptocysteine (SAMC) | NAFLD induced by high unsaturated fat diet (30% fish oil) for 8 wk in female Sprague-Dawley rats | - | Intraperitoneal injection of 200 mg/kg SAMC, 3 times per week for 8 wk | - | Reduced lipogenesis (SREBP-1c), fibrosis (TGF-β1, α-SMA, PC-1), oxidative stress (CYP2E1), inflammation (TNF-α, IL-1β, iNOS, COX-2, MCP-1, MIP-2, KC) and induced lipolysis (adiponection), antioxidative stress (CAT, GPx) | [67] |
| NAFLD induced by high unsaturated fat diet (30% fish oil) for 8 wk in female Sprague-Dawley rats | - | Intraperitoneal injection of 200 mg/kg SAMC, 3 times per week for 8 wk | - | Reduced intrinsic apoptosis (Bcl-2, Bcl-XL, Bakl, Bax) and extrinsic apoptosis (Fas, TRAIL, FADD, cleaved caspase-8), induced autophagy (vps34, beclin1, Atg12, LC3II, phosphorylated mTOR and p62) | [68] | |
Resveratrol (trans-3,4,5-trihydroxystilbene) is a naturally polyphenolic compound found in edible plants, such as grapes, peanuts and berries. Due to its anti-inflammatory, antioxidant and anti-cancer effects, resveratrol is widely used to help prevent cardiovascular and cerebrovascular diseases, treat cancer, and reduce steatosis[37-39]. Several clinical trials have reported that orally administered resveratrol inhibits the progression of NAFLD[40-42].
In a randomized, double-blind, controlled clinical trial, 50 NAFLD patients were given one 500 mg capsule of resveratrol per day for 12 wk while eating an energy-balanced diet. Some parameters, such as anthropometric measurements (weight, body mass index and waist circumference), liver enzymes (ALT and AST), and biomarkers of inflammation (hs-CRP, TNF-α and IL-6) and hepatocellular apoptosis (cytokeratin-18 fragment M30), as well as the histological characteristics (steatosis and fibrosis) of the patients, were significantly improved compared with the patients who received a placebo capsule[41]. These data suggest that resveratrol prevents NAFLD by inhibiting the inflammatory response, apoptosis and fibrotic process.
Other studies have shown that resveratrol decreases lipogenesis by suppressing expression of acetyl-CoA carboxylase (ACC), peroxisome proliferator-activated receptor γ (PPAR-γ) and sterol regulatory element-binding protein-1 (SREBP-1)[43,44]. Additionally, resveratrol was reported to reduce the levels of proinflammatory cytokines TNF-α, IL-6 and IL-1β in mice fed a high-fat diet by affecting the NF-κB pathway[43,45]. Finally, results of another study suggested that resveratrol could significantly increase autophagy and SIRT1 activity, and might improve the symptoms of NAFLD partially by inducing autophagy via the cAMP-PRKA-AMPK-SIRT1 signaling pathway[46].
When male C57BL/6 mice fed with MCD diet were administered resveratrol (100 mg/kg or 250 mg/kg) or AML12 cells cultured with MCD medium were treated with resveratrol (50 μmol/L or 100 μmol/L), certain autophagic markers (LC3II) became significantly up-regulated while certain autophagic negative regulators (p62) became down-regulated; steatosis and inflammatory response (IL-6, IL-1β and TNFα) also became down-regulated. Then, AML12 cells treated with chloroquine showed blockade of autophagy, with inflammatory response (IL-6, IL-1β and TNFα) and oxidative stress (reactive oxygen species, ROS) being accumulated in the cells. However, autophagy was up-regulated while inflammatory response and oxidative stress were attenuated when the AML12 cells were further treated with resveratrol[47]. These facts indicate that resveratrol protects against NAFLD partially through regulating autophagy[46,47].
The Lycium barbarum polysaccharides (LBPs) consist of fibrous-like proteoglycan molecules that are extracted from the rare but traditional medicinal herb, Chinese wolfberry (Lycium barbarum L.). Recent evidence has confirmed that LBPs are composed of arabinose, glucose, galactose, mannose, xylose and rhamnose. Due to their antioxidant, anti-cancer, anti-aging, neuroprotective, anti-hyperlipemia and anti-hyperglycemia properties, LBPs are increasingly consumed by elderly individuals[48,49]. In a NASH rat model, LBPs displayed therapeutic effects when used to treat steatosis, inflammation and hepatic fibrosis. Moreover, LBPs were shown to reduce steatosis by reducing mRNA expression of SREBP-1c while regulating inflammatory cytokines (TNF-α, IL-1β and MCP-1), partially by inhibiting activation of the NF-κB pathway[50,51]. LBPs were also shown to alleviate hepatic fibrosis by affecting the TGF-SMAD signaling pathway. Finally, when LBPs were administered to female Sprague-Dawley rats fed with a high-fat diet, certain autophagic markers (Atg5 and LC3II) became significantly up-regulated while certain autophagic negative regulators [phosphorylated (p)mTOR and p62] became down-regulated[51]. Rat obesity, insulin resistance, hepatic injury (inflammatory foci and cellular necrosis), oxidative stress (antioxidant enzymes CAT and GPx) were also improved. Autophagy was reported to have the effects of improving insulin resistance and oxidative stress. We speculate that LBPs improve NAFLD/NASH via several different mechanisms, including autophagy[51].
Dioscin is a natural steroidal saponin compound found in dietary foods especially Dioscoreaceae (Dioscorea opposite Thunb), which is widespread throughout Asian countries such as China, North Korea and Japan. Pharmacological studies have confirmed that dioscin can reduce inflammation, decrease blood sugar and lipid levels, protect hepatocytes and promote digestion[52,53]. Due to these effects, dioscin is now widely consumed in China.
Dioscin was found to promote β-oxidation of fatty acids by up-regulating ACADM, ACADS, PPARα, ACSL1, ACSL5, CPT1 and ACO expression. It can also inhibit triglyceride and cholesterol synthesis by down-regulating SREBP-1c, FAS, ACC1 and SCD1 expression, which might help to prevent lipid deposition[54,55]. Dioscin was also shown to increase oxygen consumption and energy expenditure. The levels of HO-1, Nrf2, GSS and SOD2 expression were found to be up-regulated and KEAP1 expression was down-regulated in a dose-dependent manner in ob/ob and C57BL/6J mice pretreated with dioscin, strongly suggesting that dioscin has an anti-oxidative effect. Additionally, the levels of p-mTOR/mTOR, Beclin-1, Atg5 and LC3 II/I protein expression were all up-regulated by dioscin[55]. Dioscin might regulate autophagy through the mTOR-independent pathway. These findings indicate that dioscin protects against NAFLD, partially by inducing autophagy[54,55].
The bergamot polyphenol fraction (BPF) consists of bioactive molecules extracted from Bergamot (Citrus bergamia Risso Poiteau), which is like Buddha’s-hand. While bergamot is native to Italy, it is now widely distributed throughout the subtropical regions of China, including Guangdong, Guangxi, Fujian and Yunnan. Bergamot has anti-inflammatory, anti-hypertensive and hepatic protective effects, and also promotes digestion[56,57]. A clinical study found reduced total low-density lipoprotein, cholesterol, triglyceride and blood glucose levels in 237 patients who had taken oral BPF for 30 d[58].
Due to its pharmacological profile, BPF may be useful for treating hyperlipemic and hyperglycemic disorders. In a cafeteria diet-induced rat model of metabolic syndrome, BPF significantly reduced steatosis by decreasing total serum lipid levels. Moreover, the expression levels of two autophagy markers (LC3 II/I and Beclin-1) were increased while SQSTM1/p62 expression was reduced, indicating that BPF could stimulate autophagy[59]. The specific mechanism by which BPF prevents NAFLD remains unclear. However, enhancement of lysosomal function via transcription factor EB, and activation of ULK1 kinase by AMPK might help to up-regulate autophagy[59].
Capsaicin (8-methyl-N-vanillylnonanamide) is a major chemical component of hot peppers (Capsicum annuum L.), which is originally from Mexico but has become a favorite seasoning food in China. Odorless and colorless dietary capsaicin is a potent agonist of transient receptor potential vanilloid 1 (TRPV1), which is a non-selective cation channel with a preference for positive ions that transmit sensations of pain[60]. Long-term intake of dietary capsaicin can lower blood pressure, reduce cholesterol accumulations, and accelerate the decomposition and excretion of cholesterol[61,62].
Furthermore, appropriate amounts of dietary capsaicin have beneficial effects on obesity and NAFLD[63,64]. A survey indicated that dietary capsaicin could reduce lipid accumulation and triglyceride levels in mice fed with a high-fat diet by up-regulating the levels of uncoupling protein 2 (UCP2)[64]. UCP2 was thought to play an important role in mitochondrial lipolysis and oxidative stress. Another study showed that capsaicin-activated TRPV1 raised the levels of hepatic phosphorylated hormone-sensitive lipase (phospho-HSL) and carnitine palmitoyl transferase 1 (CPT1), which were critical regulators of lipolysis. This effect may be TRPV1-dependent because it was absent in TRPV1 (-/-) mice. At the same time, the levels of hepatic FAS, SREBP-1, PPARα and liver X receptor remained unchanged, which was important for lipogenesis. These findings suggest that capsaicin promotes lipolysis without inhibiting fat synthesis in NAFLD patients.
On the other hand, capsaicin was shown to enhance the expression levels of PPARδ and several autophagy-related proteins, including LC3 II, Beclin1, Atg5 and Atg7 in HepG2 cells, which had been pretreated with free fatty acids (oleate/palmitate, 2:1). Furthermore, autophagy induced by capsaicin was further increased by PPARδ agonist (GW0742) in steatosis HepG2 cells. Autophagy inhibited by capsazepine (inhibition of capsaicin) was further reduced by PPARδ antagonist (GSK0660) in steatosis HepG2 cells. It is suggested that chronic dietary capsaicin appears to prevent NAFLD by enhancing PPARδ-dependent autophagy[65].
S-allylmercaptocysteine (SAMC) is the major active component of garlic (Allium sativum L.), which is one of the most favorite seasonings of food in China. Garlic is originally from the western plateau of Asia, but is now widely planted in low-wet areas of China, including Henan, Shandong, Jiangsu, etc. Garlic has the effects of sterilization, antioxidant and anti-cancer. A randomized, double-blind, controlled clinical trial found that body weight and body fat mass were decreased in 55 NAFLD patients who had orally taken two garlic tablets per day (containing 400 mg of garlic powder)[66]. Furthermore, some pharmacological studies had confirmed that SAMC could ameliorate NAFLD. A survey indicated that SAMC could reduce steatosis, fibrosis, oxidative stress and inflammation in female rats fed with a highly unsaturated fat diet (30% fish oil) by up-regulating the levels of lipolysis markers (adiponectin), antioxidative stress markers (CAT and GPX), and down-regulating the levels of lipogenesis markers (SREBP-1c), fibrosis markers (TGF-β1, α-SMA and PC-1), oxidative stress markers (CYP2E1) and inflammatory markers (TNF-α, IL-1β, iNOS, COX-2, MCP-1, MIP-2 and KC). The protective effect of SAMC was partly through regulation of p38 MAPK, NF-κB and AP-1 signaling pathways[67]. Another survey suggested that hepatic autophagic negative regulators (phosphorylated mTOR and p62), intrinsic apoptotic markers (phosphorylated p53, Bcl-2, Bcl-XL, Bakl and Bax) and extrinsic apoptotic markers (Fas, TRAIL, FADD and cleaved caspase-8) were reduced while hepatic autophagic markers (vps34, beclin1, Atg12 and LC3II) induced in NAFLD rat models after intraperitoneal injection of SAMC (200 mg/kg 3 times per week)[68]. These findings indicate that SAMC prevents NAFLD, partially by inducing autophagy[68].
Traditional Chinese herbal extracts are widely used to prevent cancer, neurodegeneration and metabolic syndrome, as well as cardiovascular and cerebrovascular diseases. Furthermore, many people use them as “first choice” medications for maintaining health[69,70]. Traditional Chinese herbal extracts have beneficial effects in treating NAFLD, as they could reduce steatosis and inhibit inflammation and oxidative stress[71-73]. In addition, these medicines appear to reverse histologic changes in the livers of NAFLD patients, which may prevent NAFLD from progressing to hepatic cirrhosis and even carcinoma.
Autophagy is a protective response that helps to maintain homeostasis and to promote survival. Lipophagy is a special kind of autophagy by which the double membrane wraps lipid droplets and sends them to lysosomes for degradation. The fundamental function of lipophagy is the degradation of abnormal lipid droplets deposited in cells and the maintenance of steady state. But, autophagy is partially suppressed in NAFLD/NASH patients and animal models, and restoring autophagy may slow the progression of NAFLD. Moreover, autophagy is a double-edged sword. It protects hepatocytes by inhibiting oxidative stress and inflammation[74,75]; yet, its over-stimulation may result in autophagic cell death that aggravates any existing liver damage[76].
As we have discussed above, it is strongly suggested that some traditional Chinese herbal extracts, such as resveratrol, LBPs, dioscin, BPF, capsaicin and SAMC, should have beneficial effects on NAFLD/NASH, partially due to their ability to activate autophagy (Figure 2). However, additional studies are needed to elucidate the molecular mechanisms by which traditional Chinese herbal extracts protect from NAFLD. Finally, prospective, randomized, double-blind, controlled clinical trials should be conducted to evaluate the specific therapeutic effects and safety of traditional Chinese herbal extracts for NAFLD patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ikura Y, Ji G, Peltec A, Tipoe GL S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Boustière C, Gauthier A. [Non-alcoholic hepatic steatosis]. Presse Med. 1985;14:1147-1150. [PubMed] |
| 2. | Williams T. Metabolic Syndrome: Nonalcoholic Fatty Liver Disease. FP Essent. 2015;435:24-29. [PubMed] |
| 3. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1330] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 4. | Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 6. | Fusillo S, Rudolph B. Nonalcoholic fatty liver disease. Pediatr Rev. 2015;36:198-205; quiz 206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Karim MF, Al-Mahtab M, Rahman S, Debnath CR. Non-alcoholic Fatty Liver Disease (NAFLD)--A Review. Mymensingh Med J. 2015;24:873-880. [PubMed] |
| 8. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 9. | Sharma M, Mitnala S, Vishnubhotla RK, Mukherjee R, Reddy DN, Rao PN. The Riddle of Nonalcoholic Fatty Liver Disease: Progression From Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J Clin Exp Hepatol. 2015;5:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Martins MJ, Ascensão A, Magalhães J, Collado MC, Portincasa P. Molecular Mechanisms of NAFLD in Metabolic Syndrome. Biomed Res Int. 2015;2015:621080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Noureddin M, Rinella ME. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis. 2015;19:361-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 13. | Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3678] [Cited by in RCA: 4869] [Article Influence: 347.8] [Reference Citation Analysis (0)] |
| 14. | Law BY, Mok SW, Wu AG, Lam CW, Yu MX, Wong VK. New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy. Molecules. 2016;21:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Lavallard VJ, Gual P. Autophagy and non-alcoholic fatty liver disease. Biomed Res Int. 2014;2014:120179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr Mol Med. 2008;8:78-91. [PubMed] |
| 17. | Oral O, Akkoc Y, Bayraktar O, Gozuacik D. Physiological and pathological significance of the molecular cross-talk between autophagy and apoptosis. Histol Histopathol. 2016;31:479-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 18. | Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 652] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 19. | Gracia-Sancho J, Guixé-Muntet S, Hide D, Bosch J. Modulation of autophagy for the treatment of liver diseases. Expert Opin Investig Drugs. 2014;23:965-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Zhi X, Zhong Q. Autophagy in cancer. F1000Prime Rep. 2015;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Bhatt HB, Smith RJ. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr. 2015;4:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 96] [Reference Citation Analysis (0)] |
| 22. | Berk PD, Verna EC. Nonalcoholic Fatty Liver Disease: Lipids and Insulin Resistance. Clin Liver Dis. 2016;20:245-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 493] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 24. | Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 25. | Martinez-Lopez N, Singh R. Autophagy and Lipid Droplets in the Liver. Annu Rev Nutr. 2015;35:215-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 26. | Ivanov VV, Shakhristova EV, Stepovaya EA, Nosareva OL, Fedorova TS, Novitsky VV. [Molecular mechanisms of modulation of lipolysis in adipose tissue and development of insulinresistance in diabetes]. Patol Fiziol Eksp Ter. 2014;111-119. [PubMed] |
| 27. | Wang CW. Lipid droplets, lipophagy, and beyond. Biochim Biophys Acta. 2016;1861:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Cai N, Zhao X, Jing Y, Sun K, Jiao S, Chen X, Yang H, Zhou Y, Wei L. Autophagy protects against palmitate-induced apoptosis in hepatocytes. Cell Biosci. 2014;4:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Jiang P, Huang Z, Zhao H, Wei T. Hydrogen peroxide impairs autophagic flux in a cell model of nonalcoholic fatty liver disease. Biochem Biophys Res Commun. 2013;433:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3239] [Cited by in RCA: 3111] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 31. | Wang Y, Shi M, Fu H, Xu H, Wei J, Wang T, Wang X. Mammalian target of the rapamycin pathway is involved in non-alcoholic fatty liver disease. Mol Med Rep. 2010;3:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | González-Rodríguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillón J, Lo Iacono O, Corazzari M, Fimia GM. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5:e1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 33. | Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1058] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 34. | Ilyas G, Zhao E, Liu K, Lin Y, Tesfa L, Tanaka KE, Czaja MJ. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1β. J Hepatol. 2016;64:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Xiao J, Fai So K, Liong EC, Tipoe GL. Recent advances in the herbal treatment of non-alcoholic Fatty liver disease. J Tradit Complement Med. 2013;3:88-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Liu ZL, Xie LZ, Zhu J, Li GQ, Grant SJ, Liu JP. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;CD009059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Heebøll S, Thomsen KL, Pedersen SB, Vilstrup H, George J, Grønbæk H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J Hepatol. 2014;6:188-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015;1852:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 39. | Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: Challenges for clinical translation. Biochim Biophys Acta. 2015;1852:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33:2895-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Müller M, Schrauwen P, Mariman EC, Blaak EE. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond). 2014;38:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 42. | Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 43. | Andrade JM, Paraíso AF, de Oliveira MV, Martins AM, Neto JF, Guimarães AL, de Paula AM, Qureshi M, Santos SH. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 44. | Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 45. | Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, Weng X. Resveratrol modulates autophagy and NF-κB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol. 2014;63:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Zhang Y, Chen ML, Zhou Y, Yi L, Gao YX, Ran L, Chen SH, Zhang T, Zhou X, Zou D. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr Food Res. 2015;59:1443-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Ji G, Wang Y, Deng Y, Li X, Jiang Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015;14:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Zhu X, Hu S, Zhu L, Ding J, Zhou Y, Li G. Effects of Lycium barbarum polysaccharides on oxidative stress in hyperlipidemic mice following chronic composite psychological stress intervention. Mol Med Rep. 2015;11:3445-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Tang WM, Chan E, Kwok CY, Lee YK, Wu JH, Wan CW, Chan RY, Yu PH, Chan SW. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology. 2012;20:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Song MY, Jung HW, Kang SY, Kim KH, Park YK. Anti-inflammatory effect of Lycii radicis in LPS-stimulated RAW 264.7 macrophages. Am J Chin Med. 2014;42:891-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Xiao J, Xing F, Huo J, Fung ML, Liong EC, Ching YP, Xu A, Chang RC, So KF, Tipoe GL. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep. 2014;4:5587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 52. | Kwon CS, Sohn HY, Kim SH, Kim JH, Son KH, Lee JS, Lim JK, Kim JS. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem. 2003;67:1451-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Zhang X, Han X, Yin L, Xu L, Qi Y, Xu Y, Sun H, Lin Y, Liu K, Peng J. Potent effects of dioscin against liver fibrosis. Sci Rep. 2015;5:9713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Poudel B, Lim SW, Ki HH, Nepali S, Lee YM, Kim DK. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int J Mol Med. 2014;34:1401-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Liu M, Xu L, Yin L, Qi Y, Xu Y, Han X, Zhao Y, Sun H, Yao J, Lin Y. Potent effects of dioscin against obesity in mice. Sci Rep. 2015;5:7973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Risitano R, Currò M, Cirmi S, Ferlazzo N, Campiglia P, Caccamo D, Ientile R, Navarra M. Flavonoid fraction of Bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-κB inhibition in THP-1 monocytes. PLoS One. 2014;9:e107431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Picerno P, Sansone F, Mencherini T, Prota L, Aquino RP, Rastrelli L, Lauro MR. Citrus bergamia juice: phytochemical and technological studies. Nat Prod Commun. 2011;6:951-955. [PubMed] |
| 58. | Mollace V, Sacco I, Janda E, Malara C, Ventrice D, Colica C, Visalli V, Muscoli S, Ragusa S, Muscoli C. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia. 2011;82:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 59. | Parafati M, Lascala A, Morittu VM, Trimboli F, Rizzuto A, Brunelli E, Coscarelli F, Costa N, Britti D, Ehrlich J. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J Nutr Biochem. 2015;26:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 60. | Li B, Yang XY, Qian FP, Tang M, Ma C, Chiang LY. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain Res. 2015;1609:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Chen D, Xiong Y, Lin Y, Tang Z, Wang J, Wang L, Yao J. Capsaicin alleviates abnormal intestinal motility through regulation of enteric motor neurons and MLCK activity: Relevance to intestinal motility disorders. Mol Nutr Food Res. 2015;59:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Wang Q, Ma S, Li D, Zhang Y, Tang B, Qiu C, Yang Y, Yang D. Dietary capsaicin ameliorates pressure overload-induced cardiac hypertrophy and fibrosis through the transient receptor potential vanilloid type 1. Am J Hypertens. 2014;27:1521-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, Azuma Y, Wang HF, Tsitsilianos N, Shafiq U. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29:3182-3192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Li L, Chen J, Ni Y, Feng X, Zhao Z, Wang P, Sun J, Yu H, Yan Z, Liu D. TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflugers Arch. 2012;463:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Li Q, Li L, Wang F, Chen J, Zhao Y, Wang P, Nilius B, Liu D, Zhu Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflugers Arch. 2013;465:1303-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Soleimani D, Paknahad Z, Askari G, Iraj B, Feizi A. Effect of garlic powder consumption on body composition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Adv Biomed Res. 2016;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Xiao J, Ching YP, Liong EC, Nanji AA, Fung ML, Tipoe GL. Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur J Nutr. 2013;52:179-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Xiao J, Guo R, Fung ML, Liong EC, Chang RC, Ching YP, Tipoe GL. Garlic-Derived S-Allylmercaptocysteine Ameliorates Nonalcoholic Fatty Liver Disease in a Rat Model through Inhibition of Apoptosis and Enhancing Autophagy. Evid Based Complement Alternat Med. 2013;2013:642920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 69. | Xiu LJ, Sun DZ, Jiao JP, Yan B, Qin ZF, Liu X, Wei PK, Yue XQ. Anticancer effects of traditional Chinese herbs with phlegm-eliminating properties - An overview. J Ethnopharmacol. 2015;172:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Li N, Ma Z, Li M, Xing Y, Hou Y. Natural potential therapeutic agents of neurodegenerative diseases from the traditional herbal medicine Chinese dragon’s blood. J Ethnopharmacol. 2014;152:508-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Shi KQ, Fan YC, Liu WY, Li LF, Chen YP, Zheng MH. Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: a systematic review and meta-analysis. Mol Biol Rep. 2012;39:9715-9722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 72. | Yang SY, Zhao NJ, Li XJ, Zhang HJ, Chen KJ, Li CD. Ping-tang Recipe () improves insulin resistance and attenuates hepatic steatosis in high-fat diet-induced obese rats. Chin J Integr Med. 2012;18:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Yang Q, Xu Y, Feng G, Hu C, Zhang Y, Cheng S, Wang Y, Gong X. p38 MAPK signal pathway involved in anti-inflammatory effect of Chaihu-Shugan-San and Shen-ling-bai-zhu-San on hepatocyte in non-alcoholic steatohepatitis rats. Afr J Tradit Complement Altern Med. 2014;11:213-221. [PubMed] |
| 74. | Kwanten WJ, Martinet W, Michielsen PP, Francque SM. Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: a controversial issue. World J Gastroenterol. 2014;20:7325-7338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Yuk JM, Jo EK. Crosstalk between autophagy and inflammasomes. Mol Cells. 2013;36:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14:1631-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |