Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2647
Peer-review started: November 2, 2015
First decision: December 11, 2015
Revised: December 23, 2015
Accepted: January 11, 2016
Article in press: January 11, 2016
Published online: March 7, 2016
Processing time: 121 Days and 13.7 Hours
Elastographic techniques are new ultrasound-based imaging techniques developed to estimate tissue deformability/stiffness. Several ultrasound elastographic approaches have been developed, such as static elastography, transient elastography and acoustic radiation force imaging methods, which include point shear wave and shear wave imaging elastography. The application of these methods in clinical practice aims at estimating the mechanical tissues properties. One of the main settings for the application of these tools has been liver stiffness assessment in chronic liver disease, which has been studied mainly using transient elastography. Another field of application for these techniques is the assessment of focal lesions, detected by ultrasound in organs such as pancreas, prostate, breast, thyroid, lymph nodes. Considering the frequency and importance of the detection of focal liver lesions through routine ultrasound, some studies have also aimed to assess the role that elestography can play in studying the stiffness of different types of liver lesions, in order to predict their nature and thus offer valuable non-invasive methods for the diagnosis of liver masses.
Core tip: Elastography is a new ultrasound technique that allows the non-invasive assessment of tissue stiffness. Some elastographic techniques have already been validated and are widely used to evaluate liver stiffness in order to assess hepatic fibrosis and cirrhosis. Elastography is also useful in the diagnosis and characterization of different solid tumors. Considering that only a few studies have so far focused on the role of elastography in the evaluation of focal liver lesions, the present review aims to evaluate the role of real-time sonoelastography, strain elastography and ultrasound-based elastrographic techniques in the differential diagnosis and characterization of focal liver lesions.
- Citation: Conti CB, Cavalcoli F, Fraquelli M, Conte D, Massironi S. Ultrasound elastographic techniques in focal liver lesions. World J Gastroenterol 2016; 22(9): 2647-2656
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2647.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2647
Elastography is an ultrasonographic technique that provides a non-invasive assessment of tissue stiffness through the assessment of subtle changes across the entire organ being investigated and also in focal parenchymal lesions. Several studies have recently made use of different elastographic techniques, such as transient and real-time elastography, mainly to evaluate liver stiffness, in order to predict hepatic fibrosis and cirrhosis[1].
Elasticity imaging has been reported to be of value in diagnosing and characterizing various tumors, which have been found to be usually stiffer than the surrounding tissues: e.g., breast lesions, prostate cancer, lymph nodes and pancreatic masses. However, only a few studies have focused on the stiffness of focal liver lesions (FLL)[2-11]: their detection by ultrasound (US) is a crucial step for chronic liver disease surveillance, being the first-line reference technique in the early detection of hepatocellular carcinoma (HCC). The FLL characterization mostly needs further more invasive techniques, such as computed tomography (CT) and/or magnetic resonance imaging (MRI) with the administration of contrast agents, in order to evaluate the morphology and vascularization patterns of those lesions. Both techniques show high diagnostic accuracy, especially as to the diagnosis of HCC in cirrhotic patients, even if fine needle biopsy remains presently mandatory in some cases[12,13].
The recent implementation of US equipment with a variety of elastographic software, has improved the study of organ stiffness and FLL, by providing additional information on their features and aid to characterize them and predict their nature and behavior. Our review aimed to elucidate the principles of real-time sonoelastography, strain elastography, transient elastography and acoustic radiation force imaging and to critically discuss the main advantages and limitations of the above different techniques in characterizing FLL.
Elastography is based on the application of slight external tissue compression on the structures examined, to produce strain (displacement) within the tissue, and then the subsequent calculation of a strain profile along the compression axis. This profile, i.e., the tissue elasticity distribution, is converted into an elastic modulus image called elastogram. By measuring the tissue strain induced by static or dynamic stress, we can estimate the tissue hardness, thus discriminate benign from malignant lesions.
Sonoelastography can be performed with different methods of measuring the stiffness of an organ and its lesions, distinguishing “quasi-static” or “dynamic” elastography, both qualitative and quantitative.
In strain elastography, which is one of the most popular commercially available techniques, a constant stress is applied to the tissue being investigated. Tissue compression is generated by an external mechanical or an internal endogenous force: during the tissue deformation, several images are obtained recording the time of delay between two subsequent images during consecutive compressions applied in the local region of interest (ROI). The strength and duration of the deformation should be visually controlled and compression is to be performed at least twice in order to make elastograms reproducible.
This technique is easy to apply, but the unknown stress distribution prevents any quantitative estimation of the stiffness of the target zone. The quality of the examination is significantly improved by the experience of the investigator in choosing the right angle to apply an adequate level of compression strength in order to avoid artifacts. However, the very fact that stress is directly applied by the operator limits the application of this technique to superficial organs, such as breast or thyroid.
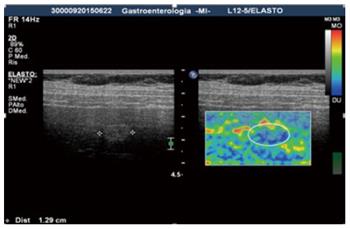
Real-time ultrasound elastography: Recently developed, real-time ultrasound elastography is an example of strain elastography used for the study of different lesions. Since the software measures the ratio between a nodule and the normal parenchyma, it gives a semi-quantitative result. The results of the calculation for every pixel is visualized on the screen in different ways: side-by-side on the conventional B-mode image or overlaid on the B-mode image. However, it is still a relative assessment and offers no precise values for the viscoelastic parameters[2,14] (Figure 1).
As of dynamic methods, a time-varying force, i.e., a short transient mechanical stress or an oscillatory force with a fixed frequency, is applied to the region under examination. Dynamic elastographic techniques, which rely on shear wave propagation, produce quantitative and higher-resolution results compared to the quasi-static methods. However, the use of shear waves requires a more complex system that is able to generate the waves through mechanical vibration or US radiation pressure, and to detect the subsequent small displacements.
Transient elastography: Transient elastography (TE) is a reliable rapid user-friendly technique, which is easy to perform in everyday practice with immediate results and good reproducibility[15]. TE is recognized as a quantitative method to assess the stiffness of organs, by means of mechanically generated low-frequency (50 Hz) shear waves, which propagate in the tissue at a speed that is directly related to the tissue elasticity, being slower in soft tissue and faster in stiffer regions[16-18]. TE fails in less than 5% of cases, mainly in obese patients. To date TE has been validated mostly in chronic hepatitis C, with a diagnostic performance comparable to that of serum markers in diagnosing significant fibrosis. In addiction, the combination of TE with serum markers increases the diagnostic accuracy, allowing to avoid liver biopsy in most patients with chronic hepatitis C. TE appears to be an excellent tool for the early detection of cirrhosis: it may have prognostic value in this setting and is useful for monitoring fibrosis progression and fibrosis regression[17], although more support data is here required (Figure 2).
Vibro-acoustography: Vibro-acoustography (VA) is a speckle-free US-based imaging modality that allows to visualize both normal and abnormal soft tissues, by mapping the acoustic response of the target tissue to a harmonic radiation force induced by US itself[2,14,19]. The method is based on the acoustic emission generated by focusing two US beams of slightly different frequencies at the same point and generating a vibration in the tissue, with a final frequency resulting from the difference between the frequencies of the primary US beams. The acoustic emission is normally detected by a hydrophone and the brightness of each image pixel is proportional to the amplitude of the acoustic signal. In medical imaging, vibro-acoustography has been tested on breast, prostate, arteries, liver, and thyroid. Pertinent studies have shown that vibro-acoustic data can be used for the quantitative evaluation of elastic properties[2,19].
Above all, VA represents an imaging method, but some viscoelastic parameters can be derived.
Acoustic radiation force impulse: Acoustic radiation force impulse (ARFI) imaging provides quantitative and qualitative measurements in a real-time mode, giving innovative information supplementary to conventional US, including elastograms and tissue parameters, such as the time to peak displacement, the peak displacement itself and the recovery time[20]. Based on the mechanical excitation of the tissue, the system allows the propagation of the shear wave away from the region of excitation by using a localized impulsive acoustic radiation force. Thus, the machine measures the tissue response to the displacement induced by excitation and uses it to generate the elastogram. ARFI is a one-dimensional technique with some limitations, such as the inability to provide an elasticity map of tissues, and the absence of real-time measurements. The only value calculated is the average value in the ROI[20,21].
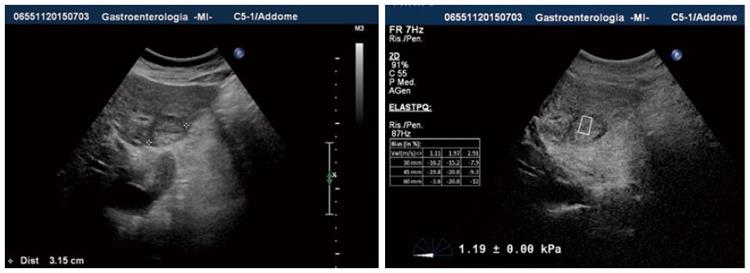
Shear wave elasticity imaging and supersonic elasticity imaging: These quantitative methods are based on measuring the shear wave propagation velocity in soft tissue[2,22,23]. A particular type of shear wave elasticity imaging (SWEI) used in clinical practice is the supersonic imaging (SSI), where the US probe generates a localized radiation force, which induces shear waves propagating from the focal point directly to the tissue of interest. This technique requires the very fast acquisition of US images, reducing the risk of artifacts created by the breath movements of the patient or by the investigator movements of the probe[5]. The SSI real-time technique creates a two-dimensional color map, where colors codes the speed of waves in meters per second or elasticity of the tissue in kilopascals. The limit of SWEI is the intensity used to avoid both mechanical and thermal bio-effects, causing some difficulty when analyzing deeper-located tissues. Nonetheless, with these methods liver stiffness samples can be acquired during a routine US examination of the liver and focused directly on a focal area (Figure 3).
Other elastographic methods: Other sonoelastographic methods include endoscopic and trans-rectal ultrasound elastography, which represent promising techniques for the study of lymph nodes, pancreatic cancer and inflammatory bowel diseases[2]. Two different methods deserve consideration: the Intravascular Ultrasound Elastography (IVUSE)[24], used for evaluating the stiffness of atheromatous plaques, and the new three-dimensional shear wave elastography (3D-SWE), which helps differentiate breast lesions when applied in combination with B-mode ultrasound[20,22].
US devices equipped with the sonoelastography option enable the more accurate imaging and evaluation of the nature of superficial focal lesions of many different organs. As an example, Endoscopic ultrasonography (EUS), which is the method of choice for the diagnosis of pancreatic lesions, has improved with EUS-elastography (EUS-EG), providing additional information on the pancreatic masses[25-27]. Also in breast lesions[3,4], thyroid nodules[6], lymph nodes[3,7,8] and prostatic nodules[9-11], elastography provides many informations. As regards the role of US elastographic techniques in FLL examination and characterization by stiffness quantification, there are to date only a few papers[28-46] that have described the application of such techniques, mostly using ARFI technology. However, the results show many interesting points and the methods can indeed have a relevant role in clinical practice.
With the aim to investigate the role of US elastography in focal liver lesions examination, an extensive bibliographical search was performed in PubMed via MeSH using the following keywords and free terms: elastography, sonoelastography, elastosonography, acoustic radiation force impulse imaging, focal liver lesions, hepatocellular carcinoma, diagnosis, follow-up. We identified all the pertinent articles published between 2000 and 2015. The reference lists from the selected studies were manually examined to identify further relevant reports. Non-English language papers were excluded. The level of evidence and strength of recommendations were graded according to the Oxford Centre of Evidence-Based Medicine system as of the March 2009 update (http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009).
The numbers of results (hits) returned for each parameter were as follows: (1) for sonoelastography AND focal liver lesion, 8; (2) for Transient Elastography AND focal liver lesion, 2; (3) for Acoustic radiation force impulse (ARFI) AND focal liver lesion, 4; (4) for Strain Elastography AND focal liver lesion, 3; (5) for Vibro-Acoustography AND focal liver lesion, 7; (6) for Wave Elasticity Imaging AND focal liver lesion, 3; (7) for elastographic techniques AND focal liver lesions, 40; (8) for elastographic techniques AND focal liver lesions AND diagnosis, 34; and (9) for elastographic techniques AND focal liver lesions AND follow-up 1.
After filtering for range of years, human studies and article type, a manual screening was carried out for full-text articles and documents specific to the scope of this systematic review, and any duplicate was removed. Among a total of 102 results, 22 pertinent articles with the strongest level of evidence were eventually identified. Their level of evidence and strength of recommendations were graded according to the Oxford Centre of Evidence-Based Medicine system as of the March 2009 update (http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009).
ARFI or SWEI and focal liver lesion: ARFI technology turned out as the most widely used method for the evaluation of liver masses. Park et al[28] examined a cohort of patients with at least a focal liver lesion well visualized on conventional US. Only one mass of interest for each patient was chosen, and was diagnosed against histological examination or clinical diagnostic criteria. Expressing as velocity, they checked the stiffness, by ARFI technique, of both the mass and the surrounding liver parenchyma (using 10 valid measurements for each stiffness). Overall, a total of 47 focal mass lesions were tested, including 83% of different malignant nodules - 24 HCC, 7 cholangiocellular carcinomas (CCC), and 8 liver metastases - and 17% of benign masses - 5 hemangiomas and 3 focal nodular hyperplasias (FNH). The mean velocity was 2.48 m/s ± SD in HCCs, 1.65 m/s in CCCs, 2.35 m/s in metastases, 1.83 m/s in hemangiomas, and 0.97 m/s in FNHs. Although considerable overlap was noted between FLL of different etiology, a significant difference in ARFI values was observed between malignant and benign masses (mean 2.31 ± SD m/s vs 1.51 SD m/s, P = 0.047), as well as between HCCs and benign masses (mean 2.48 ± SD m/s vs 1.51 ± SD m/s, P = 0.006). The areas under the receiver operating characteristics curves (AUROC) for discriminating malignant masses from benign ones and HCCs from benign lesions were 0.724 (95%CI: 0.566-0.883, P = 0.048), and 0.813 (95%CI: 0.649-0.976, P = 0.008), respectively. According to the above results, the Authors proposed a cut-off value for ARFI of 1.82 m/s to differentiate malignant from benign liver lesions, and also of 1.82 m/s for distinguishing HCCs from benign masses. Also Gallotti et al[29] studied the stiffness of the focal liver lesions, of different nature, and the liver surrounding parenchyma by ARFI technology. The main finding was a great variability in stiffness of hemangiomas, with a mean wave velocity value of 2.30 m/s and mean wave velocity value of the surrounding parenchyma of 1.45 m/s. Those authors justified this variability with the hypothesis that different amounts of fibrotic septa among the dilated vascular space of the lesions were present. Furthermore FNH resulted the stiffest lesion after metastases and CCC (mean wave velocity value of the lesion 2.75 m/s; mean wave velocity value of the surrounding parenchyma 1.57 m/s), while adenomas resulted as the softer lesions (mean wave velocity value of the lesion was 1.25 m/s; the mean wave velocity value of the surrounding parenchyma was 1.40 m/s). Gallotti et al[29] interpreted the data on the basis of the high and the low fibrotic content of FNH and adenomas, respectively. The HCCs resulted softer as compared to the surrounding cirrhotic liver (mean wave velocity value of the lesion 2.17 m/s; mean wave velocity value of the surrounding parenchyma 2.99 m/s).
A larger Asiatic study on 134 focal liver lesions by Guo et al[30], evaluated the difference of the mean velocity of the mass and the ratio of focal lesion to the surrounding liver parenchyma. A total of 55 (41%) lesions were malignant and 79 (59%) were benign, the shear wave velocity (SWV) of malignant and benign masses being 2.95 ± 1.00 m/s and 1.69 ± 0.89 m/s, respectively (P < 0.001) and the SWV ratio of each lesion to the surrounding parenchyma being 1.83 ± 1.32 and 1.26 ± 0.78 for malignant and benign ones, respectively (P < 0.001). In distinguishing malignant from benign lesions the area under the ROC curve was 0.824 for the SWV and 0.660 for the SWV ratio of focal lesion to the surrounding parenchyma. Thus, those authors proposed cut-off values of 2.13 m/s for the SWV value and 1.37 for the SWV ratio of focal lesion to the parenchyma in the differential diagnosis of malignant and benign masses. The estimation of sensitivity, specificity, LR+ and LR- resulted: 83.3%, 77.9%, 3.7 and 0.2 for SWV and 59.6%, 77.3%, 2.6 and 0.5 for the SWV ratio, respectively. The authors concluded that the elastographic method provides more information on FLLs and would help discriminate malignant vs benign masses, particularly with regard to patients unsuitable for contrast-enhanced imaging. However, the above data should be verified in prospective studies with larger patient samples in order to evaluate the diagnostic accuracy of this technique.
All the studies[28-30], show homogeneous findings about the observed metastatic lesions and cholangiocarcinomas, which resulted stiffer than the surrounding liver parenchyma: this outcome can be explained assuming the presence of a substantial amount of fibrous content in the lesions.
Moreover, Zhang et al[31] performed ARFI on 140 patients with 154 FLLs, of which 28 hemangiomas, 14 FNHs, 61 HCCs, 39 metastases and 12 CCCs, using as reference standards: histopathology, CT, MRI or contrast-enhanced Ultrasound (CEUS). The virtual touch tissue quantification (VTTQ) median values resulted: 1.30, 1.80, 2.52, 3.08 and 3.89 m/s for hemangiomas, FNH, HCC, metastasis and CCC, respectively, from the softest to stiffest one (P < 0.001). The AUROC (95%CI) of VTTQ values was 0.94 (0.90-0.98) for hemangiomas, with sensitivity, specificity and corresponding positive and negative likelihood ratios (i.e., LR+ and LR-) of 86.5%, 89.3%, 7.8 and 0.1 respectively; 0.91 (0.87-0.96) for malignant lesions, with sensitivity of 81.3% and specificity of 92.9%, LR+ of 11.1 and LR- of 0.2, respectively, and 0.87 (0.79-0.94) with sensitivity of 91.7%, specificity of 72.5% and LR+ 2.8 and LR- 0.1 for CCC. The same authors identified VTTQ cut-off values of 1.76 and 2.22, for the diagnosis of hemangiomas and all malignancies (except for CCC) respectively. Conversely, the cut-off value proposed for the diagnosis of CCC was 3.00 m/s. Ronot et al[32] used ARFI to carry out the evaluation of 105 lesions (60 FNH and 17 HCAs, 20 hemangiomas, 2 cholangiocarcinomas, one HCC and 5 focal fatty sparing areas) in 73 patients (61 women, 84%, mean age of 44.8, range 20-75). The characterization of the lesions relied on MRI and/or CEUS, or biopsy. The mean stiffness value was 33.3 ± 12.7 kPa, 19.7 ± 9.8 kPa, 17.1 ± 7 kPa, 34.1 ± 7.3 kPa, 19.6 kPa and 11.3 ± 4.3 kPa (P < 0.0001) for FNHs, HCAs, hemangiomas, CCCs, HCCs and focal fatty sparing areas, respectively. The most important finding was that there was no difference between the benign and the malignant groups (P = 0.64). Secondly, those authors focused their study on focal FNH and hepatocellular adenoma (HCA) values, as an accurate diagnosis was a key point for the differential management of patients, mostly conservative for FNHs and surgically aggressive for many HCAs. The best imaging techniques for the differentiation between FNHs and HCAs and for the subtyping of HCAs were: magnetic resonance imaging (MRI) and also CEUS. MR-hepatospecific contrast agents clarified the difficult cases. At elastographic examination FNHs were significantly stiffer than HCAs (P < 0.0001), the AUROC for differentiating FNHs from other lesions was 0.86 ± 0.04; there was also a difference among HCAs: telangiectatic/inflammatory ones, significantly stiffer, vs steatotic ones, less stiff (P = 0.014). The study concluded that SWE can provide additional information toward the characterization of FLLs, and helps in differentiating FNHs from HCAs and subtyping HCAs, although further studies are required.
Other elastographic methods in the study of focal liver lesion: Sandulescu et al[33] studied 39 liver tumors by real-time elastography (RTE). RTE was performed by EUS or trans-abdominal US. The ROC analysis of the mean hue obtained through histogram analysis of the region of interest (liver lesion) after averaging individual pixels over a 10-s elastography movie, was used to assess the color information inside the region of interest and to consequently differentiate benign vs malignant liver lesions. Using a cut-off value of 170 for the mean hue histogram values recorded on the region of interest, the sensitivity, specificity, LR+ and LR- and overall accuracy of the technique in the differentiation between benign and malignant masses were 92.5%, 88.8%, with LR+ of 8.3, and LR- of 0.08, and 88.6%, respectively. This led to conclude about the possible role of RTE as a promising technique to improve the characterization and differentiation of benign vs malignant focal liver lesions.
Transient elastography and focal liver lesion: In the study of FLL TE needs separate consideration. In fact, to date its application has been validated mostly in chronic hepatitis B and C settings and its technical features do not allow the elastographic measurement of stiffness on a single focal lesion. However it has to be mentioned that several studies focused on the role of TE in identifying patients with focal liver lesions, but always among cirrhotic populations, aiming at predicting with TE the development of HCC and/or prognosis and survival. These studies found a significant correlation between higher-baseline TE values and the risk of the development of HCC in chronic hepatitis B and C settings[36-46]. Accordingly, a recent meta-analysis[38] reported a significant association between baseline TE values and the risk of the development of HCC in 8 out of the 9 included studies (214 cases of HCC)[39-45]. Interestingly, a previous study reported the elevated IQR of TE measurements, unrelated to technical reasons, a good indicator of HCC presence[47].
Ultrasound elastography and thermal liver lesions: A different interesting field of application of US elastography - and evaluation of its reliability - is about delineating necrosis post radiofrequency ablation (RFA) or directly studying the changes of the lesion stiffness during the process of percutaneous ablation. In fact, the local recurrence rates of tumors are in part attributable to the inability of US to accurately visualize the zone of necrosis (thermal lesion). Only a few preliminary studies, most of which in vitro, have concentrated on this context. A German study[34] compared the size of liver lesions using RTE with CEUS in 21 malignant liver tumors, previously percutaneously ablated using RFA, in order to detect any thermo-ablation defects. During CEUS examination the operator took the measurements of the lesions (long axis, short axis, and area) and compared the measurements from the elastograms, concluding that elastography had slightly underestimated the lesion size, as judged by the CEUS images. Varghese et al[35] tried to work out the application of a method for in vivo elastographic visualization of the ablated regions in the liver during and after thermal therapy, as in their population there was a correspondence between elastographic image features and pathology. However, those authors[35] spotted a challenge in liver elastography due to the difficulty of providing controlled and reproducible compression. Further studies with large-size samples are required, but the elastographic technique surely represents a new important tool also in this field.
All the studies reported some technical limitations of elastography in examining focal liver lesions: the size of focal liver lesions, being in some cases smaller than the ROI and the location of the mass, could not be determined by the operator as it was sited deep in the liver. Another possible limitation would be the poor compliance of the patients in holding their breath as requested. In addition, the presence of a significant necrotic degeneration of the lesion can impair the assessment of its stiffness and such a limitation would be overcome, whenever possible, through the accurate choice of a region of interest (ROI) for the stiffness calculation out of the necrotic portion.
The data analyzed in the present review indicate that the elastographic US-based technique is a potentially promising accurate tool for differentiating benign from neoplastic lesions in patients without liver cirrhosis.
However, there is a great amount of heterogeneity shown in the studies published to date in terms of study design, study population, prevalence of FLL included, stiffness measurement methods. In addition, most of the studies analyzed have shown some methodological shortcomings: firstly, the lack of an adequate assessment of reproducibility (via inter- and intra-observer variability) and, secondly, the lack, within the single study, of an adequate diagnostic confirmation of the FLL nature against the same reference standard applied to all the patients, with the resulting constraint of methodological quality. In addition, all the studies have shown a case-series design that carried a low level of evidence, with reduced strength of their results.
Thus, there is no availability to date of an unbiased assessment of the actual impact of elastography when differentiating the nature of focal liver lesions. Nevertheless, most of the studies reviewed found a significant difference in stiffness values between malignant and benign lesions (Table 1). Also the diagnostic accuracy of elastography is fair[28,30] to good[31,33] in excluding as well as in confirming the malignancy of the lesion[31,33]. Thus, elastography seems to be a promising set of techniques for predicting the nature of liver lesions. Only one study[32] did not found any diagnostic power of the ARFI technique in predicting the malignant nature of the masses, but the study included only three malignant lesions. Consideration is given to the large overlap of values for the stiffness of the different masses, among the study subgroups (i.e., benign or malignant), showing the poor role of elastography in differentiating the masses. Some authors justify such a variability with the hypothesis of the presence of different amounts of fibrotic septa tissue between hemangiomas[29] or with the different telangiectatic/inflammatory vs steatoic nature of HCAs[32]. However, all the studies lack of an accurate comparison between elastographic values and the histology of the single nodule. The US elastographic techniques (Table 2) seem to hold a promising role in the discrimination between benign and malignant lesions, probably increasing the accuracy of routine US, but do not help to discriminate among the different lesions. There is a potential role too in improving the accuracy of the US guide during an invasive procedure. Toward such a goal the study of the thermal lesions represents a further relevant use of elastography, which needs further application and studies.
| Ref. | Method | Study design | Cut-off1 | Sens (%) | Spec (%) | LR (+ve) | LR (-ve) | Level of evidence2 |
| Park et al[28], 2013 | ARFI | Prospective, | 1.82 | 71.8 | 75 | 2.9 | 0.38 | 4 |
| (m/s) | monocentric | |||||||
| Sandulescu et al[33], 2012 | RTE | Retrospective, | 170 | 92.5 | 89 | 8.3 | 0.08 | 4 |
| Hue | monocentric | |||||||
| Guo et al[30], 2015 | ARFI-SWV | Prospective, | 2.13 | 83.3 | 78 | 3.7 | 0.20 | 4 |
| (m/s) | monocentric | (ratio 1.37) | (ratio 59.6) | (ratio 77.3) | (ratio 2.6) | (ratio 0.5) | ||
| Ronot et al[32], 2015 | ARFI | Prospective, | - | - | - | - | - | 4 |
| (m/s) | Monocentric | |||||||
| Zhang et al[31], 2013 | ARFI-VTTQ | Retrospective, | 2.22 | 81.3 | 93 | 11.1 | 0.20 | 4 |
| (m/s) | monocentric |
| Type of elastography | Elastographic method | Commercial implementation | Studies investigating the role of elastography in FLL |
| Strain | RTE | Hitachi 6500® | Sandulescu et al[33] 2012 |
| LOGIQ E9, GE® | Wiggermann et al[34] 2013 | ||
| Acuson 128XP® | Varghese et al[35] | ||
| Dynamic | TE | Echosens® | Masuzaki et al[36] 2009 |
| Jung et al[37] 2011 | |||
| Singh et al[38] 2013 | |||
| Akima et al[39] 2011 | |||
| Fernández-Montero et al[40] 2013 | |||
| Fung et al[41] 2011 | |||
| Chon et al[42] 2013 | |||
| Merchante et al[43] 2012 | |||
| Salmon et al[44] 2012 | |||
| Calvaruso et al[45] 2012 | |||
| Narita et al[46] 2012 | |||
| Feier et al[47] 2013 | |||
| ARFI and SWEI | Acuson S2000 Siemens® | Park et al[28] 2013 | |
| Aixplorer SuperSonic Imagine® | Gallotti et al[29] 2012 | ||
| Guo et al[30] 2015 | |||
| Zhang et al[31] 2013 | |||
| Ronot et al[32] 2015 | |||
| VA | None available |
Further larger prospective studies with a stronger level of evidence are required to assess the potential role of these techniques in discriminating the benign vs malignant nature of lesions and among different lesions. Importantly, studies are also needed among liver diseases patients, in particular, cirrhotic patients, in order to assess the diagnostic performance of these techniques in discriminating between malignant and benign nodules in this large setting.
P- Reviewer: Frider B, Sugimoto K S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | De Robertis R, D’Onofrio M, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Noninvasive diagnosis of cirrhosis: a review of different imaging modalities. World J Gastroenterol. 2014;20:7231-7241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 2. | Zaleska-Dorobisz U, Pawluś A, Szymańska K, Łasecki M, Ziajkiewicz M. Ultrasound Elastography--Review of Techniques and Its Clinical Applications in Pediatrics--Part 2. Adv Clin Exp Med. 2015;24:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341-350. [PubMed] |
| 4. | Zhi H, Ou B, Luo BM, Feng X, Wen YL, Yang HY. Comparison of ultrasound elastography, mammography, and sonography in the diagnosis of solid breast lesions. J Ultrasound Med. 2007;26:807-815. [PubMed] |
| 5. | Bhatia KS, Tong CS, Cho CC, Yuen EH, Lee YY, Ahuja AT. Shear wave elastography of thyroid nodules in routine clinical practice: preliminary observations and utility for detecting malignancy. Eur Radiol. 2012;22:2397-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Sporea I, Sirli R, Bota S, Vlad M, Popescu A, Zosin I. ARFI elastography for the evaluation of diffuse thyroid gland pathology: Preliminary results. World J Radiol. 2012;4:174-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952-957. [PubMed] |
| 8. | Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Hiraoka M, Insana MF, Brill AB, Saga T, Togashi K. Cervical lymph node metastases: diagnosis at sonoelastography--initial experience. Radiology. 2007;243:258-267. [PubMed] |
| 9. | Brock M, Eggert T, Löppenberg B, Braun K, Roghmann F, Palisaar RJ, Noldus J, von Bodman C. Value of real-time elastography to guide the systematic prostate biopsy in men with normal digital rectal exam. Aktuelle Urol. 2013;44:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Salomon G, Köllerman J, Thederan I, Chun FK, Budäus L, Schlomm T, Isbarn H, Heinzer H, Huland H, Graefen M. Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy. Eur Urol. 2008;54:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Tsutsumi M, Miyagawa T, Matsumura T, Kawazoe N, Ishikawa S, Shimokama T, Shiina T, Miyanaga N, Akaza H. The impact of real-time tissue elasticity imaging (elastography) on the detection of prostate cancer: clinicopathological analysis. Int J Clin Oncol. 2007;12:250-255. [PubMed] |
| 12. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 13. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 14. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 15. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] |
| 16. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 17. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1071] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 18. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 19. | Aguiló MA, Aquino W, Brigham JC, Fatemi M. An inverse problem approach for elasticity imaging through vibroacoustics. IEEE Trans Med Imaging. 2010;29:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 21. | Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 507] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 23. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 24. | Chen S, Sanchez W, Callstrom MR, Gorman B, Lewis JT, Sanderson SO, Greenleaf JF, Xie H, Shi Y, Pashley M. Assessment of liver viscoelasticity by using shear waves induced by ultrasound radiation force. Radiology. 2013;266:964-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Park MK, Jo J, Kwon H, Cho JH, Oh JY, Noh MH, Nam KJ. Usefulness of acoustic radiation force impulse elastography in the differential diagnosis of benign and malignant solid pancreatic lesions. Ultrasonography. 2014;33:26-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Dyrla P, Gil J, Florek M, Saracyn M, Grala B, Jędrzejewski E, Wojtuń S, Lubas A. Elastography in pancreatic solid tumours diagnoses. Prz Gastroenterol. 2015;10:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | D’Onofrio M, Crosara S, Canestrini S, Demozzi E, De Robertis R, Salvia R, Bassi C, Mucelli RP. Virtual analysis of pancreatic cystic lesion fluid content by ultrasound acoustic radiation force impulse quantification. J Ultrasound Med. 2013;32:647-651. [PubMed] |
| 28. | Park H, Park JY, Kim do Y, Ahn SH, Chon CY, Han KH, Kim SU. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J Gastroenterol. 2013;19:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Gallotti A, D’Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012;81:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Guo LH, Wang SJ, Xu HX, Sun LP, Zhang YF, Xu JM, Wu J, Fu HJ, Xu XH. Differentiation of benign and malignant focal liver lesions: value of virtual touch tissue quantification of acoustic radiation force impulse elastography. Med Oncol. 2015;32:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Zhang P, Zhou P, Tian SM, Qian Y, Deng J, Zhang L. Application of acoustic radiation force impulse imaging for the evaluation of focal liver lesion elasticity. Hepatobiliary Pancreat Dis Int. 2013;12:165-170. [PubMed] |
| 32. | Ronot M, Di Renzo S, Gregoli B, Duran R, Castera L, Van Beers BE, Vilgrain V. Characterization of fortuitously discovered focal liver lesions: additional information provided by shearwave elastography. Eur Radiol. 2015;25:346-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Sandulescu L, Padureanu V, Dumitrescu C, Braia N, Streba CT, Gheonea DI, Cazacu S, Ciurea T, Rogoveanu I, Saftoiu A. A pilot study of real time elastography in the differentiation of focal liver lesions. Curr Health Sci J. 2012;38:32-35. [PubMed] |
| 34. | Wiggermann P, Brünn K, Rennert J, Loss M, Wobser H, Schreyer AG, Stroszczynski C, Jung EM. Monitoring during hepatic radiofrequency ablation (RFA): comparison of real-time ultrasound elastography (RTE) and contrast-enhanced ultrasound (CEUS): first clinical results of 25 patients. Ultraschall Med. 2013;34:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Varghese T, Zagzebski JA, Lee FT. Elastographic imaging of thermal lesions in the liver in vivo following radiofrequency ablation: preliminary results. Ultrasound Med Biol. 2002;28:1467-1473. [PubMed] |
| 36. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 37. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [PubMed] |
| 38. | Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573-84.e1-2; quiz e88-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res. 2011;41:965-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Fernández-Montero JV, Barreiro P, Vispo E, Labarga P, Sánchez-Parra C, Soriano V. Liver stiffness predicts liver-related complications and mortality in HIV patients with chronic hepatitis C on antiretroviral therapy. AIDS. 2013;27:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Fung J, Lai CL, Seto WK, Wong DK, Yuen MF. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat. 2011;18:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Chon YE, Jung ES, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Jung KS, Kim SU. The accuracy of noninvasive methods in predicting the development of hepatocellular carcinoma and hepatic decompensation in patients with chronic hepatitis B. J Clin Gastroenterol. 2012;46:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Merchante N, Rivero-Juárez A, Téllez F, Merino D, José Ríos-Villegas M, Márquez-Solero M, Omar M, Macías J, Camacho A, Pérez-Pérez M. Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology. 2012;56:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Salmon D, Bani-Sadr F, Loko MA, Stitou H, Gervais A, Durant J, Rosenthal E, Quertainmont Y, Barange K, Vittecoq D. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: results from ANRS CO13 HEPAVIH. J Hepatol. 2012;56:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Calvaruso V, Di Marco V, Bavetta MG, Cabibi D, Conte E, Bronte F, Simone F, Burroughs AK, Craxì A. Quantification of fibrosis by collagen proportionate area predicts hepatic decompensation in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2015;41:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Narita Y, Genda T, Tsuzura H. Liver stiffness as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients receiving interferon-based anti-viral therapy. Hepatology. 2012;56:451A. |
| 47. | Feier D, Lupsor Platon M, Stefanescu H, Badea R. Transient elastography for the detection of hepatocellular carcinoma in viral C liver cirrhosis. Is there something else than increased liver stiffness? J Gastrointestin Liver Dis. 2013;22:283-289. [PubMed] |