Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2789
Peer-review started: October 1, 2015
First decision: November 13, 2015
Revised: December 14, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: March 7, 2016
Processing time: 154 Days and 17.8 Hours
AIM: To assess human cytomegalovirus-encoded US28 gene function in colorectal cancer (CRC) pathogenesis.
METHODS: Immunohistochemical analysis was performed to determine US28 expression in 103 CRC patient samples and 98 corresponding adjacent noncancerous samples. Patient data were compared by age, sex, tumor location, histological grade, Dukes’ stage, and overall mean survival time. In addition, the US28 gene was transiently transfected into the CRC LOVO cell line, and cell proliferation was assessed using a cell counting kit-8 assay. Cell cycle analysis by flow cytometry and a cell invasion transwell assay were also carried out.
RESULTS: US28 levels were clearly higher in CRC tissues (38.8%) than in adjacent noncancerous samples (7.1%) (P = 0.000). Interestingly, elevated US28 amounts in CRC tissues were significantly associated with histological grade, metastasis, Dukes’ stage, and overall survival (all P < 0.05); meanwhile, US28 expression was not significantly correlated with age, sex or tumor location. In addition, multivariate Cox regression data revealed US28 level as an independent CRC prognostic marker (P = 0.000). LOVO cells successfully transfected with the US28 gene exhibited higher viability, greater chemotherapy resistance, accelerated cell cycle progression, and increased invasion ability.
CONCLUSION: US28 expression is predictive of poor prognosis and may promote CRC.
Core tip: Human cytomegalovirus (HCMV) is strongly correlated to colorectal cancer (CRC), although it remains unclear whether the virus contributes to CRC pathogenesis. We examined the expression of HCMV-encoded US28 protein in 103 CRC patient samples and 98 corresponding adjacent noncancerous samples using immunohistochemistry; the relationship between US28 expression and clinicopathological features was also analyzed. We found that US28 expression differed significantly between colorectal carcinoma and adjacent noncancerous colorectal tissues and that US28 expression was correlated with histological grade, metastasis, Dukes’ stage, and survival. After successful transfection with the US28 gene, LOVO cells exhibited higher viability, greater chemotherapy resistance, accelerated cell cycle progression, and increased invasion. These findings indicate that US28 expression predicts a poor prognosis and may promote the pathogenesis of CRC. Thus, targeting specific HCMV proteins (e.g., US28) in endogenously infected CRC may constitute a novel antitumor approach.
- Citation: Cai ZZ, Xu JG, Zhou YH, Zheng JH, Lin KZ, Zheng SZ, Ye MS, He Y, Liu CB, Xue ZX. Human cytomegalovirus-encoded US28 may act as a tumor promoter in colorectal cancer. World J Gastroenterol 2016; 22(9): 2789-2798
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2789.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2789
Colorectal cancer (CRC) is ranked third among cancers in terms of prevalence and cause of death in the United States[1]. Globally, CRC is newly diagnosed in > 1 million individuals per year[2], resulting in approximately 715000 deaths in 2010, up from 490000 in 1990[3]. To improve therapeutic approaches for CRC, its etiology and pathogenesis should be further understood.
Growing evidence suggests that infectious agents significantly affect the pathogenesis of various cancers. Indeed, de Martel et al[4] reported that 16.1% of newly diagnosed cancers are caused by infectious organisms; this means that about 2 million newly detected cancers result from infection. The major organisms implicated are Helicobacter pylori, hepatitis B and C viruses, and human papillomaviruses, which cause largely gastric, liver, and cervical/uterine carcinomas.
Human cytomegalovirus (HCMV), a β-herpesvirus, is common in the human population. A number of recent reports suggest the specific involvement of HCMV in certain human cancers. For example, HCMV molecules were found in glioblastomas and medulloblastomas, prostate, breast, and colon cancers, as well as muco-epidermoid carcinomas of the salivary glands[5-13]. Furthermore, HCMV proteins are consistently absent in healthy tissues surrounding HCMV-positive tumors. Interestingly, HCMV has been shown to dysregulate multiple signaling pathways important in cancer pathogenesis[14].
It remains unclear if HCMV might play an oncomodulatory role in CRC. Huang et al[15] was the first to report that HCMV infections are associated with CRC; however, other studies have reported discrepant findings[16-19]. Non-detection of HCMV could result from inadequate assay conditions, which substantially affect the data[20,21]. Although increasing evidence suggests that HCMV infection is strongly correlated to CRC[11,22,23], it is unknown whether the virus contributes to CRC pathogenesis.
HCMV encoded G protein-coupled receptors, including US27, US28, UL33, and UL78, display a significant degree of homology to human chemokine receptors[24]. These receptors are important immune factors, although a few (such as CXCR4) are implicated in malignancy[25]. To date, US28, more so than the other three viral G protein-coupled receptors, has been extensively studied[26] and was shown to activate signaling networks associated with cell proliferation and migration, both in vitro and in vivo[27,28]. Accordingly, US28 may markedly subvert cell signaling and promote oncogenesis[29]. Here, immunohistochemistry was performed on samples from 103 patients previously infected with HCMV in order to assess the possible roles of US28 in CRC pathology and prognosis. Furthermore, we studied the effects of US28 overexpression on proliferation, chemotherapy sensitivity, cycle distribution, and invasion of CRC LOVO cells. Our findings provide a basis for understanding the tumor-promoting effects of US28 in the pathogenesis of CRC.
One hundred and three formalin-fixed, paraffin-embedded CRC samples and 98 corresponding adjacent noncancerous specimens were obtained from the Department of Pathology, The Second Affiliated Hospital of Wenzhou Medical University, China. All patients had been infected with HCMV, as confirmed by blood testing. Sixteen additional CRC cases not infected with HCMV were used as controls. No patient had received radiotherapy or chemotherapy before the operation. Tissue samples were assessed by two or more expert histopathologists. Tumor staging was performed according to the Dukes classification system, with the histological type determined based on World Health Organization classification criteria. There were 56 male and 47 female cancer patients, with an age range of 22-85 years old (mean age, 58.2 years). The main characteristics of the patients are summarized in Table 1. All colorectal tumor tissues samples were collected using protocols approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University; informed consent was provided by each patient.
| Clinicopathological feature | Parameter | US28 | P value | |
| Low | High | |||
| Age | ≥ 60 | 36 | 19 | 0.339 |
| < 60 | 27 | 21 | ||
| Sex | Male | 35 | 21 | 0.762 |
| Female | 28 | 19 | ||
| Tumor site | Colon | 41 | 24 | 0.603 |
| Rectum | 22 | 16 | ||
| Histologic grade | High | 29 | 5 | 0.001 |
| Moderate to low | 34 | 35 | ||
| Metastasis | Yes | 23 | 25 | 0.010 |
| No | 40 | 15 | ||
| Dukes’stage | A-B | 40 | 15 | 0.010 |
| C-D | 23 | 25 | ||
| Survival | Month | 55.5 ± 1.4 | 47.4 ± 2.4 | 0.003 |
Tissue sections (4 μm thick) from paraffin-embedded CRC samples were placed onto slides coated with polylysine. US28 expression in CRC samples was examined following a standard immunohistochemistry protocol. Briefly, deparaffinized and rehydrated slides were submitted for antigen retrieval in boiling ethylenediamine tetraacetic acid (EDTA) buffer (pH 8.0). After treatment with 3% hydrogen peroxide (10 min, room temperature) for endogenous peroxidase quenching and blocking with normal bovine serum albumin, samples were treated with anti-US28 antibody (Santa Cruz Biotechnology, Dallas, TX, United States; 1:100) at 4 °C overnight. Then, the samples were sequentially incubated with biotinylated secondary antibodies for 2 h and streptavidin-horseradish peroxidase complex and stained with 3,3-diaminobenzidine (DAB). Mayer’s hematoxylin was used for counterstaining. US28 expression in colorectal tumor samples was evaluated by two independent clinical pathology experts. Immunohistochemical evaluation of the US28 protein was carried out as described previously, taking into account positive signal intensity (graded from 0 to 3) and extent (0 to 4)[30]; final scores were the products of both individual scores and ranged between 0 and 12, with values ≥ 4 reflecting high expression.
Human CRC LOVO cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences, and maintained in Dulbecco’s Modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco, Walthan, MA, United States), 100 U/mL penicillin G, and 10 μg/mL streptomycin (Gibco). For transfection, LOVO cells were seeded at approximately 4 × 105/well in six-well culture plates; after 24 h, the cells were rinsed twice with SFM, and 2 mL of fresh serum-free medium (SFM) was added. Transfection was carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) following the manufacturer’s protocol. Briefly, 250 μL SFM was added to each 1.5-mL extreme pressure (EP) tube, followed by the addition of 4 μg pCMV6-entry-US28 (Origene, Rockville, MD, United States, VC101344) or the pCMV6-entry empty vector. The resulting solution (solution A) was mixed for 5 min. Next, 250 μL SFM and 10 μL Lipofectamine 2000 were added to each EP tube and mixed for 5 min (solution B). Solution B was then added to solution A for 20 min at ambient temperature. The resulting transfection complex was added to cells for 48 h.
Total RNA was purified with TRIzol (Invitrogen) following the manufacturer’s protocol. First strand cDNA was prepared using a RevertAid™ First Strand cDNA Synthesis Kit from Fermentas (Waltham, MA, United States) with 2 μg total RNA in 20 μL reaction. Quantitative real time polymerase chain reaction (qRT-PCR) was carried out using SYBR® Premix Ex Taq™ (Perfect Real Time) PCR kit from TaKaRa (Shiga, Japan) on a LightCycler 480 (Roche Biochemicals, Basel, Switzerland). The following primers were used in this study: US28, forward 5’-TCGCGCCACAAAGGTCGCAT-3’, reverse 5’-GACGCGACACACCTCGTCGG-3’; β-actin, forward 5’-CGTGGACATCCGCAAAGAC-3’, reverse 5’-AAGAAAGGGTGTAACGCAACTAAG-3’. qRT-PCR was performed as follows: 5 min, 95 °C; 35 cycles of 94 °C for 40 s, 55 °C for 40 s, and 72 °C for 1 min; 72 °C, 10 min. One percent agarose gel electrophoresis was used to examine the PCR products, with an expected amplicon of 390 bp. Three independent experiments were run in triplicate. Relative mRNA amounts of the target gene were normalized to those of the control (β-actin).
Harvested cells were lysed with radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime Bio, Haimen, China); after clearing the resulting cell lysates by centrifugation, total protein amounts in supernatants were measured with Beyotime bicinchoninic acid (BCA) Protein Assay Kit (Beyotime Bio). Total protein (30 μg) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred onto polyvinylidene fluoride (PVDF) membranes (Beyotime Bio). After overnight incubation (4 °C) with anti-US28 primary antibody (Santa Cruz Biotechnology, sc-28042; diluted 1:600) or anti-β-actin (Beyotime, 1:2000), membrane were incubated at room temperature for 2 h with appropriate horseradish peroxidase (HRP)-labeled secondary antibodies (Santa Cruz Biotechnology, sc-2020, 1:4000; Beyotime Bio, aa208, 1:1000). Protein signals were visualized by enhanced chemiluminescence detection reagents (Applygen Technologies, Beijing, China) and analyzed using the AlphaEaseFC 4.0 software (Alpha Innotech Co., San Leandro, CA, United States). All experiments were carried out in triplicate and repeated three times. β-actin was used as loading control for normalization.
The CRC LOVO cell line was prepared and transfected as described above (see the “Cell culture and transfection” section). Cell viability was assessed using Cell Counting Kit-8 (CCK-8) (Dojindo, Tokyo, Japan). For cell proliferation evaluation, 5000 cells were plated per well in 96-well plates. Then, transfection complex, including pCMV6-entry-US28 (transfection group, OE), pCMV6-entry empty vector (negative control group, NC), or SFM (non-transfected control group, CON), was added to wells for 24, 48, or 72 h. This was followed by incubation with CCK-8 reagent for 4 h. Optical density (OD) at 450 nm was recorded using a microplate reader (ELX800; Bio-Tek, Winooski, VT, United States). All experiments were carried out in triplicate and repeated three times.
For cytotoxicity assays, samples were setup as described above for cell proliferation, except that a chemotherapeutic agent (cisplatin) was added to the normal growth medium after transfection for 48 h. Cytotoxicity was also measured using the CCK-8 method described above. Relative drug resistance was assessed by half maximal inhibitory concentration (IC50) values.
LOVO cell cycle was assessed using flow cytometry. Briefly, cells were washed and resuspended in staining buffer containing 10 μg/mL propidium iodide. Analysis was performed on a FACSVantage flow cytometer using CellQuest (BD Biosciences, San Jose, CA, United States). Cell debris, doublets, and clumps were excluded from analysis.
Transfected LOVO cells in 0.2 mL Roswell Park Memorial Institute (RPMI)-1640 containing 5% FBS were seeded into upper wells of Matrigel precoated (BD) transwell plates (Corning Costar Corp., Lowell, CA, United States). Lower chambers had 0.6 mL RPMI 1640 containing 20% FBS. After 30 h the membranes were submitted to 2% crystal violet staining (10 min). Cells that moved across the transwell membrane were counted by microscopy in 10 randomly selected high power fields.
Statistical analyses were carried out with SPSS 22.0 (SPSS, Armonk, NY, United States). Differences among treatment groups were assessed by one-way analysis of variance (ANOVA). Oher statistical methods included Fisher’s exact, Pearson’s χ2, and Spearman’s correlation coefficient tests; the Kaplan-Meier method was used to evaluate survival, with log-rank test or Cox regression employed to determine associations of US28 expression with disease parameters. P < 0.05 was considered statistically significant.
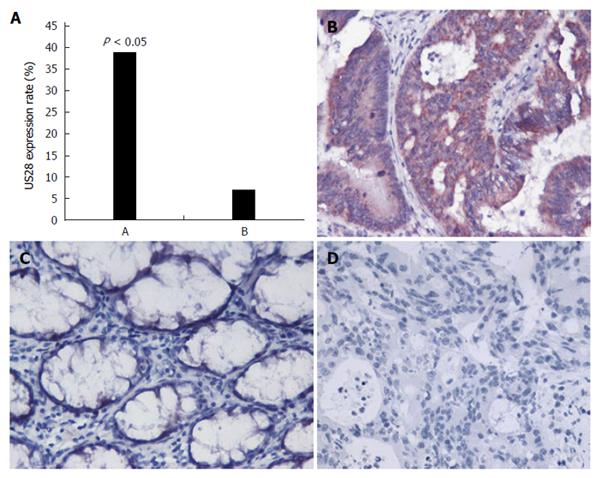
US28 expression was detected by immunohistochemical analysis using a specific antibody against US28. As shown in Figure 1, significant differences in US28 levels were observed between colorectal carcinoma (40/103, 38.8%) and adjacent noncancerous colorectal tissues (7/98, 7.1%, P = 0.000) (Figure 1A). In forty carcinoma samples, the US28 protein was highly expressed or diffusely positive in the cytoplasm and membrane of colorectal carcinoma cells (Figure 1B). In contrast, most adjacent noncancerous colorectal tissues exhibited negative expression (Figure 1C). All cases of colorectal carcinoma without HCMV infection exhibited negative expression (Figure 1D).
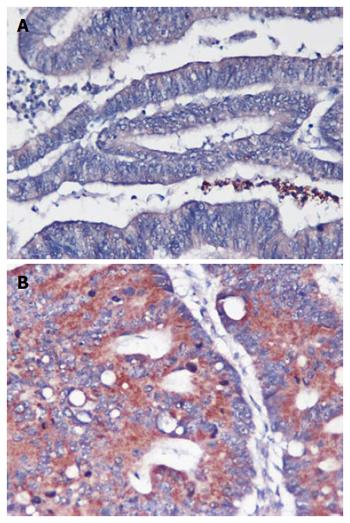
Statistical analysis indicated that among the 103 colorectal tumor samples, high US28 level was related to histological grade (P = 0.001), metastasis status (P = 0.01), Dukes’ stage (P = 0.01), and survival (P = 0.003); a significant association was not obtained with age, sex, or tumor site (Table 1). In addition, the intensity of US28 expression was inversely associated with histological grade (Figure 2), with high US28 expression associated with metastasis, an advanced stage and a poor prognosis.
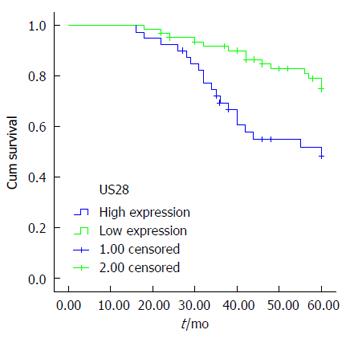
Overall survival in individuals harboring tumors showing low US28 expression (55.5 ± 1.4 mo), and survival in those with high US28 expression (47.4 ± 2.4 mo) was significantly different (P = 0.003) (Figure 3). These results suggest that high US28 expression was significantly related to shorter mean survival (P = 0.000) in these cancer patients. Multivariate Cox regression analysis revealed that US28 expression was an independent marker for CRC prognosis (P = 0.000).
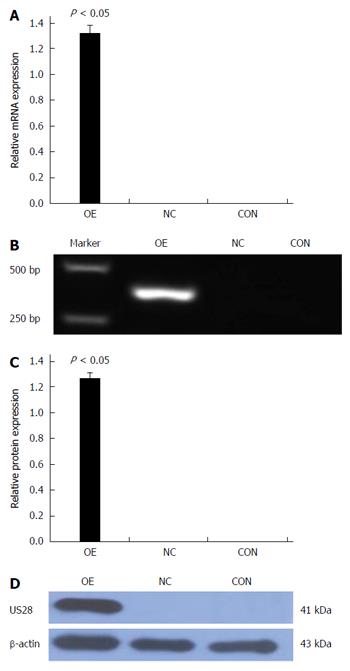
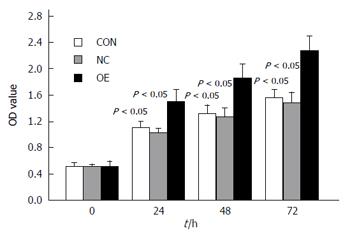
LOVO cells were transfected with pCMV6-entry-US28 for exogenous US28 gene expression. As shown in Figure 4, western blot and qRT-PCR results indicated US28 expression was significantly higher in transfected LOVO cells than in control cells. The negative control and non-transfected control groups exhibited no US28 expression. These data indicated that the US28 gene was successfully transfected into LOVO cells. We then determined cell proliferation-promoting effects in this overexpression model using a CCK-8 assay. As shown in Figure 5, overexpression of US28 increased the viability of LOVO cells at 24, 48, and 72 h (P < 0.05).
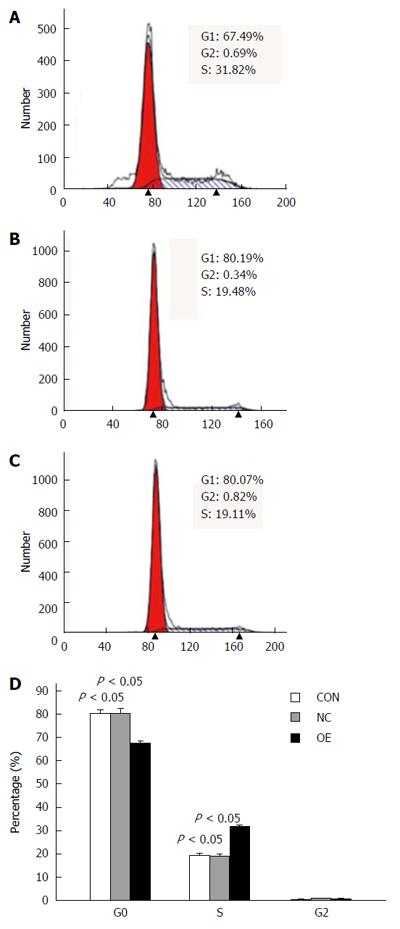
LOVO cells were transiently transfected with pCMV6-entry-US28 for 48 h, and cell cycle distribution was assessed flow-cytometrically. As shown in Figure 6, cells overexpressing US28 were less likely to be in stage G0/G1 (67.49%) compared with the control group (80.19%) (P = 0.004); meanwhile, the percentage of US28-overexpressing cells in stage S (31.82%) was remarkably higher compared to control group values (19.48%) (P = 0.002). These data indicate that US28 overexpression potentiated LOVO cell cycle progression.
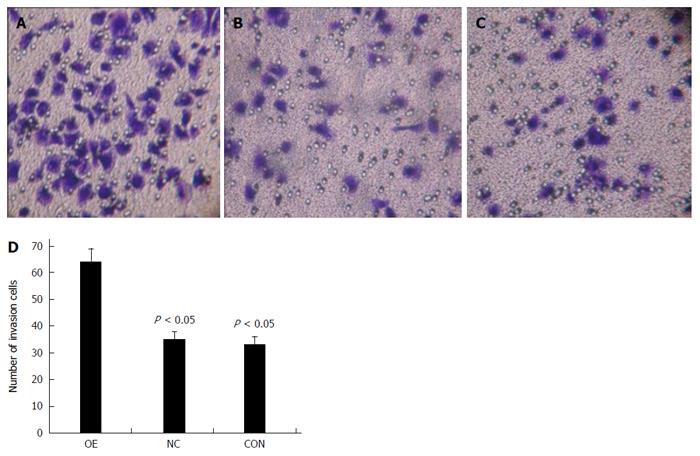
Next, a transwell assay was used to evaluate the impact of US28 gene transfection on LOVO cell invasion. As shown in Figure 7, the invasion capacity of LOVO cells in the pCMV6-entry-US28 group (OE) was 82.9% (P = 0.001) and 93.9% (P = 0.000) greater than that of non-transfected control (CON) and negative control groups (NC), respectively.
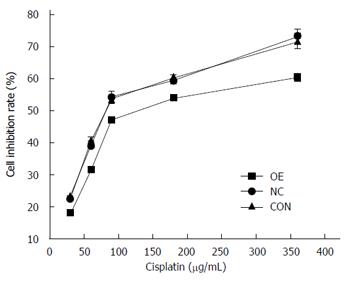
IC50 values of cisplatin were found to be 120.06 ± 1.48 μg/mL, 75.01 ± 0.86 μg/mL, and 72.71 ± 2.12 μg/mL for the OE, NC, and CON groups, respectively. In comparison with the CON and NC groups, fold changes in drug resistance levels for the OE group were 1.60 (OE/CON) and 1.65 (OE/NC), respectively (Figure 8).
Although there is some controversy regarding the relationship between HCMV infection and colorectal tumors[16-19], HCMV is found in multiple malignancies. Indeed, previous reports have detected HCMV in CRC specimens[11,15,31]. Chen et al[31] reported that 42.3% of tumor specimens were positive for HCMV, significantly higher than 5.6% obtained for adjacent non-cancerous samples. In our study, immunohistochemistry demonstrated that 38.8% of CRC specimens were positive for US28 compared to only 7.1% for adjacent noncancerous colorectal tissues. Furthermore, we found that differential US28 levels might contribute to the pathogenesis of CRC.
Bongers et al[32] reported that US28 induced intestinal dysplasia and malignancy in a transgenic mouse model. Clinical data also suggest that HCMV in tumors is linked to disadvantageous outcome in aged individuals with CRC, predicting reduced period of disease-free survival, regardless of TNM stage[33]. However, another study reported that HCMV is not related to CRC malignancy. Based on CRC patients with follow-up data, our results demonstrated that high US28 expression is inversely correlated with histological grade and Dukes’ stage and directly correlated with metastasis, indicating that US28 may be involved in the progression of CRC. Furthermore, our data demonstrated that high US28 protein expression is highly associated with overall survival, suggesting that expression of this gene might be a potential prognostic factor for colorectal tumors. Thus, US28 may play a role in promoting CRC.
The mechanism through which HCMV promotes tumor development is complex. In theory, HCMV can upregulate multiple host cellular signaling networks, enhancing malignancy[34]. This has been confirmed in animal experiments[35]. Strååt et al[36] also reported that US28 promoted tumor proliferation in HCMV-infected individuals via activation of IL-6-STAT3 signaling. We evaluated the cell proliferation-promoting effects of US28 overexpression in LOVO cells and found increased viability at 24, 48, and 72 h. Cell cycle analysis also indicated a dramatic increase in S phase entry in transfected cells, which indicates that US28 overexpression might enhance LOVO cell cycle progression. Further studies are needed to explore the underlying mechanisms.
HCMV infection is linked to significantly altered matrix metalloproteinase (MMP) levels and function[36]. Among these proteins, matrix metalloproteinase-9 (MMP-9) is negativity correlated with poor survival; high MMP-9 levels predict a favorable outcome in CRC[37]. Additionally, HCMV infection was shown to reduce MMP-9 expression and activity and increase levels of tissue inhibitor of matrix proteinase (TIMP)-1[36]. Moreover, increased gene expression of MMP-1, a CRC metastasis marker[38], was found in HCMV-positive samples[39]. Taken together, these findings suggest that HCMV might promote tumor invasion and metastasis; Soroceanu et al[40] recently reported that HCMV-encoded US28 in glioblastomas promoted an invasive and angiogenic phenotype[24]. In this study, we used a transwell assay to examine the impact of the US28 gene on LOVO cell invasion. Compared to the non-transfected control and negative control groups, overexpression of US28 resulted in 82.9% and 93.9% increases, respectively, in invasion, indicating that US28 expression can promote invasion in LOVO cells.
Nonetheless, whether HCMV infection-induced gene expression in tumors can influence therapy remains unknown. A report by Cinatl et al[41] demonstrated for the first time that HCMV infection protects tumor cells against the cytotoxic effects of anticancer drugs. Meanwhile, Soroceanu et al[42] reported that CMV proteins enhance stemness in glioblastomas. As shown above, overexpression of US28 in LOVO cells decreased cell sensitivity to cisplatin. Therefore, we can speculate that HCMV infection may induce chemotherapy resistance in CRC.
Taken together, our data suggest that US28 expression predicts poor prognosis and may promote CRC pathogenesis. Accordingly, targeting specific HCMV proteins (e.g., US28) in endogenously infected CRC may constitute a novel antitumor approach.
Human cytomegalovirus (HCMV) is a beta-herpesvirus that is common in the human population. A number of recent reports suggest that HCMV may be specifically associated with certain human malignancies, although some contrary results have also been published. Currently, growing evidence suggests that HCMV is strongly correlated with colorectal cancer (CRC); however, it is unclear whether the virus contributes to CRC pathogenesis.
This study demonstrated that the expression of US28 is significantly different between colorectal carcinoma and adjacent noncancerous colorectal tissues, with US28 expression being correlated with histological grade, metastasis, Dukes’ stage, and survival. Thus, US28 expression may predict poor prognosis. Furthermore, after successful transfection with the US28 gene, LOVO cells exhibited higher viability, greater chemotherapy resistance, accelerated cell cycle progression, and increased invasion, indicating that US28 may promote the pathogenesis of colorectal tumors.
The results indicate that HCMV is specifically associated with CRC and may act as an oncomodulatory virus. Targeting specific HCMV proteins (e.g., US28) in endogenously infected CRC may constitute a novel antitumor approach.
US28 expression can be a predictive factor for poor prognosis in colon cancer. In the future, it may be possible to target specific HCMV proteins in endogenously infected CRC to treat CRC.
HCMV-encoded chemokine receptor US28 is the most well characterized of the four chemokine receptor-like molecules found in the HCMV genome.
In this study, the authors explored the role of the HCMV-encoded US28 in the pathogenesis of CRC. To this purpose, they performed both ex vivo and in vitro studies. In the ex vivo study, they analyzed by immunohistochemistry the expression of the US28 protein in CRC samples and in adjacent noncancerous samples and correlated US28 levels to the clinicopathological features (histological grade, metastasis, Dukes’ stage, and survival). In the in vitro study, they analyzed the effect of US28 gene overexpression in LOVO CRC cells on viability, resistance to chemotherapy, cell cycle, and invasion of cells. They found that LOVO cells transfected with US29 exhibited higher viability, greater chemotherapy resistance, accelerated cell cycle progression, and increased invasion. They found that US28 expression was increased in CRC tissues compared with the adjacent noncancerous tissues. They conclude that US28 expression predicts a poor prognosis and may act to promote the pathogenesis of CRC.
P- Reviewer: Serafino A S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
| 1. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2073] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 2. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1178] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 3. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9583] [Article Influence: 737.2] [Reference Citation Analysis (0)] |
| 4. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1720] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 5. | Strååt K, Liu C, Rahbar A, Zhu Q, Liu L, Wolmer-Solberg N, Lou F, Liu Z, Shen J, Jia J. Activation of telomerase by human cytomegalovirus. J Natl Cancer Inst. 2009;101:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116:79-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol. 2003;170:998-1002. [PubMed] |
| 8. | Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med. 2008;359:539-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, Sampson JH. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10-18. [PubMed] |
| 10. | Melnick M, Sedghizadeh PP, Allen CM, Jaskoll T. Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp Mol Pathol. 2012;92:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002;360:1557-1563. [PubMed] |
| 12. | Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347-3350. [PubMed] |
| 13. | Baryawno N, Rahbar A, Wolmer-Solberg N, Taher C, Odeberg J, Darabi A, Khan Z, Sveinbjörnsson B, FuskevÅg OM, Segerström L. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest. 2011;121:4043-4055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Huang ES, Roche JK. Cytomegalovirus D.N.A. and adenocarcinoma of the colon: Evidence for latent viral infection. Lancet. 1978;1:957-960. [PubMed] |
| 16. | Akintola-Ogunremi O, Luo Q, He TC, Wang HL. Is cytomegalovirus associated with human colorectal tumorigenesis? Am J Clin Pathol. 2005;123:244-249. [PubMed] |
| 17. | Boguszaková L, Hirsch I, Brichácek B, Faltýn J, Fric P, Dvoráková H, Vonka V. Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol. 1988;32:303-308. [PubMed] |
| 18. | Brichácek B, Hirsch I, Závadová H, Procházka M, Faltýn J, Vonka V. Absence of cytomegalovirus DNA from adenocarcinoma of the colon. Intervirology. 1980;14:223-227. [PubMed] |
| 19. | Hart H, Neill WA, Norval M. Lack of association of cytomegalovirus with adenocarcinoma of the colon. Gut. 1982;23:21-30. [PubMed] |
| 20. | Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J Virol. 2012;86:854-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Chen HP, Lin JC, Yang SP, Lan YC, Weng WS, Tsai CH, Ho DM, Liu CY, Cho WL, Chan YJ. The type-2 variant of human cytomegalovirus glycoprotein N (gN-2) is not the rarest in the Chinese population of Taiwan: influence of primer design. J Virol Methods. 2008;151:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Dimberg J, Hong TT, Skarstedt M, Löfgren S, Zar N, Matussek A. Detection of cytomegalovirus DNA in colorectal tissue from Swedish and Vietnamese patients with colorectal cancer. Anticancer Res. 2013;33:4947-4950. [PubMed] |
| 23. | Tafvizi F, Fard ZT. Detection of human cytomegalovirus in patients with colorectal cancer by nested-PCR. Asian Pac J Cancer Prev. 2014;15:1453-1457. [PubMed] |
| 24. | Chee MS, Satchwell SC, Preddie E, Weston KM, Barrell BG. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344:774-777. [PubMed] |
| 25. | Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540-550. [PubMed] |
| 26. | Vischer HF, Leurs R, Smit MJ. HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends Pharmacol Sci. 2006;27:56-63. [PubMed] |
| 27. | Casarosa P, Menge WM, Minisini R, Otto C, van Heteren J, Jongejan A, Timmerman H, Moepps B, Kirchhoff F, Mertens T. Identification of the first nonpeptidergic inverse agonist for a constitutively active viral-encoded G protein-coupled receptor. J Biol Chem. 2003;278:5172-5178. [PubMed] |
| 28. | Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, Mattison K, Altschuler Y, Nelson JA. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511-520. [PubMed] |
| 29. | Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA. 2006;103:13068-13073. [PubMed] |
| 30. | Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP. Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer Res. 2000;60:18-21. [PubMed] |
| 31. | Chen HP, Jiang JK, Chen CY, Chou TY, Chen YC, Chang YT, Lin SF, Chan CH, Yang CY, Lin CH. Human cytomegalovirus preferentially infects the neoplastic epithelium of colorectal cancer: a quantitative and histological analysis. J Clin Virol. 2012;54:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Bongers G, Maussang D, Muniz LR, Noriega VM, Fraile-Ramos A, Barker N, Marchesi F, Thirunarayanan N, Vischer HF, Qin L. The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest. 2010;120:3969-3978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Chen HP, Jiang JK, Lai PY, Chen CY, Chou TY, Chen YC, Chan CH, Lin SF, Yang CY, Chen CY. Tumoral presence of human cytomegalovirus is associated with shorter disease-free survival in elderly patients with colorectal cancer and higher levels of intratumoral interleukin-17. Clin Microbiol Infect. 2014;20:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Chen HP, Chan YJ. The oncomodulatory role of human cytomegalovirus in colorectal cancer: implications for clinical trials. Front Oncol. 2014;4:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Price RL, Bingmer K, Harkins L, Iwenofu OH, Kwon CH, Cook C, Pelloski C, Chiocca EA. Cytomegalovirus infection leads to pleomorphic rhabdomyosarcomas in Trp53+/- mice. Cancer Res. 2012;72:5669-5674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Strååt K, de Klark R, Gredmark-Russ S, Eriksson P, Söderberg-Nauclér C. Infection with human cytomegalovirus alters the MMP-9/TIMP-1 balance in human macrophages. J Virol. 2009;83:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Koskensalo S, Hagström J, Linder N, Lundin M, Sorsa T, Louhimo J, Haglund C. Lack of MMP-9 expression is a marker for poor prognosis in Dukes’ B colorectal cancer. BMC Clin Pathol. 2012;12:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | O’connell JB, Maggard MA, Liu JH, Etzioni DA, Ko CY. A report card on outcomes for surgically treated gastrointestinal cancers: are we improving? J Surg Res. 2004;121:214-221. [PubMed] |
| 39. | Botero JE, Contreras A, Parra B. Effects of cytomegalovirus infection on the mRNA expression of collagens and matrix metalloproteinases in gingival fibroblasts. J Periodontal Res. 2008;43:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, Greene M, Soteropoulos P, Cobbs CS. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011;71:6643-6653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Cinatl J, Cinatl J, Vogel JU, Kotchetkov R, Driever PH, Kabickova H, Kornhuber B, Schwabe D, Doerr HW. Persistent human cytomegalovirus infection induces drug resistance and alteration of programmed cell death in human neuroblastoma cells. Cancer Res. 1998;58:367-372. [PubMed] |
| 42. | Soroceanu L, Matlaf L, Khan S, Akhavan A, Singer E, Bezrookove V, Decker S, Ghanny S, Hadaczek P, Bengtsson H. Cytomegalovirus Immediate-Early Proteins Promote Stemness Properties in Glioblastoma. Cancer Res. 2015;75:3065-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |