Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1891
Peer-review started: May 8, 2015
First decision: June 19, 2015
Revised: July 14, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: February 7, 2016
Processing time: 264 Days and 24 Hours
AIM: To assess the utility and safety of single-operator cholangiopancreatoscopy (SOCPS) using the SpyGlass system in widespread clinical application for biliary and pancreatic diseases.
METHODS: This study was a prospective case series conducted in 20 referral centers in Japan. There were 148 patients who underwent SOCPS; 124 for biliary diseases and 24 for pancreatic diseases. The attempted interventions were SOCPS examination, SOCPS-directed tissue sampling, and therapy for stone removal, among others. The main outcomes were related to the procedure success rate in terms of visualizing the target lesions, SOCPS-directed adequate tissue sampling, and complete stone removal.
RESULTS: A total of 148 patients were enrolled for the diagnosis of indeterminate biliary and pancreatic lesions or treatment of biliary and pancreatic disease. The overall procedure success rate of visualizing the target lesions was 91.2% (135/148). The overall procedural success rates of visualizing the target lesions of diagnostic SOCPS in the bile duct and pancreatic duct were 95.5% (84/89) and 88.2% (15/17), respectively. Diagnosis: the overall adequate tissue for histologic examination was secured in 81.4% of the 86 patients who underwent biopsy under SOCPS (bile duct, 60/75, 80.0%; pancreatic duct, 10/11, 90.9%). The accuracy of histologic diagnosis using SOCPS-directed biopsies in indeterminate bile duct lesions was 70.7% (53/75). In the pancreatic duct, the accuracy of SOCPS visual impression of intraductal papillary mucinous neoplasm was 87.5% (14/16). Stone therapy: complete biliary and pancreatic stone clearance combined with SOCPS-directed stone therapy using electrohydraulic lithotripsy or laser lithotripsy was achieved in 74.2% (23/31) and 42.9% (3/7) of the patients, respectively. Others: SOCPS using the SpyGlass system was used in cannulation of the cystic duct in two patients and for passing across the obstructed self-expandable metallic stent for a malignant biliary stricture in two patients. All procedures were successful in both SOCPS-guided therapies. The incidence of procedure-related adverse events was 5.4% (8/148).
CONCLUSION: SOCPS with direct visualization and biopsy for diagnosis and SOCPS-directed therapy for biliary and pancreatic diseases can be safely performed with a high success rate.
Core tip: This investigation was a prospective, multicenter study in Japan involving 148 enrolled patients in whom the utility and safety of single-operator cholangiopancreatoscopy (SOCPS) with the SpyGlass system in pancreatobiliary disorders were analyzed. SOCPS with direct visualization and biopsy for diagnosis, and SOCPS-directed therapy for biliary and pancreatic diseases can be safely performed with a high success rate.
- Citation: Kurihara T, Yasuda I, Isayama H, Tsuyuguchi T, Yamaguchi T, Kawabe K, Okabe Y, Hanada K, Hayashi T, Ohtsuka T, Oana S, Kawakami H, Igarashi Y, Matsumoto K, Tamada K, Ryozawa S, Kawashima H, Okamoto Y, Maetani I, Inoue H, Itoi T. Diagnostic and therapeutic single-operator cholangiopancreatoscopy in biliopancreatic diseases: Prospective multicenter study in Japan. World J Gastroenterol 2016; 22(5): 1891-1901
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1891.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1891
The usefulness of peroral cholangiopancreatoscopy (POCPS) in the direct endoscopic diagnosis and treatment of biliary and pancreatic diseases has been reported[1-6]. Direct visualization of the bile and pancreatic ducts by POCPS is valuable in the diagnosis of biliary and pancreatic abnormalities that may not be detectable by cholangiography. Distinct advantages of POCPS are that it enables more precisely targeted biopsy in direct visualization and difficult stone therapy by electrohydraulic lithotripsy (EHL) or laser lithotripsy. However, POCPS has several technical limitations involving the POCP scope. For example, the restricted cholangioscope tip deflection hampers observation of the entire biliary and pancreatic ducts. Suboptimal irrigation capabilities have also rendered POCPS examination and therapy more difficult. Moreover, the POCPS system is fragile, and its repair cost is high. In addition, POCPS requires two endoscopists, one to operate the duodenoscope, the other to operate the cholangioscope. These disadvantages prevent POCPS from being widely used for the diagnosis and treatment of pancreatobiliary diseases.
The SpyGlass Direct Visualization system (Microvasive Endoscopy; Boston Scientific Corp., Natick, MA, United States) was, therefore, developed to overcome these disadvantages. The SpyGlass system is a single-operator POCPS technique. This system consists of a reusable optical probe and a disposable access catheter. In addition, the SpyGlass system has distinct characteristics, such as two separate, dedicated irrigation channels and four-way tip deflection for complete observation. To date, there have been numerous studies on the usefulness of the SpyGlass system[7-20]. However, there is presently only one multicenter, prospective study involving a large case series[9].
The aim of this study was to prospectively assess the efficacy of single-operator cholangiopancreatoscopy (SOCPS) using the SpyGlass system in a widespread clinical application for biliopancreatic diseases.
This prospective case series was conducted at 20 referral centers in Japan. Patients with biliopancreatic diseases who underwent SOCPS using the SpyGlass system were prospectively evaluated between October 2011 and September 2012. A total of 154 patients were prospectively enrolled and retrospectively analyzed. Eligibility for study entry was based on an age of 20 years or older and the existence of an indication for cholangiopancreatography. In the diagnostic procedure, the main patient inclusion criteria were indeterminate biliary and pancreatic duct stricture and filling defect. In the therapeutic procedure, the main inclusion criteria were biliary and pancreatic stones that were treatable by conventional lithotripsy. The exclusion criteria included age younger than 20 years, pregnancy, bleeding diatheses, distorted anatomy, severe jaundice or cholangitis, critically ill patients, and inability to provide informed consent.
The protocol was approved by the institutional review boards or ethics committees of each participating institution. The study was performed at 20 Japanese referral centers according to the guidelines described in the Declaration of Helsinki for biomedical research involving human subjects. The protocol appears on UMIN CTR (UMIN000015155).
The SpyGlass Direct Visualization system consists of capital equipment, including an irrigation pump, light source, monitor, access and delivery catheter (SpyScope), and an optical probe (SpyGlass). The part used as the observation scope is composed of two modularized components. One component is a disposable SpyScope 10 Fr access and delivery catheter with a 1.2-mm-diameter working channel and dedicated irrigation channels. This catheter is introduced through a therapeutic duodenoscope with a working channel diameter of 4.2 mm. The catheter is capable of tip deflection of at least 30 degrees in four directions, thus facilitating visualization of the entire circumference of the biliary tree. The other component is a reusable SpyGlass Fiber Optic Probe that provides 6000 pixel images and a 70-degree field of view. This probe is available as a multiple-use device that may be reprocessed after each use. The SpyBite biopsy forceps is a single-use device with jaws at the tip that open to 4.1 mm, obtaining adequate tissue for histology.
This study was designed to document the performance of the SOCPS system in the setting of routine clinical practice. The procedure was performed with patients under sedation using intravenous midazolam or flunitrazepam, with continuous monitoring of the pulse rate, oxygen saturation, and blood pressure. The procedure using the SpyGlass system was performed by a single operator. The decision whether to attempt SOCPS during the initial endoscopic retrograde cholangiopancreatography (ERCP) procedure was at the discretion of the endoscopist. The endoscopist could elect to defer performing SOCPS, for instance, to limit the length of the session. Endoscopic sphincterotomy (EST) or endoscopic papillary balloon dilation (EPBD) was performed before SOCPS without a dilated papillary orifice for mucin, which is produced from intraductal papillary mucinous neoplasms (IPMNs). For the SOCPS, the SpyScope was advanced through the 4.2-mm working channel of the therapeutic duodenoscopes (TJF-240 or TJF-260V; Olympus Medical Systems, Tokyo, Japan) into the bile duct or pancreatic duct. The SpyScope was inserted through the papilla under direct endoscopic and fluoroscopic imaging and advanced toward the target lesion with or without the use of a guidewire. Before observation using the SpyGlass, the contrast agent and bile were aspirated, then the bile duct was irrigated through the dedicated irrigation channel with sterile saline.
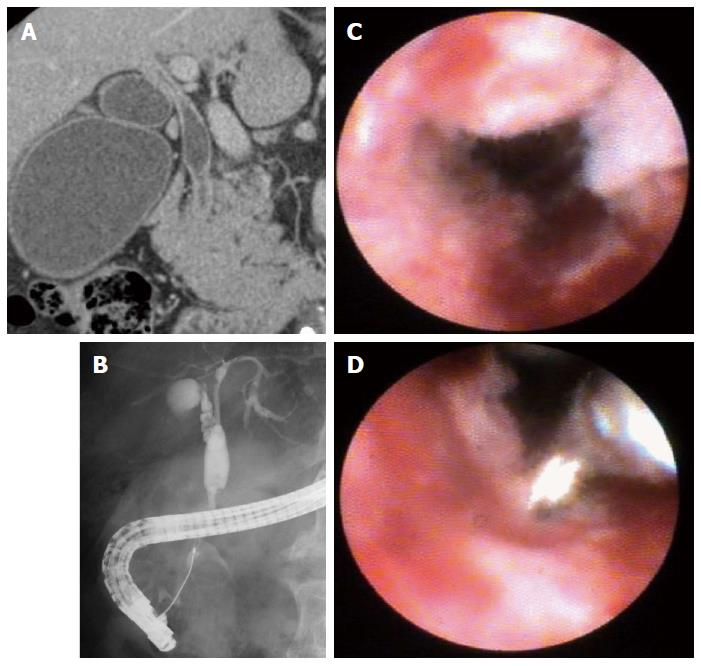
In the diagnostic procedure, we initially examined the image of the biliary or pancreatic duct stricture and filling defect using the SpyGlass system. During the procedure, the quality of target lesion visualization using SOCPS was rated as able or unable to visualize. SOCPS findings were classified as benign or malignant impression. The definition of SOCPS findings was evaluated according to a previous report[8]. In the bile duct, a lesion was defined as malignant on the basis of the presence or absence of a mass, an irregular granular stricture with a thin to thick tortuous vessel, or papillary or villous projections. Benign lesions were defined by a smooth surface without neovascularization or scar. If the target lesion could be observed by SOCPS, biopsies from the target lesion were attempted under cholangioscopic or pancreatoscopic guidance using the SpyBite (Figure 1). Adequate tissue was defined as sufficient quantity of tissue enabling a pathologist to make a histologic diagnosis. If necessary, fluoroscopy-guided transpapillary biopsy using conventional biopsy forceps was also performed. In biopsy patients without tissue evidence of malignancy, follow-up was scheduled every 2 mo using imaging examinations until 6 mo.
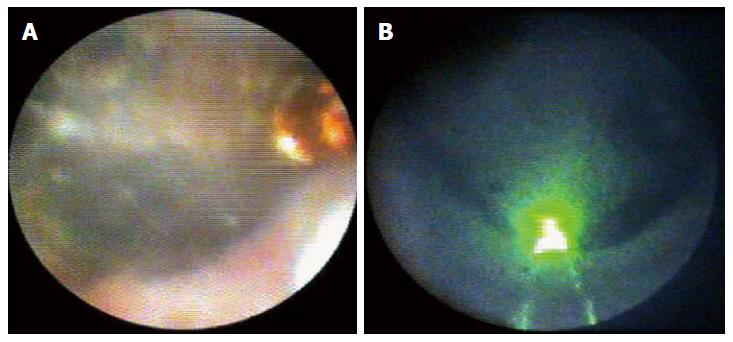
In the stone therapy, after EST or EPBD, a conventional stone extraction procedure was initially attempted using a basket, a balloon, and a mechanical lithotriptor. In some cases, sphincteroplasty via a small sphincterotomy and using a 12-mm to 18-mm controlled radial expansion balloon (Boston Scientific Corp.) was attempted. SOCPS-guided lithotripsy was then considered when these stone removal techniques failed. We used the YAG-laser or EHL technology for SOCPS-guided stone therapy. We confirmed under SOCPS view whether the stone and YAG-laser or EHL probe came in contact with each other (Figure 2). YAG-laser and EHL bursts were delivered under continuous saline irrigation via the SpyScope’s irrigation channels for the bile duct stone. On the other hand, intermittent irrigation at the minimal setting was delivered for the pancreatic stone. Stone fragmentation was confirmed on SOCPS and cholangiography. When sufficient stone fragmentation was achieved, a stone fragment was extracted using a conventional basket and balloon catheter. Complete stone clearance was determined by SOCPS and cholangiography.
The main outcomes were related to the procedure success rate in terms of the following: (1) visualization of the target lesions; (2) SOCPS-directed adequate tissue sampling; (3) complete stone removal; and (4) others. The secondary outcomes were the sensitivity and specificity of SOCPS impression in accordance with the histologic diagnosis of SOCPS-directed biopsies, and the occurrence of adverse events. Adverse events were assessed according to established criteria[21].
Summary statistics consisted of the mean, standard deviation, and median. The diagnostic accuracy, sensitivity, and specificity of the SOCPS examination, and the histologic diagnosis of SOCPS-directed biopsy were determined together with those in the final diagnosis. The final diagnoses were made on the basis of the surgical specimen and clinical follow-up for more than 6 mo. The success rate of complete stone removal in the therapeutic procedure was calculated.
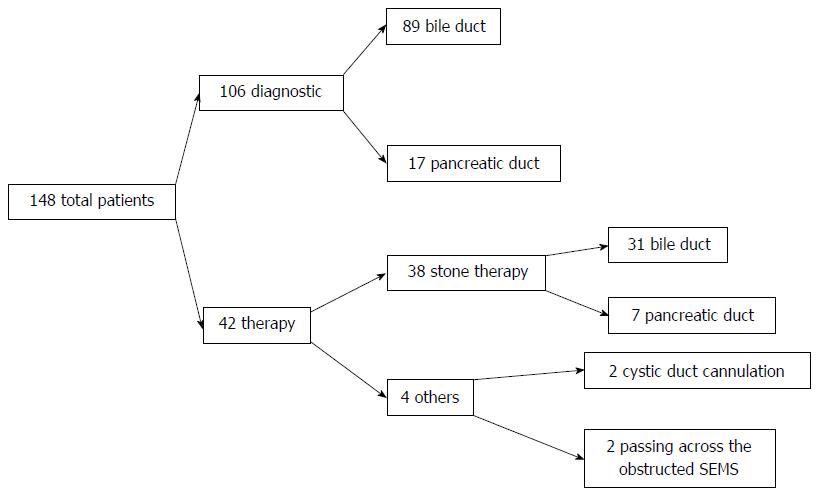
A total of 154 patients were enrolled during the study period, with 148 completing the study. The evaluation of six patients was discontinued prematurely because of loss to follow-up. Among the 148 patients, 106 patients were classified for diagnostic purposes and 42 for therapeutic purposes. The detailed breakdown of the particular conditions and numbers of patients was as follows: indeterminate bile duct lesion (n = 89), indeterminate pancreatic lesions (n = 17), stone therapy by YAG-laser or EHL (n = 38), assistance of cystic duct cannulation (n = 2), and passing across the obstructed self-expandable metal stent (SEMS) (n = 2) (Figure 3). The characteristics of all the patients in this study are shown in Table 1. The overall success rate of visualizing the target lesions was 91.2% (135/148).
| Characteristic | Value |
| Male/female, n | 98/50 |
| Mean age (yr), (range) | 68.4 (38-93) |
| Previous examination, n (%) | |
| CT | 146 (98.6) |
| MRCP | 96 (64.9) |
| ERCP | 148 (100) |
| Preparation of the papilla, n (%) | |
| EST | 130 (87.8) |
| EPBD | 3 (2.1) |
A total of 106 patients underwent diagnostic SOCPS. These patients were grouped into 89 patients with bile duct lesions and 17 with pancreatic lesions.
Bile duct access: Of the 89 patients with bile duct abnormalities, the success rate of visualizing the target lesions was 95.5% (84/89). The hilar and superior common bile ducts were the most frequent target sites.
In the 89 patients with indeterminate bile duct lesions, the final diagnoses are shown in Table 2. Among the 89 patients suspected with bile duct cancer, 56 cases (62.9%) showed an irregular granular mucosal surface with thin to thick tortuous vessels in the bile duct lumen. The sensitivity and specificity of SOCPS impression were 94.7% and 92.6%, respectively, with an accuracy of 94.0% (Table 3). The SOCPS-guided targeted biopsy was attempted in 75 patients with indeterminate bile duct lesions. Adequate tissue for histologic examination was secured in 80.0% (60/75) of these patients (Table 4). A final diagnosis of a malignant or benign tumor was established on the basis of surgical findings in 27 patients and of pathologic findings and clinical follow-up for more than 6 mo in 48 patients. The accuracy of histologic diagnosis using SOCPS-directed biopsies in indeterminate bile duct lesions was 70.7% (53/75) (Table 5).
| Bile duct target site | n |
| Intrahepatic | 10 |
| Hilar | 26 |
| Superior | 19 |
| Mid | 17 |
| Inferior | 15 |
| Mobile filling defect | 2 |
| Suspected disease | |
| Bile duct cancer | 58 |
| Cystic duct cancer | 2 |
| IPNB | 2 |
| IgG4-related cholangitis | 3 |
| PSC | 3 |
| Inflammatory change | 19 |
| Bile duct stone | 2 |
| Diagnosis by SOCPS impression | Final diagnosis | |
| Malignant | Benign | |
| Malignant | 54 | 2 |
| Benign | 3 | 25 |
| Bile duct targetsite | n | Success rate of visualizing target site, n (%) | Biopsy attempted(n) | Adequate tissue forhistologic examination, n (%) |
| Intrahepatic | 10 | 8 (80.0) | 6 | 5 (83.3) |
| Hilar | 26 | 26 (100) | 26 | 21 (80.8) |
| Superior | 19 | 19 (100) | 19 | 15 (78.9) |
| Mid | 17 | 16 (94.1) | 14 | 12 (85.7) |
| Inferior | 15 | 13 (86.7) | 10 | 7 (70.0) |
| Mobile filing defect | 2 | 2 (100) | 0 | |
| Overall | 89 | 84 (95.5) | 75 | 60 (80.0) |
| Diagnosis by SOCPS-biopsy | Final diagnosis (n) | |
| Malignant | Benign | |
| Malignant | 37 | 0 |
| Benign | 20 | 16 |
| Inadequate | 0 | 2 |
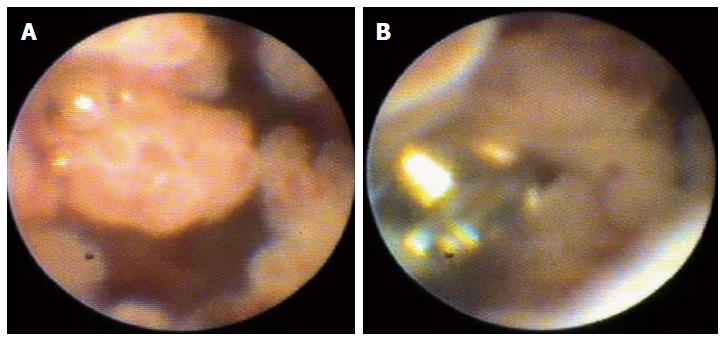
Pancreatic duct access: Pancreatoscopy using SOCPS for diagnostic purposes was attempted in 17 patients. Fifteen patients underwent SOCPS for further evaluation of the dilated main pancreatic duct and a suspected IPMN, and the remaining two patients for indeterminate main pancreatic duct stricture. Among these patients, IPMN was finally diagnosed in 16 cases and pancreatic carcinoma in one case. Before SOCPS, endoscopic imaging showed an enlarged orifice of the papilla in 15 patients, and a dilated main pancreatic duct (> 5 mm) in the same 15 patients. Therefore, the number of patients who required EST or EPBD before SOCPS was only two patients. The most frequent target site was the head of the pancreas in 14 patients. Pancreatic lesions were located in the main pancreatic duct in all of the 17 patients (Table 6). The procedural success rate of visualizing target lesions was 88.2% (15/17) (Table 7). Pancreatoscopy using SOCPS failed in two patients because of the inability to advance the SpyScope to the target site. In patients with IPMN in the main pancreatic duct, the lesion could be seen as a papillary stricture or fish-egg-like lesion in 87.5% (14/16) of the patients (Figure 4). The SOCPS-guided targeted biopsy was attempted in 11 patients, and adequate tissue sampling for histologic examination was secured in 90.9% (10/11). Of the 15 patients in whom visualization of the target lesion was successfully achieved, SOCPS-directed biopsy was not attempted in four patients because SOCPS direct visualization enabled an obvious diagnosis of IPMN.
| Location of main lesion of pancreatic duct | n |
| Head of pancreas | 14 |
| Body | 2 |
| Tail | 1 |
| Suspected disease | |
| Pancreatic cancer | 1 |
| IPMN | 16 |
| Preparation of the papilla | |
| EST | 1 |
| EPBD | 1 |
| Pancreatic duct target site | n | Successrate of visualizing target site | Biopsy performed,n | Adequatetissue for histologic examination |
| Head | 15 | 14 (93.3) | 10 | 9 (90.0) |
| Body | 1 | 0 (0) | 0 | 0 (0.0) |
| Tail | 1 | 1 (100.0) | 1 | 1 (100) |
| Overall | 17 | 15 (88.2) | 11 | 10 (90.9) |
SOCPS-guided stone therapy: SOCPS-guided stone therapy using EHL or laser lithotripsy was attempted in 38 patients. Bile duct stone and pancreatic duct stone therapies were carried out in 31 and seven patients, respectively. Stone extraction previously failed in all patients using conventional stone therapies such as EST, EPBD, use of a basket catheter, mechanical lithotripsy, and occasionally large balloon sphincteroplasty. The mean sizes of the bile duct stone and pancreatic duct stone were 20.6 mm and 13.7 mm, respectively. EST or EPBD had been performed before SOCPS in 97.4% (37/38) or 2.6% (1/38) of the patients, respectively (Table 8).
| Characteristic | Value |
| Male/female, n | 23/15 |
| Mean age (yr), (range) | 65.8 (38-93) |
| Bile duct stones, n | 31 |
| Mean size of stone (mm), (range) | 20.6 (5-48) |
| Cause of difficulty of conventional treatment, n | |
| Confluence stone | 14 |
| Large impact stone > 20 mm | 14 |
| Intrahepatic stone | 3 |
| Pancreatic stone, n | 7 |
| Mean size of stone (mm), (range) | 13.9 (7-30) |
| Cause of difficulty of conventional treatment, n | |
| Impact stone | 6 |
| Large stone > 20 mm | 1 |
| Pretreatment of the papilla, n | |
| EST | 37 |
| EPBD | 1 |
| Devices used during stone therapy | |
| EHL | 15 |
| YAG laser | 20 |
The stone locations were the common bile duct in 28 patients and the intrahepatic bile duct in three patients. The bile duct stones that were difficult to retrieve were: a large common bile duct stone that was impacted (n = 14 patients), a confluence stone (n = 14), and an intrahepatic impacted stone (n = 3). The procedure success rate of visualizing a target bile duct stone was 96.8% (30/31). The success rate of complete stone removal was 74.2% (23/31). Of the 23 patients with successful stone removal, complete stone clearance was achieved in one session in three patients and in two sessions in 18 patients. Of the remaining two patients, one needed two sessions and the other needed eight sessions. Of the 20 patients who required more than one SOCPS session, ten had an impacted stone, six had a hard and/or multiple large stones, and four had a confluence stone. After SOCPS-guided stone therapy, five patients had additional mechanical lithotripsy to further aid in the fragmentation and extraction of the remaining stones. SOCPS-guided stone therapy in the bile duct failed in eight patients. One patient had an intrahepatic stone that could not be visualized, and four had a confluence stone. In the remaining patient, stone therapy could not be carried out successfully because of the inability to sufficiently target the stone using EHL and a laser probe. In the failed bile duct stone therapy using SOCPS, 5/8 patients were treated by permanent stenting with a regular stent exchange and the remaining three patients were treated by surgery.
SOCPS-guided pancreatic duct stone therapy was attempted in seven patients; the procedure success rate of visualizing the target stone was 71.4% (5/7). In the two patients for whom visualizing the target stone failed, the SpyScope could not be advanced to the area of interest because the downstream pancreatic duct was tightly bent and had a small duct diameter. SOCPS-guided stone therapy for complete stone clearance was successfully achieved in 42.9% (3/7) of the patients. Despite the successful visualization of the target stone, complete stone clearance failed in two patients, because of a huge stone (> 30 mm) in one patient, and a hard impacted stone in the other. However, permanent stent insertion with a regular stent exchange was achieved in these two patients as aided by pancreatoscopy-directed stone fragmentation.
Others: SOCPS with the SpyGlass system used in cannulation of the cystic duct was attempted in two patients. Cystic duct cannulation was intended to enable gallbladder cytology for the diagnosis of a suspected gallbladder carcinoma. SOCPS-guided cystic duct cannulation was performed because conventional cystic duct cannulation failed. SOCPS enabled direct visualization of a branch of the cystic duct and a guidewire was inserted into the cystic duct from the SpyScope working channel. The guidewire was then advanced to the gallbladder under fluoroscopic guidance. Finally, a 5 Fr endoscopic naso-gallbladder drainage tube of the appropriate length was retrogradely inserted into the gallbladder.
Passing across the obstructed SEMS for a malignant biliary stricture under SOCPS guidance was performed in two patients who had unresectable hilar bile duct carcinomas. They underwent bilateral biliary drainage using uncovered SEMS deployment, which formed a partial stent-in-stent. In these patients, stent occlusion due to tumor ingrowth resulted in obstructive jaundice and acute cholangitis. Necessitating reintervention for cholangitis, bilateral inner plastic stent placement was attempted. However, a guidewire could not be passed through the mesh space of the uncovered SEMS, thus SOCPS-guided reintervention was considered. In these two patients, a guidewire was successfully passed across the hilar bile duct stricture under direct visualization of SOCPS, which revealed the mesh space of the SEMS. Finally, inner plastic stent placement was achieved in both patients.
The incidence of procedure-related adverse events among the patients was 5.4% (8/148). All adverse events occurred in the bile duct access. Cholangitis developed in four of these patients, mild pancreatitis in two patients, post-EST bleeding in one patient, and aspiration pneumonia in one patient. All adverse events resolved without sequelae.
Pancreatocholangioscopy has some advantages for pancreatobiliary disorders, as reported previously[1-6]. However, pancreatocholangioscopy has not been widely performed for clinical use because of its technical and practical limitations. SOCPS using the SpyGlass system was developed to overcome those limitations. In this prospective multicenter case series conducted in Japan, SOCPS using the SpyGlass system with direct visualization and biopsy for the diagnosis and therapy of biliary and pancreatic diseases was safely performed with a high success rate. One of the advantages of cholangioscopy is its ability to precisely identify the location of the lesion for subsequent biopsy sampling under direct visualization. In the present study, adequate tissue sampling and accurate SOCPS-directed biopsy sampling was achieved in 80.0% and 70.7%, respectively, of the patients in whom previous ERC impression and ERC-guided biopsies and cytology had yielded indeterminate diagnoses.
Tissue sampling of malignant and benign bile duct lesions remains a challenging procedure. Generally, the accuracy of standard biopsy under fluoroscopic guidance was previously reported to be approximately 50% of all the patients[8-10]. The diagnostic accuracy of cytology is as low as 20% to 30%. On the other hand, SOCPS-guided direct tissue sampling was reported to have a high success rate and a high accuracy[8-10]. In view of these results, SOCPS-guided direct tissue sampling was considered technically feasible and clinically useful. Generally, the intrahepatic and inferior bile ducts were considered difficult sites to visualize by cholangiography. In this study, the success rates of visualizing the target lesion in the intrahepatic bile duct and inferior bile duct were 83.3% and 70.0%, respectively. The SpysScope features of four-way tip deflection and smaller diameter (10 Fr) provide the possibility to achieve a high success rate of visualizing any site. These potential abilities have not been particularly reported to date.
The present study reveals the potential value of SOCPS for biliary disorders. However, one big issue is that the quality of visualization by the SpyGlass is less than the quality of visualization by videocholangiography. Particularly in the bile duct, the presence of many fragments and concentrated bile juice prevent good visualization because the focus distance of the SpyGlass is short and the volume of light and resolution are limited. Therefore, sufficient irrigation by the dedicated irrigation channel and absorption of the bile using a 1.2-mm-diameter working channel are needed to achieve optimum SpyGlass visualization.
Despite the technical difficultly in observing the pancreatic duct by pancreatoscopy (because of the narrow and curved duct), the success rate of visualizing the target lesion in this area was 88.2% among the patients in the present study, which was equivalent to the success rate of visualization of the target lesion in the bile duct. However, there are some limitations in the present study, such as the small number of patents (n = 17) examined. Moreover, in the case of IPMN, the occasional presence of a large amount of mucin in the pancreatic duct prevents good visualization. In such cases, sufficient irrigation and mucin absorption are needed. In IPMN cases, a papillary stricture or fish-egg-like lesion in the main pancreatic duct could be seen with a high success rate of 93% (14/15) among the patients in this study (Figure 3). Taken together, the present study provides evidence of the feasibility and usefulness of SOCPS using the SpyGlass system for the diagnosis of IPMN in the main pancreatic duct.
Stone clearance in the bile duct can be achieved using a conventional endoscopic procedure in up to 90% of patients with stone disease[22-24]. To improve the outcome and ease of lithotripsy by ERCP, mechanical lithotripsy and endoscopic papillary large-balloon dilation after sphincterotomy were developed[25,26]. Bile duct stones that are huge, impacted, and located behind a stricture or the intrahepatic bile duct and confluence stones are difficult to extract conventionally. To achieve further improvement of the success rate of stone clearance, several therapies for difficult bile duct stones have been introduced such as extracorporeal shockwave lithotripsy (ESWL) and EHL or laser lithotripsy under direct visual control with a cholangioscope. The success rate of complete stone removal using ESWL has been reported to be about 80% with complications of up to 35%[27]. Patients also need to undergo endoscopic treatment repeatedly. Therefore, ESWL has not been authorized as a first-line treatment for difficult bile duct stones. EHL and laser lithotripsy via a peroral cholangioscope for difficult bile duct stones have been reported, and their success rate of complete stone removal ranges from 88% to 90%[28-30]. In the present series, the success rate of complete stone removal under SOCPS with EHL and laser lithotripsy was 74.2% (23/31). This success rate is lower than that previously reported mainly because of the substantial proportion of confluence stones (14/31). In some cases, it is beyond the capability of cholangioscopy-directed stone therapy to extract confluence stones. However, the confluence stones in 71.4% (10/14) of the patients were successfully and completely extracted in this series. This result suggests that SOCPS-guided lithotripsy with EHL or YAG-laser is an alternative for difficult-to-extract confluence stones. In the present study, complete stone clearance in the pancreatic duct was successfully achieved in 42.9% (3/7) of the patients. This success rate is lower than that previously reported[20]. After SOCPS-guided lithotripsy fragmentation, there is limited success using mechanical lithotripsy and a conventional basket catheter for clearance of large and hard pancreatic stones because stone capture and extraction are difficult and the risk of adverse events increases. In the present series, despite the successful visualization of the target stone, complete stone clearance failed in two patients. However, permanent stenting with a regular stent exchange was achieved in these patients as facilitated by pancreatoscopy-directed stone fragmentation.
In the present series, SOCPS using the SpyGlass system had wide clinical applications, such as in the cannulation of the cystic duct and for passing across an obstructed SEMS. In patients with an unresectable malignant hilar biliary stricture, SEMS is expected to show long-term stent patency. However, complications of SEMS placement in the hilar bile duct, such as stent occlusion, are still encountered at an unacceptably high frequency. Reintervention is thus required in cases of stent dysfunction. If a guidewire cannot be passed through a mesh space of an uncovered SEMS by conventional ERCP, SOCPS-directed visualization can be used to reveal the mesh space of the SEMS and a guidewire can subsequently be passed into the SEMS mesh via the working channel. We believe that the SOCPS-directed procedure is very useful for enabling passage through the narrow stricture and hole.
In conclusion, SOCPS-guided SpyBite biopsies have a high accuracy in patients with indeterminate biliary and pancreatic lesions, as well as a high success rate for the complete clearance of bile duct stones. Our study further demonstrates the efficacy, safety, and possible wide clinical application of the SOCPS-guided procedure for the diagnosis and therapy of pancreatobiliary disorders. However, there are also several limitations in this study. Although this was a prospective study, randomization was not carried out and there was a lack of controls. All participating institutions were also tertiary-care and high-volume referral centers.
The authors are indebted to Dr. Edward Barroga, Associate Professor and Senior Medical Editor of the Department of International Medical Communications of Tokyo Medical University for reviewing and editing the manuscript.
The authors also thank Hirofumi Kogure, Yousuke Nakai, and Tsuyoshi Hamada of the Department of Gastroenterology, Graduate School of Medicine, The University of Tokyo, and Atsushi Sofuni, Fumihide Itokawa, Kentaro Ishii, Syujiro Tsuji, Takayoshi Tsuchiya, Junko Umeda, Reina Tanaka, Nobuhito Ikeuchi, Ryosuke Tonozuka, Syuntaro Mukai, Mitsuyoshi Honjo, and Mitsuru Fujita of the Department of Gastroenterology and Hepatology, Tokyo Medical University.
Direct visualization of the bile and pancreatic ducts is valuable in the diagnosis and therapy of biliary and pancreatic abnormalities. However, operator pancreatocholangioscopy (POCPS) has several technical limitations involving the POCP scope. The SpyGlass Direct Visualization system was therefore developed to overcome these technical limitations. This study was conducted to assess the utility and safety of single-operator cholangiopancreatoscopy (SOCPS) using the SpyGlass system for biliary and pancreatic diseases.
Peroral cholangiopancreatoscopy became familiar to endoscopists with the invention of the SpyGlass system. This study suggests the efficacy, safety, and possible wide clinical application of the SpyGlass system for the diagnosis and therapy of pancreatobiliary disorders.
Reports in the literature suggest high success rates of visualizing target lesions in the intrahepatic and inferior bile ducts. The SpyScope features four-way tip deflection and a smaller diameter (10 Fr) than the previously used cholangioscopes. This potentially achieves a high success rate of visualizing any site.
This study provides additional evidence supporting the investigation of the SpyGlass system’s potential role in the diagnosis and therapy of pancreatobiliary diseases.
Conventional POCPS requires two experienced endoscopists. The Spyglass system is a single-operator POCPS technique.
The authors have performed a good study; the manuscript is interesting.
P- Reviewer: Moon JH S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
| 1. | Siddique I, Galati J, Ankoma-Sey V, Wood RP, Ozaki C, Monsour H, Raijman I. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50:67-73. [PubMed] |
| 2. | Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374-382. [PubMed] |
| 3. | Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219-225. [PubMed] |
| 4. | Tajiri H, Kobayashi M, Ohtsu A, Ryu M, Yoshida S. Peroral pancreatoscopy for the diagnosis of pancreatic diseases. Pancreas. 1998;16:408-412. [PubMed] |
| 5. | Yamao K, Ohashi K, Nakamura T, Suzuki T, Sawaki A, Hara K, Fukutomi A, Baba T, Okubo K, Tanaka K. Efficacy of peroral pancreatoscopy in the diagnosis of pancreatic diseases. Gastrointest Endosc. 2003;57:205-209. [PubMed] |
| 6. | Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Arisaka Y, Moriyasu F. Initial experience of peroral pancreatoscopy combined with narrow-band imaging in the diagnosis of intraductal papillary mucinous neoplasms of the pancreas (with videos). Gastrointest Endosc. 2007;66:793-797. [PubMed] |
| 7. | Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841. [PubMed] |
| 8. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 2011;74:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Draganov PV, Lin T, Chauhan S, Wagh MS, Hou W, Forsmark CE. Prospective evaluation of the clinical utility of ERCP-guided cholangiopancreatoscopy with a new direct visualization system. Gastrointest Endosc. 2011;73:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Gutkin E, Hussain SA, Kim SH. The Successful Treatment of Chronic Cholecystitis with SpyGlass Cholangioscopy-Assisted Gallbladder Drainage and Irrigation through Self-Expandable Metal Stents. Gut Liver. 2012;6:136-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Siddiqui AA, Mehendiratta V, Jackson W, Loren DE, Kowalski TE, Eloubeidi MA. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol. 2012;10:466-71; quiz e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Seelhoff A, Schumacher B, Neuhaus H. Single operator peroral cholangioscopic guided therapy of bile duct stones. J Hepatobiliary Pancreat Sci. 2011;18:346-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kantsevoy SV, Frolova EA, Thuluvath PJ. Successful removal of the proximally migrated pancreatic winged stent by using the SpyGlass visualization system. Gastrointest Endosc. 2010;72:454-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Barkay O, Bucksot L, Sherman S. Endoscopic transpapillary gallbladder drainage with the SpyGlass cholangiopancreatoscopy system. Gastrointest Endosc. 2009;70:1039-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Bhat YM, Kochman ML. Novel management of complex hilar biliary strictures with the Spyglass Direct Visualization System (with video). Gastrointest Endosc. 2009;69:1182-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Draganov P. The SpyGlass® Direct Visualization System for Cholangioscopy. Gastroenterol Hepatol (N Y). 2008;4:469-470. [PubMed] |
| 18. | Wright H, Sharma S, Gurakar A, Sebastian A, Kohli V, Jabbour N. Management of biliary stricture guided by the Spyglass Direct Visualization System in a liver transplant recipient: an innovative approach. Gastrointest Endosc. 2008;67:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Kalaitzakis E, Webster GJ, Oppong KW, Kallis Y, Vlavianos P, Huggett M, Dawwas MF, Lekharaju V, Hatfield A, Westaby D. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol. 2012;24:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 20. | Maydeo A, Kwek BE, Bhandari S, Bapat M, Dhir V. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos). Gastrointest Endosc. 2011;74:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] |
| 22. | Binmoeller KF, Brückner M, Thonke F, Soehendra N. Treatment of difficult bile duct stones using mechanical, electrohydraulic and extracorporeal shock wave lithotripsy. Endoscopy. 1993;25:201-206. [PubMed] |
| 23. | Cipolletta L, Costamagna G, Bianco MA, Rotondano G, Piscopo R, Mutignani M, Marmo R. Endoscopic mechanical lithotripsy of difficult common bile duct stones. Br J Surg. 1997;84:1407-1409. [PubMed] |
| 24. | Chang WH, Chu CH, Wang TE, Chen MJ, Lin CC. Outcome of simple use of mechanical lithotripsy of difficult common bile duct stones. World J Gastroenterol. 2005;11:593-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Minami A, Hirose S, Nomoto T, Hayakawa S. Small sphincterotomy combined with papillary dilation with large balloon permits retrieval of large stones without mechanical lithotripsy. World J Gastroenterol. 2007;13:2179-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic sphincterotomy combined with large balloon dilation can reduce the procedure time and fluoroscopy time for removal of large bile duct stones. Am J Gastroenterol. 2009;104:560-565. [PubMed] |
| 27. | Neuhaus H, Zillinger C, Born P, Ott R, Allescher H, Rösch T, Classen M. Randomized study of intracorporeal laser lithotripsy versus extracorporeal shock-wave lithotripsy for difficult bile duct stones. Gastrointest Endosc. 1998;47:327-334. [PubMed] |
| 28. | Arya N, Nelles SE, Haber GB, Kim YI, Kortan PK. Electrohydraulic lithotripsy in 111 patients: a safe and effective therapy for difficult bile duct stones. Am J Gastroenterol. 2004;99:2330-2334. [PubMed] |
| 29. | Kim TH, Oh HJ, Choi CS, Yeom DH, Choi SC. Clinical usefulness of transpapillary removal of common bile duct stones by frequency doubled double pulse Nd: YAG laser. World J Gastroenterol. 2008;14:2863-2866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |