Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2855
Peer-review started: August 21, 2015
First decision: September 9, 2015
Revised: October 13, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: March 7, 2016
Processing time: 195 Days and 17.4 Hours
A case in which implantation of esophageal squamous cell carcinoma onto a post-dissection gastric ulcer was strongly suspected is presented. A 72-year-old man with alcoholic liver cirrhosis underwent esophagogastroduodenoscopy (EGD). Esophageal cancer (EC) (Mt, 20 mm, 0-Is) and gastric cancer (GC) (antrum, 15 mm, 0-IIc) were identified. Biopsy specimens revealed moderately differentiated squamous cell carcinoma (SCC) and differentiated adenocarcinoma, respectively. The GC was resected by endoscopic submucosal dissection (ESD) [14 mm × 9 mm, type 0-IIc, tub1, pT1a(M), ly0, v0, HM(-), VM(-)]. Two months after ESD, radiation therapy was started for the EC, and an almost complete response was obtained. Nine months after the ESD, a follow-up EGD showed a submucosal tumor-like lesion with ulceration, located immediately under the post-ESD scar, and biopsy specimens showed moderately differentiated SCC. There were no similar lesions suggesting hematogenous or lymphatic metastasis in the stomach.
Core tip: A 72-year-old-man had gastric adenocarcinoma and esophageal squamous cell carcinoma (SCC); the gastric cancer was resected by endoscopic submucosal dissection (ESD), and the esophageal cancer was treated by radiation therapy two months after the ESD. The post ESD scar was found to be elevated and ulcerated during follow-up examination nine months after ESD, and the biopsy specimens showed SCC. It was strongly suspected that the lesion was the result of implantation of esophageal cancer onto the post-ESD ulcer, because the tumor was located immediately under the scar, and there were no similar lesions suggesting hematogenous or lymphatic metastasis.
- Citation: Asai S, Takeshita K, Kano Y, Nakao E, Ichinona T, Fujimoto N, Akamine E, Mori T, Ogawa A. Implantation of esophageal cancer onto post-dissection ulcer after gastric endoscopic submucosal dissection. World J Gastroenterol 2016; 22(9): 2855-2860
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2855.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2855
Implantation of cancer cells onto the ulcer after endoscopic submucosal dissection (ESD) is extremely rare, although implantation onto the site of a stapled anastomosis after colectomy or implantation of pharyngoesophageal malignancy onto a percutaneous endoscopic gastrostomy (PEG) site is a well-known phenomenon. There is a reported case of local recurrence thought to be from implantation of tumor cells after rectal ESD[1], and there is also a report of implantation after endoscopic mucosal resection in a patient with rectosigmoid cancer[2]. However, to the best of our knowledge, implantation of cancer cells from a malignancy of another organ to the ulcer after ESD has never been reported. Thus, a case in which implantation of esophageal squamous carcinoma onto post-dissection gastric ulcer was strongly suspected is reported.
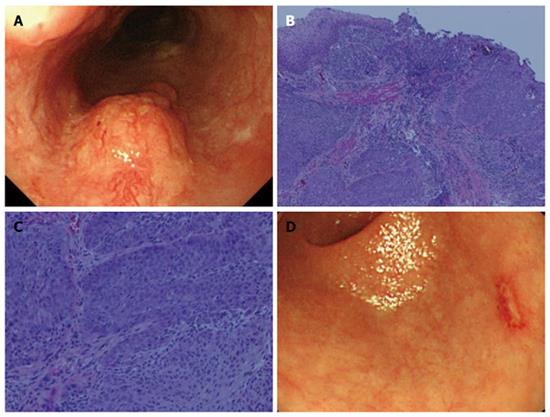
A 72-year-old man with alcoholic liver cirrhosis underwent esophagogastroduodenoscopy (EGD). Risky esophageal varices (EV), esophageal cancer (EC) (Mt, left, 20 mm, 0-Is, depth of SM2), and gastric cancer (GC) (L, post, 15 mm, 0-IIc, depth of M) were identified. Biopsy specimens revealed moderately differentiated squamous cell carcinoma (SCC) and differentiated adenocarcinoma, respectively (Figure 1).
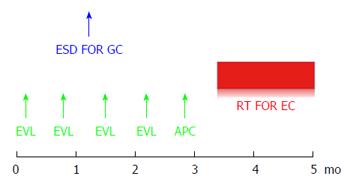
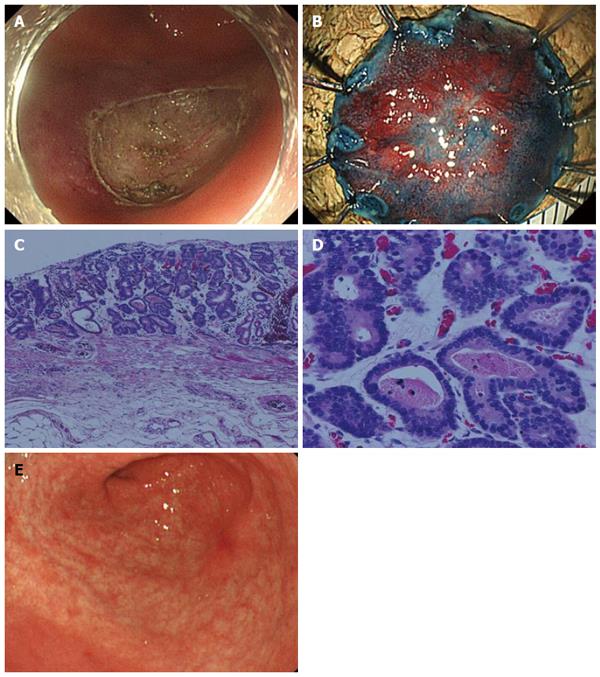
Considering his comorbidity and general condition, the treatment strategy shown in Figure 2 was planned. Repeated biweekly endoscopic variceal ligation therapy (EVL) was performed until the EV disappeared before radiation therapy for the EC. In addition, the GC was resected by ESD during the same period of time. The operation time was 63 min, and the resected specimen was 22 mm in diameter. No complications occurred. On histology, the lesion was 14 mm × 9 mm, type 0-IIc, tub1, pT1a(M), UL(-), ly0, v0, HM(-), VM(-) (Figure 3).
Two months after ESD, when the post-ESD ulcer was healed (Figure 3E) and the EV had disappeared, radiation therapy (RT) was started for the EC at 60 Gy/6 wk, and an almost complete response was obtained at the end of the RT.
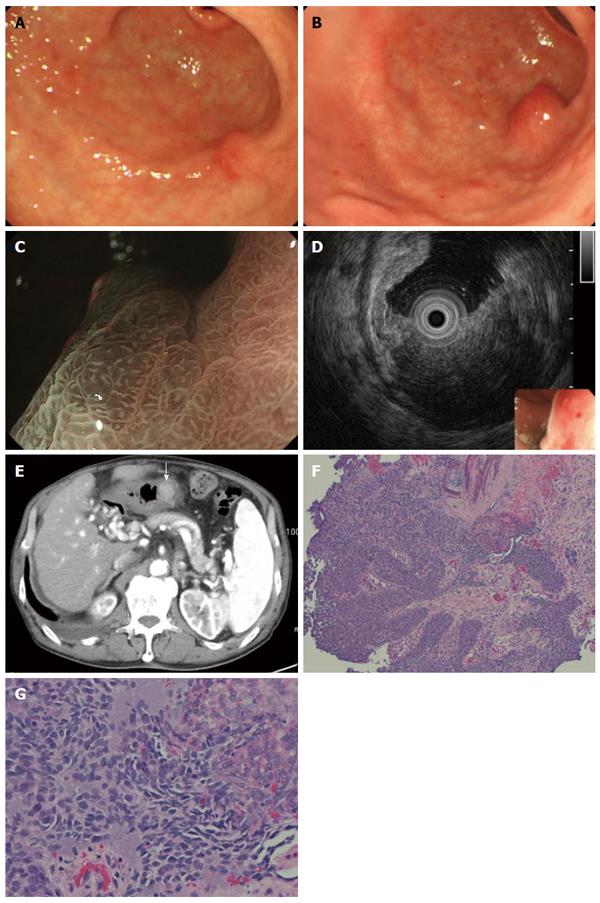
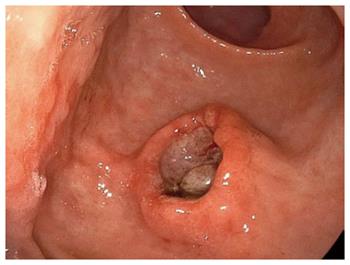
A follow-up EGD 7 mo after the ESD showed a slight elevation on the post ESD scar (Figure 4A), but biopsy specimens did not show any malignancy. Two more months later, EGD showed a submucosal tumor-like lesion with ulceration, 15 mm in size, located immediately under the post-ESD scar, and there were no other similar elevated lesions in the stomach. Magnifying endoscopy with narrow band imaging showed that the base of the elevation was covered with normal mucosa. Endoscopic ultrasonography (EUS) findings indicated that the tumor mainly involved the submucosal layer. The tumor was also recognized as a well-demarcated, early enhanced mass on CT. At that time, some swollen lymph nodes were observed around the esophagus, but no hematogenous metastases, such as lung or liver metastases, were detected. Biopsy specimens from the edge of the ulcer on the top of the elevated lesion revealed moderately differentiated SCC, which closely resembled the EC (Figure 4B-G).
It was strongly suspected that the lesion was implantation of EC onto the post-ESD ulcer after ESD for GC because the tumor was located immediately under the scar, and there were no similar lesions suggesting hematogenous metastasis. Because local recurrence of the esophageal cancer was also found by follow-up EGD at that time, the patient was managed with best supportive care. The tumor seemed to be Borrmann type 2, and there were no other lesions suggesting intramural metastases in the stomach a year after the ESD (Figure 5).
In the current case, the post ESD scar at the gastric antrum was found to be elevated and ulcerated during follow-up examination after ESD for gastric adenocarcinoma, and the biopsy specimen from that lesion revealed SCC. Although direct implantation, hematogenous metastasis, intramural metastasis (lymphatic dissemination), and primary gastric SCC were considered potential causes, implantation was thought to be the most likely because the solitary SCC grew directly under the post ESD scar in the antrum. Hematogenous metastasis to the stomach usually presents with multiple lesions in the gastric wall. In the literature[3,4], intramural metastasis to the stomach is predominantly located in the upper portion of the stomach. The possibility that primary gastric squamous cell carcinoma, which is quite a rare histological type, arose immediately under the post ESD scar was thought to be extremely low. Furthermore, the histological examination revealed that the tissue taken from the gastric lesion resembled that from the esophageal cancer. A review of 44 cases of stomal metastases after PEG from pharyngoesophageal malignancy concluded that the risk factors, such as the higher risk of stomal metastases with endoscopic rather than surgical gastrostomy, are more consistent with direct implantation of cancerous cells at the stoma than with hematogenous or lymphatic dissemination at a wound site[5]. All these previous reports strongly support the implantation hypothesis.
As the mechanism of implantation in the current case, it is possible that a relatively large amount of cancer cells were exfoliated by continuous physical contact of the scope against the EC during gastric ESD, which took one hour and reached the post-dissection ulcer, which was regarded as freshly cut tissue. It has also been reported that colorectal cancer cells could exfoliate into the intestinal lumen due to intraoperative manipulation, and such cells have high viability[6]. There is another possibility that implantation occurred at a later date following ulcer healing after ESD, for example, by EVL or food intake. Since this case required EVL and APC procedures after ESD that would be unusual in regular cases, these frequent endoscopic approaches to the esophagus may have been associated with implantation of esophageal cancer. In fact, the EVL procedures were carried out twice, followed by one APC procedure after ESD. Although the tip of the inserted flexible over tube used for the EVL procedure was far from the esophageal cancer, because it was shorter than the length from his mouth to the esophageal cancer, these frequent approaches might have caused the cancer cells to exfoliate to a certain degree. However, at the time of the procedures, the post-dissection ulcer bed was completely covered by a white coat, and freshly cut tissue was not exposed. It was assumed that implantation was not able to occur in such a situation even if many cancer cells were exfoliated.
We have to carefully develop treatment strategies for patients with double cancers, keeping in mind that implantation can occur after ESD. The present patient could not undergo surgery for EC because of his comorbidities. However, if the case were operable, esophagectomy and reconstruction by gastric tube following gastric ESD would result in SCC recurrence under the post ESD scar in the gastric tube, and he would have to undergo reoperation. On the other hand, esophagectomy prior to the treatment of gastric cancer leaves some risks. For example, there is a risk that gastric cancer may involve the suture line of the gastric tube. Even if the patient could avoid the situation above, we need to perform the technically demanding ESD in the gastric tube after esophagectomy. Moreover, we have to consider additional surgical operations in the case of GC, which is a proven contraindication after ESD.
There are some ways to reduce the risk of implantation after ESD. First, it is important to prevent exfoliation of cancer cells as much as possible. Specifically, in addition to gentle manipulation of both lesions, application of an over tube designed for double balloon endoscopy for covering another lesion may be useful. Second, washing out the post-dissected ulcer and surrounding area to remove exfoliated cancer cells may be effective, though there is no evidence for this in the field of endoscopy. It has been reported that intraluminal lavage can help prevent tumor cell implantation to the anastomotic site after rectal cancer surgery[7]. Third, closure of the post ESD ulcer by endoscopic clipping could be beneficial when another lesion exists on the oral side of the lesion to be resected by ESD, as in the present case, because implantation might occur at a later date following ulcer healing after ESD.
This was the first report of a case of implantation of esophageal SCC onto a post-dissection ulcer after ESD for early gastric adenocarcinoma. We should keep this fact in mind and develop treatment strategies for patients with double cancers carefully.
A follow-up esophagogastroduodenoscopy (EGD) for a 72-year-old man after the gastric endoscopic submucosal dissection (ESD) followed by radiation therapy against the esophageal squamous cell carcinoma (SCC) showed a submucosal tumor-like lesion immediately under the post-ESD scar.
It was strongly suspected that the lesion was implantation of esophageal SCC on to the post-ESD ulcer because biopsy specimen taken form the lesion revealed SCC.
Hematogenous or lymphatic metastasis from the esophageal cancer was taken into consideration.
It was strongly suspected on EGD that the lesion was the result of implantation of esophageal cancer onto the post-ESD ulcer, because the tumor was located immediately under the scar, and there were no similar lesions suggesting hematogenous or lymphatic metastasis.
Biopsy specimens from the edge of the ulcer on the top of the elevated lesion revealed moderately differentiated SCC, which closely resembled the esophageal cancer.
Best supportive care only.
There is a reported case of local recurrence thought to be from implantation of tumor cells after rectal ESD, and there is also a report of implantation after endoscopic mucosal resection in a patient with rectosigmoid cancer.
A case of implantation of esophageal cancer to gastric ulcer after ESD is extremely rare. However this possibility should be kept in mind and strategies to manage patients with double cancers should be developed carefully.
In this report, implantation of esophageal cancer cells is strongly suspected through the clinical course and endoscopic findings. From this point of view, the discussion paragraph is well structured and includes useful information for readers. This case report could be helpful for readers to treat double cancers of gastrointestinal tract.
P- Reviewer: Hironaka S, Tohda G, Tsuji Y S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Inoue T, Fujii H, Koyama F, Nakagawa T, Uchimoto K, Nakamura S, Ueda T, Nishigori N, Kawasaki K, Obara S. Local recurrence after rectal endoscopic submucosal dissection: a case of tumor cell implantation. Clin J Gastroenterol. 2014;7:36-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Haba S, Ogura T. A first report of tumor cell implantation after EMR in a patient with rectosigmoid cancer. Gastrointest Endosc. 2012;75:1117-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Ebihara Y, Hosokawa M, Kondo S, Katoh H. Thirteen cases with intramural metastasis to the stomach in 1259 patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2004;26:1223-1225. [PubMed] |
| 4. | Saito T, Iizuka T, Kato H, Watanabe H. Esophageal carcinoma metastatic to the stomach. A clinicopathologic study of 35 cases. Cancer. 1985;56:2235-2241. [PubMed] |
| 5. | Cappell MS. Risk factors and risk reduction of malignant seeding of the percutaneous endoscopic gastrostomy track from pharyngoesophageal malignancy: a review of all 44 known reported cases. Am J Gastroenterol. 2007;102:1307-1311. [PubMed] |
| 6. | Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71:659-663. [PubMed] |
| 7. | Matsuda A, Kishi T, Musso G, Matsutani T, Yokoi K, Wang P, Uchida E. The effect of intraoperative rectal washout on local recurrence after rectal cancer surgery: a meta-analysis. Ann Surg Oncol. 2013;20:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |