Published online Jan 7, 2017. doi: 10.3748/wjg.v23.i1.87
Peer-review started: August 16, 2016
First decision: October 11, 2016
Revised: October 19, 2016
Accepted: November 15, 2016
Article in press: November 16, 2016
Published online: January 7, 2017
Processing time: 142 Days and 7.2 Hours
To explore the effect of hydrogen sulfide (H2S) on restraint water-immersion stress (RWIS)-induced gastric lesions in rats and the influence of adenosine triphosphate (ATP)-sensitive potassium (KATP) channels and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway on such an effect.
Male Wistar rats were randomly divided into a control group, a physiological saline (PS) group, a sodium hydrosulfide (NaHS) group, a glibenclamide (Gl) group, Gl plus NaHS group, a pyrrolidine dithiocarbamate (PDTC) group, and a PDTC plus NaHS group. Gastric mucosal injury was induced by RWIS for 3 h in rats, and gastric mucosal damage was analyzed after that. The PS, NaHS (100 μmol/kg body weight), Gl (100 μmol/kg body weight), Gl (100 μmol/kg or 150 μmol/kg body weight) plus NaHS (100 μmol/kg body weight), PDTC (100 μmol/kg body weight), and PDTC (100 μmol/kg body weight) plus NaHS (100 μmol/kg body weight) were respectively injected intravenously before RWIS.
RWIS induced serious gastric lesions in the rats in the PS pretreatment group. The pretreatment of NaHS (a H2S donor) significantly reduced the damage induced by RWIS. The gastric protective effect of the NaHS during RWIS was attenuated by PDTC, an NF-κB inhibitor, and also by glibenclamide, an ATP-sensitive potassium channel blocker, in a dose-dependent manner.
These results suggest that exogenous H2S plays a protective role against RWIS injury in rats, possibly through modulation of KATP channel opening and the NF-κB dependent pathway.
Core tip: In this study, the authors demonstrate that exogenous hydrogen sulfide plays a protective role against restraint water-immersion stress injury in rats possibly through modulation of adenosine triphosphate-sensitive potassium channel opening and the nuclear factor kappa-light-chain-enhancer of activated B cells dependent pathway.
- Citation: Sun HZ, Zheng S, Lu K, Hou FT, Bi JX, Liu XL, Wang SS. Hydrogen sulfide attenuates gastric mucosal injury induced by restraint water-immersion stress via activation of KATP channel and NF-κB dependent pathway. World J Gastroenterol 2017; 23(1): 87-92
- URL: https://www.wjgnet.com/1007-9327/full/v23/i1/87.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i1.87
Restraint water-immersion stress (RWIS), considered to be a mixture of physical and psychological stress, can induce anxiety, hypothermia, and severe gastric dysfunction including gastric hypercontractility, gastric acid hypersecretion, and gastric mucosal lesions within a few hours[1-4]. This model is used to study the mechanism of gastric mucosal lesions induced by stress and filter drugs in clinical trials. As is well known, not only is gastric stress ulcera common complication in patients with clinical critical disease, but also the number of primary gastric stress ulcers seen continues to increase with the fierce competition and pressure common in modern society. Thus, clarifying the mechanism that causes gastric mucosal lesions as a result of RWIS and looking for ways to reduce these lesions are very important to prevent and cure gastric stress damage in the clinical setting.
Recent studies suggest that hydrogen sulfide (H2S) is the third gaseous mediator in mammals after nitric oxide (NO) and carbon monoxide (CO) and that it regulates a range of physiological and pathological processes in the nervous system, cardiovascular system, respiratory system, and digestive system, and regulates metabolism and immunity, etc[5-11].
Recent studies on rats suggest that H2S can protect the gastric mucosa, possibly through mechanisms that involve anti-oxidant and anti-inflammatory actions[12], but the effect of H2S on gastric mucosa damage induced by RWIS still needs further research. Previous reports have shown that H2S regulates a range of physiological and pathological processes involving KATP channels. Hydrogen sulfide has been shown to protect gastric epithelial cells from ischemia-reperfusion injury by Keap1 S-sulfhydration, mitogen-activated protein kinase (MAPK) dependent anti-apoptosis, and the NF-κB dependent anti-inflammation pathway[13]. Therefore, in this study, we evaluated the effect of H2S on RWIS-induced gastric lesions in rats and the influence of KATP channels and the NF-κB dependent pathway on this effect.
Experiments were performed on male Wistar rats (220-280 g) purchased from the Experimental Animal Center of Shandong University. Animals were maintained in a temperature-controlled environment with a 12-h light/dark cycle. They were allowed free access to food and water for one week. Prior to the experiments, the animals were fasted for 24 h but allowed free access to water. All procedures performed were according to the guidelines of the International Association for the Study of Pain[14] and were approved by the Experimental Animal Ethical Association in Qi Lu Normal University.
Chemicals used and their sources were as follows[15]: sodium hydrosulfide (NaHS, 100 μmol/kg body weight), glibenclamide (Gl, 100 or 150 μmol/kg body weight), and pyrrolidine dithiocarbamate (PDTC, 100 μmol/kg body weight), purchased from Sigma (Saint Louis, MO, United States). NaHS and PDTC were dissolved in 0.9% saline, but Gl was dissolved in dimethyl sulfoxide. All chemicals were injected intraperitoneally (IP) before inducing RWIS.
The rats were randomly divided into 7 groups with 13 rats per group: (1) in the control group, the rats were not stressed under otherwise identical conditions; (2) in the physiological saline (PS) group, the rats were given RWIS for 3 h after pretreatment with IP injection of PS; (3) in the NaHS group, the rats were given RWIS for 3 h after pretreatment with IP injection of NaHS (100 μmol/kg body weight); (4) in the Gl group, the rats were given RWIS for 3 h after pretreatment with IP injection of Gl (100 μmol/kg body weight); (5) in the Gl plus NaHS group, the rats were given RWIS for 3 h after pretreatment with IP injection of Gl (100 or 150 μmol/kg body weight) and NaHS; (6) in the PDTC group, the rats were given RWIS for 3 h after pretreatment with IP injection of PDTC (100 μmol/kg body weight); and (7) in the PDTC plus NaHS group, the rats were given RWIS for 3 h after pretreatment with IP injection of PDTC (100 μmol/kg body weight) and NaHS.
In the RWIS groups, after light ether anesthesia, the four limbs of each rat were gently bound on a wooden board securely using medical adhesive tape. After the rats were conscious, they were vertically immersed in cold water (21 °C ± 1 °C) to the level of the xiphoid for 3 h. All of the experiments were terminated by a bolus IP injection of sodium pentobarbital (100 mg/kg body weight). Then the abdomen of each rat was opened, and the stomach was removed and fixed with 1% formalin. The gastric lesions were examined with a light microscope, and a scoring system was used to assess the gastric mucosal erosion index (GMEI) of each rat[16]. Scores were given according to the length of lesions: ≤ 1 mm = 1 point, 1 to ≤ 2 mm = 2 points, and so on. The score was multiplied by 2 when the damage was more than 1 mm in width. The cumulative scores of all lesions in a rat served as the GMEI of that rat.
All values were analyzed using SPSS13.0 software (SPSS Inc.) and presented as mean ± SE. Statistical analysis was performed by the Student t-test. Significance was accepted at the level of P < 0.05.
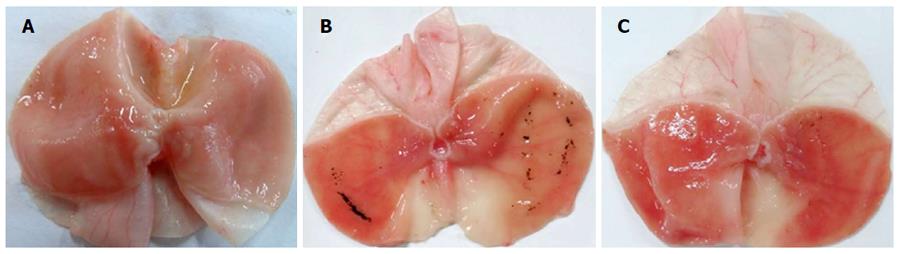
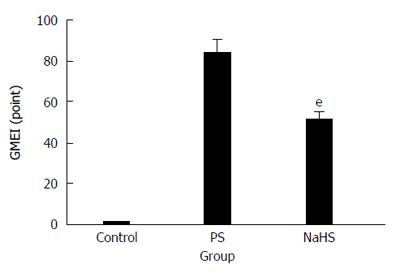
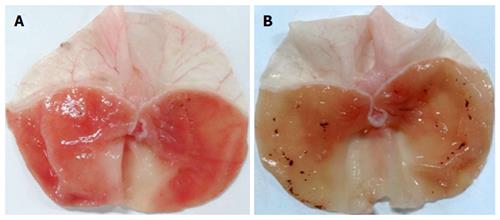
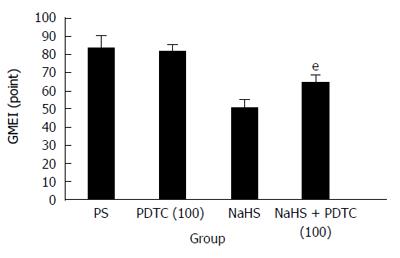
The mucosal surface of the control group was smooth, and no significant abnormality in the gastric mucosa was observed under the light microscope (Figure 1A). However, significant hemorrhage and edema and several erosions of varying depths and sizes were observed on the surface of the mucosa of the RWIS groups (Figure 1B and C). The GMEI was 1.54 ± 0.27 points in the control group, 84.38 ± 6.34 points in the PS group (compared with the control group, P < 0.001), and 51.23 ± 4.08 points in the NaHS group (compared with the control group, P < 0.001) (Figure 2).
Compared with the PS group, the injury area and the extent of mucosal damage significantly decreased in the NaHS group (Figure 1B and C). The GMEI in the NaHS group was obviously lower than that in the PS group (51.23 ± 4.08 points vs 84.38 ± 6.34 points, P < 0.001) (Figure 2).
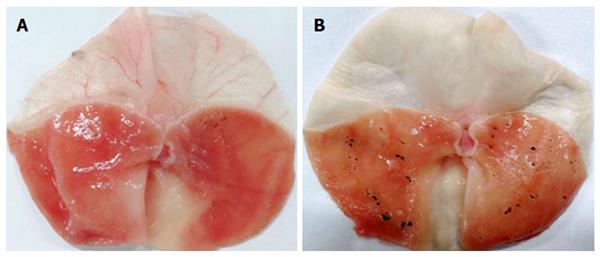
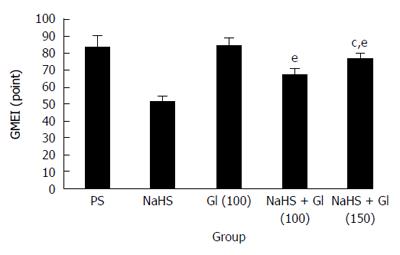
The gastric protective effect of NaHS during RWIS was abolished by Gl, an ATP-sensitive potassium channel KATP blocker (Figure 3). Under the light microscope, the GMEIs in the Gl (100 μmol/kg body weight) plus NaHS group and the Gl (150 μmol/kg body weight) plus NaHS group were much higher than those in the NaHS group (67.92 ± 4.63 points and 76.92 ± 4.71 points vs 51.23 ± 4.08 points, P < 0.001). The GMEI in the Gl (100 μmol/kg body weight) plus NaHS group was lower than that in the Gl (150 μmol/kg body weight) plus NaHS group (P < 0.05) (Figure 4). These results suggest that Gl prevented the protective effect of NaHS on RWIS-induced gastric mucosal injury in a dose-dependent manner.
The gastric protective effect of NaHS during RWIS was weakened by PDTC, an NF-κB inhibitor (Figure 5). Under the light microscope, the GMEI in the PDTC (100 μmol/kg body weight) plus NaHS group was higher than that in the NaHS group (65.00 ± 4.01 points vs 51.23 ± 4.08 points, P < 0.001) (Figure 6). These results suggest that PDTC weakened the protective effect of NaHS on RWIS-induced gastric mucosal injury.
In this study, significant hemorrhage and edema and several erosions of varying depths and sizes were observed on the surface of the mucosa in the RWIS groups. The occurrence of RWIS-induced gastric mucosal erosion is possibly related to a number of factors, including excessive production of oxygen free radicals in the mucosa[12,17], leukocyte infiltration[18], decreased release of nitric oxide[19], gastric hypercontractility, gastric acid hypersecretion, and gastric mucosa ischemia caused by a reduction in the gastric mucosal blood flow.
H2S is formed in mammalian cells by the activity of two pyridoxal phosphate-dependent enzymes: cystathionine-γ-1yase and cystathionine-β-synthase[20]. NaHS, as a H2S donor, dissociates in vivo into sodium ions and sulfhydryl group ions, and the latter bind with hydrogen ions to generate H2S. Thus, H2S and NaHS are in dynamic equilibrium[21]. Previous work has demonstrated that H2S has anti-inflammatory and antioxidant activities[22]. The gastroprotective effect of endogenous H2S against gastric ischemia-reperfusion injury may be mediated by enhancing the anti-oxidative capacity through increasing glutathione and superoxide dismutase to reduce free radical production[21]. In this study, NaHS significantly attenuates gastric mucosal injury induced by restraint water-immersion stress. We surmise that the mechanism is possibly through anti-oxidant and anti-inflammatory actions.
Previous reports showed that ATP-sensitive potassium KATP channels regulate a range of physiological and pathological processes. Dawe et al[23] found that H2S in the hypothalamus decreases blood pressure and heart rate by a KATP channel-dependent mechanism in freely moving rats. Data support the hypothesis that endogenous H2S produces cardiovascular inhibition functions in the nucleus of solitary tract, mainly mediated by KATP channel regulation or/and glutamate receptors[24]. Exogenous H2S plays a protective role against gastric ischemia-reperfusion injury in rats possibly through modulation of KATP channel opening[25]. Here, we have shown that Gl, an ATP-sensitive potassium channel blocker, reversed the protective effect of NaHS on RWIS-induced gastric damage in a dose-dependent manner. These results suggest that H2S plays a protective role against gastric RWIS injury in rats, possibly through modulation of KATP channel opening mechanisms. Data have demonstrated that the H2S-induced relaxation of mesenteric artery beds was mediated by ATP-sensitive K+ (KATP) channel activity in vascular smooth muscle cells[26]. Therefore, we speculate that on the one hand, H2S, by opening KATP channels, relaxes gastric mucosal blood vessels and increases gastric mucosal blood flow. This reduces the damage caused by RWIS by accelerating the removal of harmful substances. On the other hand, H2S, by opening the KATP channels, increases the K+ efflux, which attenuates RWIS-induced gastric mucosal injury by hyperpolarizing the oxyntic cell membrane to reduce gastric acid secretion.
H2S is a small gas molecule, which can freely pass through a variety of biological membranes, target a wide range, which may affect multiple signaling pathways such as mitogen-activated protein kinase (MAPK) signaling pathways, NF-κB signal through-road, and phosphoinositide 3-kinase (PI3K) and its downstream molecules, serine/threonine protein kinase AKT (PI3K/AKT)[27,28]. Hydrogen sulfide protected gastric epithelial cells from ischemia-reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis, and the NF-κB dependent anti-inflammation pathway[13]. Our study results show that PDTC, an NF-κB inhibitor, reversed the protective effect of NaHS on RWIS-induced gastric damage, which suggests that H2S plays a protective role against gastric RWIS injury in rats, possibly through an NF-κB dependent anti-inflammation mechanism.
In conclusion, the results of this study suggest that H2S plays a protective role against RWIS-induced gastric mucosal injury in rats, possibly through modulation of KATP channel opening and through the NF-κB dependent pathway.
Recent studies suggest that hydrogen sulfide (H2S) is the third gaseous mediator in mammals after nitric oxide and carbon monoxide and that it modulates a range of physiological and pathological processes. H2S has been found throughout the gastrointestinal tract, but little is known about the effect of H2S on restraint water-immersion stress (RWIS)-induced gastric lesions in rats and the influence of adenosine triphosphate (ATP)-sensitive potassium (KATP) channels and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway on such an effect.
Previous reports have shown that H2S is a small gas molecule, which may not only regulate a range of physiological and pathological processes involving KATP channels, but also affect multiple signaling pathways, such as mitogen-activated protein kinase signaling pathways, NF-κB signal through-road, and phosphoinositide 3-kinase and its downstream molecules serine/threonine protein kinase. In this study, the authors demonstrate that exogenous H2S plays a protective role against RWIS injury in rats possibly through modulation of KATP channel opening and the NF-κB dependent pathway.
This is the first study to report that exogenous H2S plays a protective role against RWIS injury in rats possibly through modulation of KATP channel opening and the NF-κB dependent pathway.
This study may provide a future strategy for therapeutic intervention in case of stress gastric lesions by helping understand the mechanism of action of H2S on RWIS-induced gastric lesions.
In the gastrointestinal tract, cystathionine-β-synthase and cystathionine-γ-lyase are mainly responsible for endogenous H2S synthesis. H2S is involved in gastric motility, gastric acid secretion, and gastric mucosal injury.
This is a well written and planned study demonstrating the protective effects of H2S in gastric stress lesions in rats. The protective effects of H2S seem to arise from modulation of KATP channel and the NF-κB dependent pathway.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim JS S- Editor: Yu J L- Editor: A E- Editor: Liu WX
| 1. | Mersereau WA, Hinckley EJ. Hypothermia-induced gastric hypercontracility in the genesis of the restraint ulcer. Can J Surg. 1981;24:622-625. [PubMed] |
| 2. | Arai I, Muramatsu M, Aihara H. Body temperature dependency of gastric regional blood flow, acid secretion and ulcer formation in restraint and water-immersion stressed rats. Jpn J Pharmacol. 1986;40:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Ai HB, Zhang ZD. Studies on the mechanism of gastric mucosal injury induced by water-immersion stress in rats. Sheng Li Xue Bao. 1990;42:496-502. [PubMed] |
| 4. | Ephgrave KS, Cullen JJ, Broadhurst K, Kleiman-Wexler R, Shirazi SS, Schulze-Delrieu K. Gastric contractions, secretions and injury in cold restraint. Neurogastroenterol Motil. 1997;9:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, Zhang Z, Tang C, Du J. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1608-R1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 536] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 7. | Sowmya S, Swathi Y, Yeo AL, Shoon ML, Moore PK, Bhatia M. Hydrogen sulfide: regulatory role on blood pressure in hyperhomocysteinemia. Vascul Pharmacol. 2010;53:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Tamizhselvi R, Moore PK, Bhatia M. Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas. 2008;36:e24-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther. 2008;324:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Zhi L, Moochhala SM, Moore PK, Bhatia M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J Leukoc Biol. 2007;82:894-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Aboubakr EM, Taye A, El-Moselhy MA, Hassan MK. Protective effect of hydrogen sulfide against cold restraint stress-induced gastric mucosal injury in rats. Arch Pharm Res. 2013;36:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Guo C, Liang F, Shah Masood W, Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur J Pharmacol. 2014;725:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand. 1986;128:221-233. [PubMed] |
| 15. | Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology. 1979;76:88-93. [PubMed] |
| 17. | Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39-50. [PubMed] |
| 18. | Andrews FJ, Malcontenti-Wilson C, O’Brien PE. Polymorphonuclear leukocyte infiltration into gastric mucosa after ischemia-reperfusion. Am J Physiol. 1994;266:G48-G54. [PubMed] |
| 19. | Wada K, Kamisaki Y, Ohkura T, Kanda G, Nakamoto K, Kishimoto Y, Ashida K, Itoh T. Direct measurement of nitric oxide release in gastric mucosa during ischemia-reperfusion in rats. Am J Physiol. 1998;274:G465-G471. [PubMed] |
| 20. | Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future? Trends Pharmacol Sci. 2003;24:609-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Cui J, Liu L, Zou J, Qiao W, Liu H, Qi Y, Yan C. Protective effect of endogenous hydrogen sulfide against oxidative stress in gastric ischemia-reperfusion injury. Exp Ther Med. 2013;5:689-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Mard SA, Neisi N, Solgi G, Hassanpour M, Darbor M, Maleki M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig Dis Sci. 2012;57:1496-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Dawe GS, Han SP, Bian JS, Moore PK. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience. 2008;152:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Qiao W, Yang L, Li XY, Cao N, Wang WZ, Chai C, Lu Y. The cardiovascular inhibition functions of hydrogen sulfide within the nucleus tractus solitarii are mediated by the activation of KATP channels and glutamate receptors mechanisms. Pharmazie. 2011;66:287-292. [PubMed] |
| 25. | Zou JH, Qiao WL, Wang GM, Ma HJ, Qi YJ, Sun H, Yan CD. [Exogenous hydrogen sulfide attenuates gastric ischemia-reperfusion injury via activation of K(ATP) channel]. Sheng Li Xue Bao. 2012;64:27-32. [PubMed] |
| 26. | Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316-H2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 27. | Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 28. | Bos EM, Snijder PM, Jekel H, Weij M, Leemans JC, van Dijk MC, Hillebrands JL, Lisman T, van Goor H, Leuvenink HG. Beneficial effects of gaseous hydrogen sulfide in hepatic ischemia/reperfusion injury. Transpl Int. 2012;25:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |