Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1826
Peer-review started: March 15, 2015
First decision: April 13, 2015
Revised: June 10, 2015
Accepted: August 25, 2015
Article in press: August 25, 2015
Published online: February 7, 2016
Processing time: 312 Days and 4.8 Hours
AIM: To investigate the effect of chymase inhibitor TY-51469 in the therapy of inflammatory bowel disease and the underlying mechanism.
METHODS: Seventy-five healthy Sprague-Dawley rats were randomly assigned to one of the three groups (control group, model group and TY-51469 experiment group) and each group had twenty-five rats. The rats of the model group and experiment group were subjected to treatment with 3.5% dextran sulfate sodium (DSS) 10 mg/kg to induce colitis. The control group and model group were subjected to intraperitoneal injection of saline, while the experiment group was subjected to intraperitoneal injection of 10 mg/kg TY-51469 each day. Five rats of each group were sacrificed on 0, 7, 14, 21 and 28 d, respectively. The degree of inflammation was assessed by histopathological scoring; flow cytometry was performed to detect the proportion of CD4+CD25+ Tregs in peripheral blood; colon tissues of rats were collected to measure mRNA and protein expression by PCR, Western blot and immunohistochemistry; serum levels of interleukin (IL)-10, transforming growth factor (TGF)-β1 and IL-17A were detected by ELISA.
RESULTS: The rats in the experiment group and model group had significantly more severe colitis than the ones in the control group (P < 0.05) before treatment on day 0; no significant difference was observed between the experiment group and model group (P > 0.05). After treatment with TY-51469, the rats in the experiment group had significantly less severe colitis compared with the model group on 7, 14, 21 and 28 d (P < 0.05). The proportion of CD4+CD25+ Tregs was lower in the model group and experiment group than in the control group; the experiment group had a significantly higher proportion of CD4+CD25+ Tregs than that in the model group (P < 0.05). The model group and experiment group demonstrated lower expression of Foxp3 than the control group; the experiment group had higher Foxp3 expression than the model group (P < 0.05). Cytokines IL-10, TGF-β1 and IL-17A were lower in the model group and experiment group than in the control group; the experiment group had higher expression than the model group (P < 0.05).
CONCLUSION: After treatment with chymase inhibitor TY-51469, the experiment group demonstrated more significantly reduced intestinal inflammation and higher expression of immune tolerance related cytokines (IL-10, TGF-β1, IL-17A) and Foxp3 which is specifically expressed in Tregs compared with the model group. Therefore, chymase inhibitor TY-51469 might ameliorate the progression of DSS-induced colitis possibly by increasing the expression of Tregs and cytokines.
Core tip: Until now, little is known of the role of chymase inhibitor in the immune system and no investigation has been performed in inflammatory bowel disease (IBD). In the present study, we built a Sprague-Dawley rat model of colitis induced with dextran sulfate sodium (DSS) and treated the model rats with chymase inhibitor TY-51469. The changes of Tregs were further detected to investigate the effect of chymase inhibitor TY-51469 on IBD and to explore the underlying mechanism. The results indicated that chymase inhibitor TY-51469 might ameliorate the progression of DSS-induced colitis possibly by increasing the expression of Tregs and cytokines.
- Citation: Liu WX, Wang Y, Sang LX, Zhang S, Wang T, Zhou F, Gu SZ. Chymase inhibitor TY-51469 in therapy of inflammatory bowel disease. World J Gastroenterol 2016; 22(5): 1826-1833
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1826.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1826
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine which mainly include Crohn’s disease (CD) and ulcerative colitis (UC)[1]. The underlying mechanism of the pathogenic process for IBD remains largely unknown. As a complex disease, IBD has been reported to be affected by intestinal microenvironment, immune factors and genetic predispositions[2-5]. Among various influencing factors, abnormal immune response and excessive inflammatory reactions play a critical role in the initiation and progression of IBD[6].
Regulatory T cells (Tregs) are a subtype of T cells which exert important effects of inducing and maintaining self-tolerance of the immune system[7]. It has been reported that Tregs could inhibit inflammatory reactions and relieve the development of IBD by secreting immunosuppressive factors such as interleukin (IL)-10, transforming growth factor (TGF)-β1 and IL-17A. IL-10 and IL-17A could restrict and terminate inflammatory reactions by inhibiting T lymphocytes and monocytes and activating macrophagocytes. In addition, the mechanism of the regulation of inflammatory reactions also involves the interactive effects of multiple immune cells including B lymphocytes, mast cells, and dendritic cells. The balance between T helper 1 (Th1) and Th2 are accordingly regulated after IL-10 interacts with these cells. TGF-β1 is a multidirectional regulatory cytokine which could inhibit immune reactions by regulating cell proliferation and differentiation and stimulating the secretion of extracellular matrix[8]. Also, TGF-β1 maintains the immune balance of organism by suppressing the functions of Th1 and Th2.
Chymases are a family of serine proteases involved in a variety of physiological functions[9]. Upon inflammatory stimulation, chymases are released from mast cells, mesenchymal cells and endothelial cells by secretory granules, which could convert angiotensin-1 to angiotensin-2[10]. Additionally, chymases play an essential role in repairing tissues and organs. However, over much chymases could lead to fibrosis of tissues. Chymase inhibitors can ameliorate the fibrosis of the lung. So far, the role of chymase in the heart, lung and kidney has been widely investigated.
Most previous studies focused on the association between chymase inhibitor and fibrosis of tissues or organs. However, little is known concerning its role in the immune system and no investigation has been performed in IBD. In the present study, we built a Sprague-Dawley (SD) rat model of colitis induced with dextran sulfate sodium (DSS) and treated the model rats with chymase inhibitor TY-51469. The changes of Tregs were further detected to investigate the effect of chymase inhibitor TY-51469 on IBD and to explore the underlying mechanism. It is anticipated that the results could provide new insights into clinical therapy for IBD.
This study was approved by the Institute Research Medical Ethics Committee of the First Affiliated Hospital of China Medical University. All rats in the study were used strictly in accordance with the National Institutions of Health Guide for the Care and Use of Laboratory Animals. Seventy-five eight-week-old female SD rats weighing 180-220 g were obtained from the Experiment Animal Center, China Medical University. DSS was purchased from MP Biomedicals; TGF-β1, rat IL-17A, IL-10 and Flow cytometry staining Kit for Tregs were purchased from Platinum Company; Trizol reagent was purchased from Invitrogen Company; ELISA kit was bought from eBioscience; reverse transcription and real-time PCR kits were purchased from BioTeke Corporation, Beijing, China; Western blot kit was purchased by Beyotime; the primers for Foxp3 were synthesized by Invitrogen Company and the primary antibody was purchased from CST Company. Other necessary reagents were available in our laboratory.
Seventy-five SD rats were randomly assigned to one of the three groups (control group, model group and experiment group) and each group had 25 rats. The rats in the control group were subjected to treatment with distilled water; the model group and the experiment group were subjected to treatment with 3.5% DSS for 6 d, and then distilled water for 6 d; the 3.5% DSS and distilled water were changed every 6 d. Observation, weighing and scoring were performed on each rat every day. The observation indexes of colitis included weight, diarrhea degree and colon lesions.
After the first six days of 3.5% DSS, the rats in the experiment group were subjected to intraperitoneal injection of TY-51469 at a dose of 10 mg/kg each day while rats in the control group and model group were subjected to intraperitoneal injection of saline at 10 mg/kg each day. The first day of injection of TY-51469 was 0 d. Five SD rats of each group were sacrificed on 0, 7, 14, 21 and 28 d, respectively. The blood of the caudal vein was collected before death for further detection of serum level of TGF-β1 and IL-10 and the proportion of CD4+CD25+ Tregs. The peripheral blood mononuclear cells (PBMCs) of rats on 28 d were collected for flow cytometry and PCR. The whole colon of each rat was collected and cut longitudinally along the intestinal tract. The location of inflammation was confirmed after washing the stool. The tissues were collected to investigate Foxp3 mRNA and protein expression.
The histopathological results were evaluated and scored independently by two investigators (Liu WX and Gu SZ). Histopathological scoring included macroscopic, microscopic and pathological observations. The scoring criteria were in accordance with the suggestions by Dutra et al[11]: 0, normal mucosa or no obvious inflammation; 1, a little irregular mucosa with or without minimal inflammatory cell infiltration; 2, obvious irregular mucosa with infiltration of a few inflammatory cells; 3, relatively inordinate structure of the mucosa with infiltration of a large amount of inflammatory cells, and angiopoiesis can be seen; 4, obviously inordinate structure of the mucosa with severe inflammatory cell infiltration, and abundant angiopoiesis and thickening of intestines wall can be seen.
The rats on 28 d with the largest difference of inflammation reaction were adopted for the detection of the proportion of CD4+CD25+ Tregs in rat peripheral blood by flow cytometry. PBMCs of rats (1 × 106 cells/mL) were isolated by density gradient centrifugation, and 0.3 μL CD4 antibody and CD25 antibody were added into 100 μL PBMCs. The antibody incubations were performed in a 100 μL volume for 45 min in the dark at 4 °C. After 5 min centrifugation at a speed of 300 g, the samples were washed three times with PBS and added 200 μL stationary liquid, which were followed by incubation in the dark at 4 °C for 60 min and 5 min centrifugation at a speed of 300 g. The samples were washed twice with PBS and resuspended in 500 μL FACS buffer solution, which was finally analyzed by flow cytometry.
The rats on 28 d with the largest difference of inflammation reaction were adopted to detect Foxp3 mRNA expression in colon tissues by real-time PCR. The total RNA from colon specimens was isolated using Trizol reagent. The isolated total RNA was reverse-transcribed into complementary DNA. Real-time PCR was performed using cDNA as templates. The reaction system of RT-PCR contained 2 μL cDNA, 10 μL SYBR Premix, 1 μL forward and reverse primer each, 0.4 μL ROX and 6.6 μL deionized water. The reaction condition was 95 °C for 30 s, 95 °C for 5 s, and 60 °C for 34 s. Each reaction was performed in duplicate, and no-template controls were included in each experiment. The relative quantification of Foxp3 mRNA expression was calculated using the 2−ΔΔCt method.
The rats on 28 d with the largest difference of inflammation reaction were adopted for the detection of Foxp3 protein expression by Western blot. The colon tissue was dissociated to obtain total protein. Quantitative 40 μg proteins were resolved on SDS-polyacrylamide gels, and then transferred electrophoretically to polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free milk at room temperature for 1 h, and then hybridized with primary antibodies (1:500) overnight at 4 °C, and secondary antibody for 1 h at room temperature. Immune reactive proteins were visualized using the ECL Plus Western blot detection reagent. GAPDH was detected as the loading control. All results are representatives of three independent experiments.
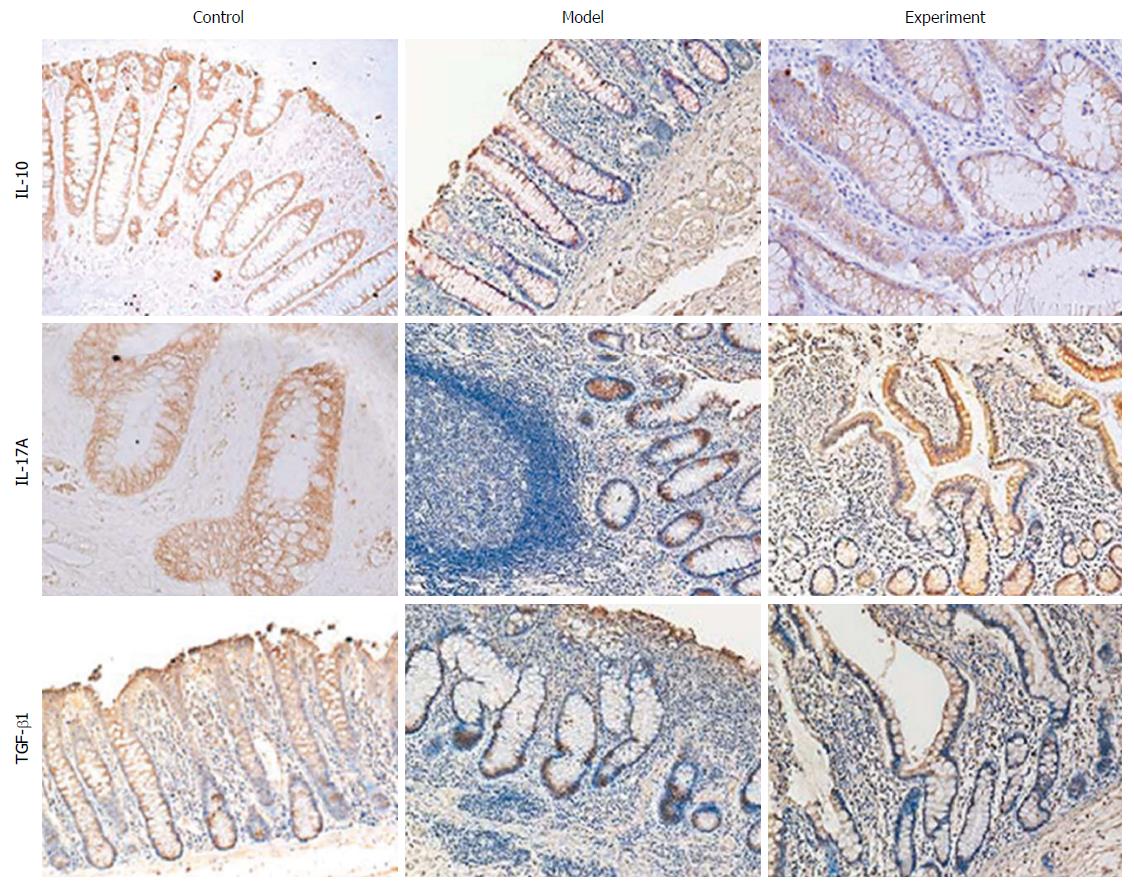
Formalin-fixed, paraffin-embedded tissues were cut into 4-μm-thick sections and mounted on poly-L-lysine-coated glass slides. Briefly, slides were deparaffinised in xylene, rehydrated in a graded alcohol series and washed in tap water. Endogenous peroxidase was blocked using 3% hydrogen peroxide for 10 min, and the sections were then washed with phosphate-buffered saline (PBS), pH 7.4. Tissue collagen was blocked to avoid nonspecific binding by the addition of 10% normal serum at 37 °C for 10 min. The antibody for IL-10, IL-17A or TGF-β1 was used as the primary antibody to detect protein expression, respectively, and incubated at 4 °C overnight. After rinsing three times with PBS for 5 min each, the sections were incubated with biotinylated secondary antibody and streptavidin-biotin peroxidase for 10 min each at 37 °C. The slides were then washed in PBS, stained with 3,3’-diaminobenzidine tetrahydrochloride and counterstained with haematoxylin. Finally, the sections were dehydrated and mounted. Primary antibodies were replaced with PBS as a negative control.
The rats on 28 d with the largest difference of inflammation reaction were adopted to detect serum levels of TGF-β1, IL-10 and IL-17a by using ELISA. The antigens were diluted into 1-10 μg/mL by coating buffer for 0.1 mL each well overnight at 4 °C. The samples were washed three times the next day, added 0.1 mL diluted sample into the wells, incubated for 1 h at 37 °C and then washed. Newly-diluted enzyme labeled secondary antibody 0.1 mL was added into the wells and incubated for 30-60 min at 37 °C followed by washes with the last wash by DDW. The TMB substrate solution 0.1 mL was added into each well for 10-30 min at 37 °C, which was terminated by 0.05 mL 2 mol/L sulfuric acid.
All the statistical analyses were carried out by using SPSS 16.0 software (SPSS, Chicago, IL, United States). Histological scores are presented with mean ± SD. For variables in accordance with normal distribution, one-way ANOVA was performed followed by LSD test. Non-parametric test was used to evaluate variables which were not in accordance with normal distribution. P values < 0.05 were considered statistically significant.
The degree of colitis before and after treatment was scored according to Dutra criteria. The results suggested that the rats in the experiment group and model group had significantly more severe colitis than the ones in the control group (P < 0.05) before treatment on day 0; no significant difference was observed between the experiment group and model group (P > 0.05). After treatment with TY-51469, the rats in the experiment group had significantly less severe colitis compared with the model group on 7, 14, 21 and 28 d (P < 0.05). The detailed information is summarized in Table 1.
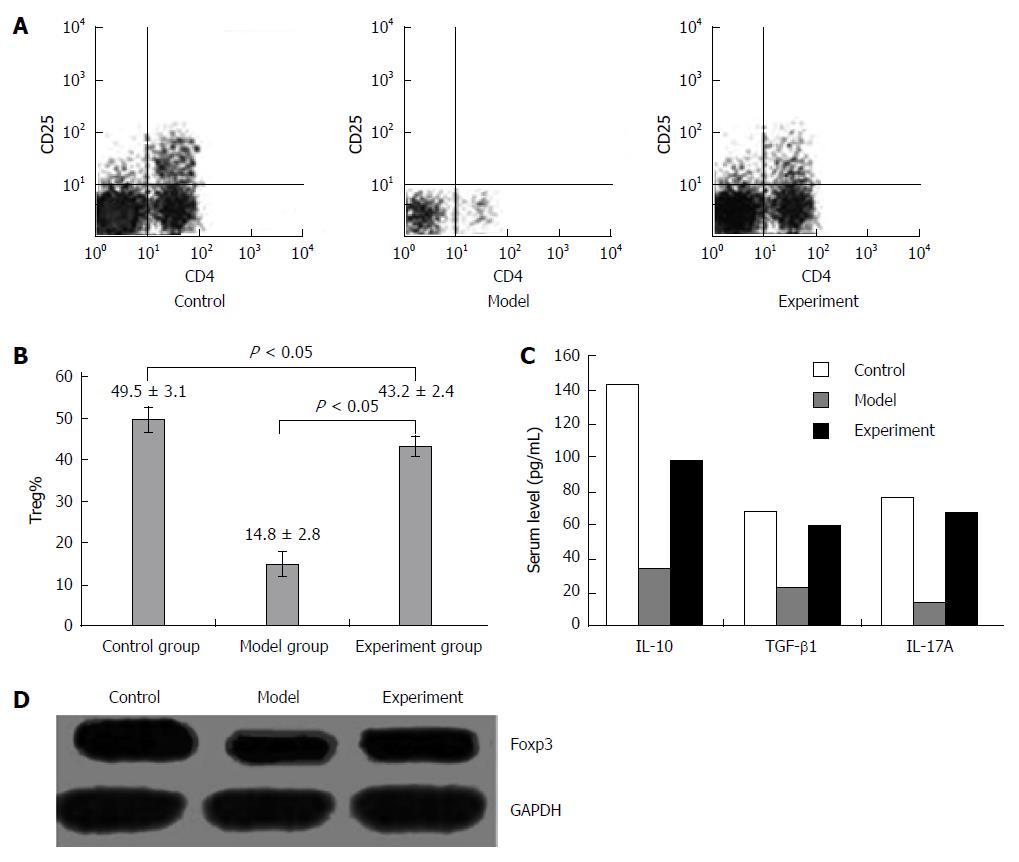
The rats on 28 d with the largest difference of inflammation reaction were adopted for the detection of the proportion of CD4+CD25+ Tregs in rat peripheral blood by flow cytometry. The proportion of CD4+CD25+ Tregs in the experiment group was significantly higher than that in the model group but lower than that in the control group (Figure 1A and B).
The results of quantitative real-time PCR indicated that after the administration of chymase inhibitor TY-51469, no significant difference was observed in IL-10, IL-17a or TGF-β1 expression in the three groups.
The results of immunohistochemistry suggested that after the administration of chymase inhibitor TY-51469, the protein expression of immune-related cytokines IL-10, IL-17A and TGF-β1 in the experiment group was significantly higher than that in the model group but lower than that in the control group (Figure 2).
The results of ELISA demonstrated that after the administration of chymase inhibitor TY-51469, the serum levels of immune-related cytokines IL-10, IL-17A and TGF-β1 were significantly higher in the experiment group than in the model group but lower than in the control group (P < 0.05) (Figure 1C, Table 2).
According to the results of Western blot, the expression of Foxp3 protein which is specifically expressed in Tregs in the experiment group was significantly higher than that in the model group but lower than that in the control group after the administration of chymase inhibitor TY-51469 (P < 0.05) (Figure 1D).
IBD is a chronic intestinal disorder typified by UC and CD[12]. The etiology of IBD remains largely unclear, even though considerable amounts of data have demonstrated that IBD is triggered by various environmental factors in genetically predisposed individuals[13]. The lesions of IBD are mainly located in the sigmoid and rectum, as well as the descending colon or even the whole colon. IBD possesses characteristics of long disease course and frequent relapse, the clinical symptoms of which mainly include diarrhea, abdominal pain, purulent stools and weight loss[14]. Generally, immunosuppressive drugs or anti-inflammatory drugs are used to treat IBD; steroids and non-steroidal anti-inflammatory drugs are effective for temporary relief of symptoms[15]. However, most of these treatments were insufficient and drug-induced severe side effects frequently occurred[16]. Chymase facilitates inflammation, tissue remodeling and matrix destruction, and thus might be implicated in IBD[17]. Rats have been widely used as the experimental models characterizing the role of chymase in the pathogenesis of a number of diseases primarily due to their availability. Aiming at elucidating the potential role of chymase inhibitors in the treatment of IBD, we used a DSS-induced SD rat model to explore the changes of Tregs and cytokines by treating the model rats with chymase inhibitor in this study.
Chymase, which can convert angiotensin-1 to angiotensin-2, has been extensively studied concerning its role in the pathophysiology of cardiovascular diseases[18]. Recently, the relation of chymase with inflammatory diseases including IBD has been described. Ishida et al[19] found that both chymase activity and MMP-9 activity were significantly increased in the DSS-induced colitis mice, which could be suppressed by chyamse inhibitor NK3201. Additionally, inhibition of chymase by use of TY-51469 has been reported to significantly attenuate indomethacin-induced small intestinal damage in rats[20]. In the present study, a DSS-induced rat colitis model was successfully built. After treatment with chymase inhibitor TY-51469, the rats in the experiment group had significantly less severe colitis compared with the model group on 7, 14, 21 and 28 d. These results suggested that chymase inhibitor TY-51469 might ameliorate DSS-induced experimental colitis in rats. Therefore, chymase inhibitor may be a novel therapy for clinical treatment of IBD in the future, although it still warrants clinical investigations.
To further explore the possible mechanism of chymase inhibitor TY-51469 acting on IBD, we investigated the changes of Tregs in different groups and found that the proportion of CD4+CD25+ Tregs in the experiment group was significantly higher than that in the model group but lower than that in the control group. The mRNA and protein expression of Foxp3, which is specifically expressed in Tregs, was also significantly higher in the experiment group than in the model group but lower than in the control group. These findings indicated that chymase inhibitor TY-51469 might promote the synthesis of Tregs. Moreover, the expression of immune tolerance related cytokines including IL-10, TGF-β1 and IL-17A in rat serum and colon tissues was also detected by ELISA and immunohistochemistry, respectively. The result of serum expression for cytokine was a supplement to the results of that at the site of inflammation. The results both demonstrated that cytokines IL-10, IL-17A and TGF-β1 protein expression was significantly higher in the experiment group than in the model group but lower than in the control group.
The imbalance of immune tolerance and immune reactions was found to be involved in the initiation of IBD[21]. Tregs have been reported to inhibit immune response by secreting cytokines, thereby preventing the occurrence and development of autoimmune diseases including IBD[22]. According to our present study, chymase inhibitor TY-51469 might reduce the progression of IBD possibly through increasing Tregs and cytokines, and thus inhibit immune and inflammatory reactions. As a kind of regulatory cells, Tregs can inhibit the activation and proliferation of T cells by directly contacting or by secretion of inhibiting cytokines such as IL-10 and TGF-β1, which ensure that the immune reactions are not excessively strong[23]. IL-10 exerts its immune regulatory function through suppressing T lymphocyte and activating monocytes; also, after interacting with B lymphocytes, mast cells, dendritic cells and keratinocytes, the balance of Th1 and Th2 cytokines is accordingly regulated, thereby inhibiting inflammatory reactions[24]. TGF-β1 inhibits immune reactions by regulating cell proliferation, differentiation and promoting the secretion of extracellular matrix; it can also maintain immune balance through suppressing the functions of Th1 and Th2[25]. In addition, CD4+CD25+ Tregs possess specific phenotypes and functions of inhibiting the proliferation and activation of self-reactive T cells by autonomic regulation[26].
It is worth noting that IL-17A was also significantly higher in the experiment group than in the model group but lower than in the control group. The proinflammatory role of IL-17A has been widely described. Its protective role may be related not to regulatory function but to a beneficial pro-inflammatory effect during acute injury. In addition, recent evidence suggested that IL-17A also exert a protective effect within the intestine. For example, researchers have revealed that IL-17A knockout mice demonstrated worsened DSS-induced colitis[27,28]. IL-17A has been reported to be involved in the host defense against fungal infection[29,30]. Considering the diverse and complex biological functions of IL-17A, further studies are still warranted to elucidate the exact role of IL-17A in IBD.
Some limitations should be acknowledged in this study. First, the finding that chymase inhibitor TY-51469 could ameliorate DSS-induced experimental colitis requires future studies to confirm in other animal model or clinical investigations. Second, our finding that chymase inhibitor TY-51469 might exert effects by increasing Tregs and cytokines was preliminary and exploratory, therefore future investigations concerning the specific mechanism of chymase inhibitor TY-51469 in relieving IBD are still needed. Additionally, the mRNA expression of IL-10, TGF-β1 and IL-17A in colon tissue did not demonstrate significant difference among the three groups. Whether it was due to limited number of samples or post-transcriptional regulation warrants further research to elucidate.
In conclusion, after treatment with chymase inhibitor TY-51469, the experiment group demonstrated more significantly reduced intestinal inflammation and higher expression of immune tolerance related cytokines (IL-10, TGF-β1, and IL-17A) and Foxp3 which is specifically expressed in Tregs compared with the model group. We therefore suggested that chymase inhibitor TY-51469 might ameliorate the progression of IBD possibly through increasing Tregs and cytokines in rats.
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine which mainly include Crohn’s disease (CD) and ulcerative colitis (UC). Until now, little is known of the role of chymase inhibitor in the immune system and no investigation has been performed in IBD.
The effects of chymase inhibitor on IBD need to be clarified.
This results demonstrated that chymase inhibitor TY-51469 might ameliorate the progression of DSS-induced colitis possibly by increasing the expression of Tregs and cytokines.
Further research concerning the role of chymase inhibitor TY-51469 in ameliorating IBD might unravel the underlying pathogenesis and novel therapy of IBD.
Chymases are a family of serine proteases involved in a variety of physiological functions.
This study investigated the effect of chymase inhibitor TY-51469 in the therapy of IBD and the underlying mechanism. The study is well designed, and the results are interesting.
P- Reviewer: Papadopoulos M, Toyonaga T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. BMJ. 2013;346:f432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 2. | Di Sabatino A, Biancheri P, Rovedatti L, Macdonald TT, Corazza GR. Recent advances in understanding ulcerative colitis. Intern Emerg Med. 2012;7:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Nielsen OH. New strategies for treatment of inflammatory bowel disease. Front Med (Lausanne). 2014;1:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Amiot A, Peyrin-Biroulet L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Therap Adv Gastroenterol. 2015;8:66-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1239] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 6. | Maul J, Zeitz M. Ulcerative colitis: immune function, tissue fibrosis and current therapeutic considerations. Langenbecks Arch Surg. 2012;397:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Dasgupta A, Saxena R. Regulatory T cells: a review. Natl Med J India. 2012;25:341-351. [PubMed] |
| 8. | Oh SA, Li MO. TGF-β: guardian of T cell function. J Immunol. 2013;191:3973-3979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Yahiro E, Miura S, Imaizumi S, Uehara Y, Saku K. Chymase inhibitors. Curr Pharm Des. 2013;19:3065-3071. [PubMed] |
| 10. | Tojo H, Urata H. Chymase inhibition and cardiovascular protection. Cardiovasc Drugs Ther. 2013;27:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Dutra RC, Cola M, Leite DF, Bento AF, Claudino RF, Nascimento AF, Leal PC, Calixto JB. Inhibitor of PI3Kγ ameliorates TNBS-induced colitis in mice by affecting the functional activity of CD4+CD25+FoxP3+ regulatory T cells. Br J Pharmacol. 2011;163:358-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] |
| 13. | Cooney R, Jewell D. The genetic basis of inflammatory bowel disease. Dig Dis. 2009;27:428-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. 2010;300:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Jani N, Regueiro MD. Medical therapy for ulcerative colitis. Gastroenterol Clin North Am. 2002;31:147-166. [PubMed] |
| 16. | Lakatos PL, Lakatos L. Ulcerative proctitis: a review of pharmacotherapy and management. Expert Opin Pharmacother. 2008;9:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Heuston S, Hyland NP. Chymase inhibition as a pharmacological target: a role in inflammatory and functional gastrointestinal disorders? Br J Pharmacol. 2012;167:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Doggrell SA, Wanstall JC. Cardiac chymase: pathophysiological role and therapeutic potential of chymase inhibitors. Can J Physiol Pharmacol. 2005;83:123-130. [PubMed] |
| 19. | Ishida K, Takai S, Murano M, Nishikawa T, Inoue T, Murano N, Inoue N, Jin D, Umegaki E, Higuchi K. Role of chymase-dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2008;324:422-426. [PubMed] |
| 20. | Kakimoto K, Takai S, Murano M, Ishida K, Yoda Y, Inoue T, Jin D, Umegaki E, Higuchi K. Significance of chymase-dependent matrix metalloproteinase-9 activation on indomethacin-induced small intestinal damages in rats. J Pharmacol Exp Ther. 2010;332:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discov Med. 2011;11:459-467. [PubMed] |
| 22. | Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1772-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Lo WC, Arsenescu RI, Friedman A. Mathematical model of the roles of T cells in inflammatory bowel disease. Bull Math Biol. 2013;75:1417-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol. 2013;19:3931-3941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 25. | Han G, Li F, Singh TP, Wolf P, Wang XJ. The pro-inflammatory role of TGFβ1: a paradox? Int J Biol Sci. 2012;8:228-235. [PubMed] |
| 26. | Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 27. | Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666-4673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55-62. [PubMed] |
| 29. | Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 30. | Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 916] [Cited by in RCA: 808] [Article Influence: 57.7] [Reference Citation Analysis (0)] |