Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9554
Peer-review started: June 17, 2016
First decision: August 29, 2016
Revised: September 8, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: November 21, 2016
Processing time: 154 Days and 12.5 Hours
To evaluate the efficacy of self-expanding metal stents (SEMS) for the palliation of malignant gastric outlet obstruction in patients with and without peritoneal carcinomatosis (PC).
We performed a retrospective analysis of 62 patients who underwent SEMS placement for treatment of malignant gastroduodenal obstruction at our hospital over a six-year period. Stents were deployed through the scope under combined fluoroscopic and endoscopic guidance. Technical success was defined as successful stent placement and expansion. Clinical success was defined as an improvement in the obstructive symptoms and discharge from hospital without additional parenteral nutrition. According to carcinomatosis status, patients were assigned into groups with or without evidence of peritoneal disease.
In most cases, obstruction was caused by pancreatic (47%) or gastric cancer (23%). Technical success was achieved in 96.8% (60/62), clinical success in 79% (49/62) of all patients. Signs of carcinomatosis were identified in 27 patients (43.5%). The diagnosis was confirmed by pathology or previous operation in 7 patients (11.2%) and suspected by CT, MRI or ultrasound in 20 patients (32.2%). Presence of carcinomatosis was associated with a significantly lower clinical success rate compared to patients with no evidence of peritoneal disease (66.7% vs 88.6%, P = 0.036). There was no significant difference in overall survival between patients with or without PC (median 48 d vs 70 d, P = 0.21), but patients showed significantly longer survival after clinical success of SEMS placement compared to those experiencing clinical failure (median 14.5 d vs 75 d, P = 0.0003).
Given the limited therapeutic options and a clinical success rate of at least 66.7%, we believe that SEMS are a reasonable treatment option in patients with malignant gastric outlet obstruction with peritoneal carcinomatosis.
Core tip: This is a retrospective study to evaluate the efficacy of self-expanding metal stents (SEMS) for the palliation of gastric outlet obstruction in patients with peritoneal carcinomatosis (PC). Between January 2008 and April 2014 we treated 62 patients with duodenal stents for palliation of malignant gastroduodenal obstruction. In most cases, obstruction was caused by pancreatic (47%) or gastric cancer (23%). Technical success was achieved in 96.8% and clinical success in 79% of all patients. Carcinomatosis was associated with a significantly lower clinical success rate (66.7% vs 88.6%, P = 0.036). Given the limited therapeutic options and a clinical success rate of at least 66.7% we believe that SEMS are a reasonable treatment option in patients with PC.
- Citation: Rademacher C, Bechtler M, Schneider S, Hartmann B, Striegel J, Jakobs R. Self-expanding metal stents for the palliation of malignant gastric outlet obstruction in patients with peritoneal carcinomatosis. World J Gastroenterol 2016; 22(43): 9554-9561
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9554
Gastric outlet obstruction (GOO) is a frequent complication of advanced gastric and pancreaticobiliary cancer[1,2]. Typically presenting with abdominal pain, serious nausea and vomiting, patients are often in a poor clinical condition[3,4]. In the past, surgical gastrojejunostomy has been the standard therapy in these patients[5]. Although associated with good functional outcome and relief of symptoms, this treatment modality carries significant morbidity[6-9].
Over the last decade endoscopic placement of self-expanding metal stents (SEMS) has increasingly been used to treat malignant GOO[4,5]. Compared to gastrojejunostomy, SEMS placement leads to a more rapid relief of obstructive symptoms and is associated with a shorter hospital stay and lower costs[6,7,10,11].
Peritoneal carcinomatosis (PC) has been generally considered a relative contraindication to SEMS placement, given the risk of multifocal obstruction and reduced gastrointestinal motility[12-16]. Mendelsohn et al[13] were the first to evaluate the impact of PC on the technical and clinical success of SEMS in the palliation of malignant GOO. They found clinical outcomes comparable to patients without peritoneal disease[13]. On the contrary, two Korean studies from the same group showed that carcinomatosis with ascites was a predictor of poor clinical success of SEMS placement in patients with gastric cancer and GOO[17,18]. Similar results were found for the impact of ascites on solid food intake after SEMS placement[19]. In another Korean study, only the ECOG scale, but not the presence of carcinomatosis was an independent predictor of clinical success regarding SEMS placement[16]. Further Korean data showed both ECOG scale and presence of PC to be related with clinical failure of palliative SEMS placement[20].
At present, there have been few reports to evaluate the effect of PC on success of SEMS for malignant GOO and the existing data is contradictory. The aim of this study was to investigate the efficacy of SEMS in patients with PC and malignant GOO.
We performed a retrospective analysis of patients who underwent stent insertion for treatment of malignant gastroduodenal obstruction at our institution between January 2008 and April 2014. The clinical records of all patients with malignant GOO who underwent palliative stent placement (institutional standard procedure: WallFlex Duodenal Stent, Boston Scientific) were evaluated and the following data was collected: demographics, type of malignancy, site of obstruction, technical outcome, clinical outcome, evidence of carcinomatosis or ascites, reinterventions, complications and overall survival.
All patients had pathologically proven malignancies and presented with symptomatic obstruction. The diagnosis of outlet obstruction was based on endoscopic and radiologic findings. Presence of PC and ascites was assessed by reviewing ultrasound, radiology, pathology and surgical reports. According to carcinomatosis status, patients were assigned into groups.
Written informed consent was obtained from every patient before the procedure. The institutional review board (Ethik-Kommission, Landesärztekammer Rheinland-Pfalz, Germany) deemed it exempt from review.
Technical success was defined as successful stent deployment and expansion. Clinical success was defined as an improvement in obstructive symptoms and discharge from the hospital without needing additional parenteral nutrition.
Confirmed PC was defined as cytologically or histologically proven involvement of the peritoneum. The definition of suspected PC was based on typical findings on a CT scan, MRI scan or ultrasound examination. In case of ascites detection without further signs of PC, we assumed peritoneal involvement if no other explaining disease could be identified (i.e., hepatic cirrhosis, portal vein thrombosis, heart failure).
In accordance to Mendelsohn et al[13], early stent failure was defined as the need for reintervention because of recurrent obstructive symptoms within 30 d; late stent failure was defined as reintervention more than 30 d after stent deployment.
Major complications were defined as life-threatening or serious events, such as hemorrhage, jaundice, stent migration or perforation, usually requiring additional treatment and hospitalization. Minor complications were defined as not life-threatening or moderately severe events, such as wound infection, mild fever or pain[10].
SEMS were deployed under combined fluoroscopic and endoscopic guidance with the patients under conscious sedation with intravenous propofol and midazolam. Stent placement was performed with a gastroscope or colonoscope (GIF-1T160, GIF-2TH180, CF-H180Ai, Olympus Europe, Hamburg, Germany) using the through-the-scope method (TTS). All patients were treated by uncovered nitinol stents (WallFlex Duodenal Stents, Boston Scientific, Natick, MA, United States). The length of the stenosis was assessed by advancing the endoscope through the stricture site, if possible, or measured fluoroscopically. After placing a guidewire through the stricture, the SEMS delivery system was advanced over the wire. Stent position was confirmed fluoroscopically and endoscopically.
Absolute numbers and percentages as well as median (with interquartile range) are computed to describe the patient population. Categorical values were compared by Fisher’s exact test or chi-square test and continuous variables were compared by two-tailed Wilcoxon rank sum test. Survival curves were compared by using the log-rank test.
P-values < 0.05 were considered significant. All P-values are results of two-tailed tests. The tests were performed using the SAS statistical package, version 9.3 (Cary, NC, United States) or SPSS, version 24.0 (Chicago, IL, United States). The statistical review of the study was performed by a biomedical statistician.
A total of 62 consecutive patients underwent SEMS placement for palliation of malignant GOO. Patient characteristics are listed in Table 1. The median patient age was 70.5 years (63-81 years); 44% of patients were female and 56% of patients were male. The most common cause of GOO was pancreatic cancer (n = 29, 47%) followed by gastric (n = 14, 23%) and biliary cancer (n = 9, 15%). Site of obstruction was predominantly the duodenal region (n = 44, 71%).
| Total | Without PC | With PC | P value | ||||
| n | % | n | % | n | % | ||
| No. of patients | 62 | 100% | 35 | 56% | 27 | 44% | |
| Gender | |||||||
| Female | 27/62 | 44% | 19/35 | 54% | 8/27 | 30% | 0.072 |
| Median age (yr) | 70.5 (63-81) | 76 (64-86) | 68 (60-74) | 0.0221 | |||
| Etiology | |||||||
| Pancreatic cancer | 29/62 | 47% | 20/35 | 57% | 9/27 | 33% | 0.077 |
| Gastric cancer | 14/62 | 23% | 4/35 | 11% | 10/27 | 37% | 0.0301 |
| GB or BD cancer | 9/62 | 15% | 4/35 | 11% | 5/27 | 19% | 0.485 |
| Ampulla of Vater cancer | 3/62 | 5% | 3/35 | 9% | 0/27 | 0% | 0.250 |
| Other | 7/62 | 11% | 4/35 | 11% | 3/27 | 11% | 1.000 |
| Site of obstruction | |||||||
| Duodenum | 44/62 | 71% | 30/35 | 86% | 14/27 | 52% | 0.0051 |
| Pylorus | 7/62 | 11% | 3/35 | 9% | 4/27 | 15% | 0.689 |
| Rest of stomach | 4/62 | 6% | 0/35 | 0% | 4/27 | 15% | 0.0311 |
| Anastomosis | 5/62 | 8% | 1/35 | 3% | 4/27 | 15% | 0.158 |
| Other | 2/62 | 3% | 1/35 | 3% | 1/27 | 4% | 1.000 |
Signs of PC were identified in 27 patients (43.5%). Peritoneal disease was suspected in 20 patients (32.2%) and cytologically or histologically proven in 7 patients (11.2%). Evidence of ascites was found in 23 of 27 patients (85%) with PC at the time of SEMS placement (Table 2).
| n | % | P value | |
| No. of patients | 27/62 | 43.5% | |
| Suspected | 20/62 | 32.2% | |
| Ultrasound | 11/62 | 17.7% | |
| CT-scan | 9/62 | 14.5% | |
| Proven | 7/62 | 11.2% | |
| Cytology | 1/62 | 1.6% | |
| Histology | 6/62 | 9.7% | |
| Ascites | 23/62 | 37.1% | |
| Clinical success | 15/23 | 65.2% | 0.0471 |
Patients in the carcinomatosis group tended to be younger compared to those without PC (P = 0.022) (Table 1). Gastric cancer and gastric obstruction site was more common in patients with carcinomatosis, whereas patients without PC experienced predominantly duodenal strictures (Table 1).
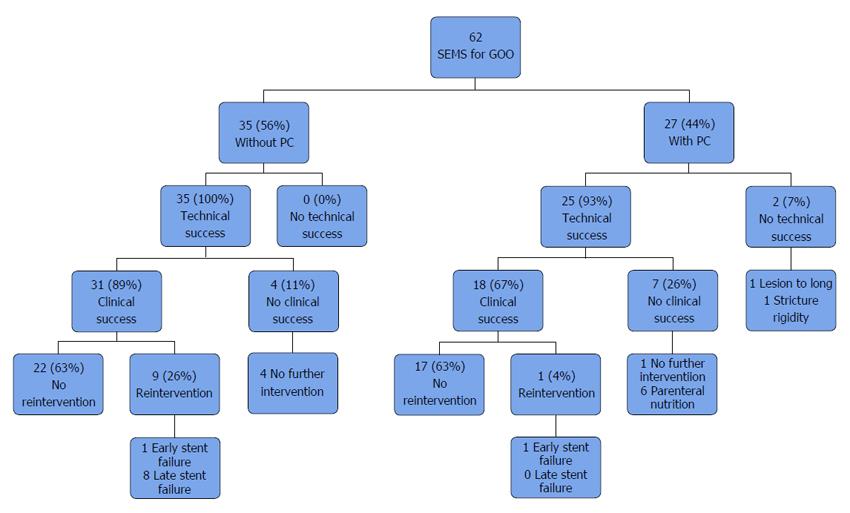
Technical success was achieved in 96.8% of all patients (60/62). In patients without carcinomatosis, the technical success rate was 100 % (35/35), while technical success rate in patients with PC was 92.6% (25/27, P = 0.186) (Table 3). Technical failure was due to insufficient stent expansion in one case and to stricture-length in another. Figure 1 shows a flowchart of clinical courses.
| Total | Without PC | With PC | P value | ||||
| n | % | n | % | n | % | ||
| No. of patients | 62 | 35 | 27 | ||||
| Technical success | 60/62 | 96.8% | 35/35 | 100.0% | 25/27 | 92.6% | 0.186 |
| Clinical success | 49/62 | 79.0% | 31/35 | 88.6% | 18/27 | 66.7% | 0.03612 |
| Reinterventions (RI) | 15/62 | 24.2% | 11/35 | 31.4% | 4/27 | 14.8% | 0.1302 |
| Early RI ( ≤ 30 d) | 7/62 | 11.3% | 3/35 | 8.6% | 4/27 | 14.8% | 0.689 |
| Late RI (> 30 d) | 8/62 | 12.9% | 8/35 | 22.9% | 0/27 | 0.0% | 0.0081 |
| Median time to early RI (d) | 6 (3.5-12.5) | 11 (8.5-15.5) | 3.5 (3.0-6.5) | ||||
| Median time to late RI (d) | 130.5 (90.75-220) | 130.5 (90.75-220) | Ø | ||||
| RI after clinical success | 10/49 | 20.4% | 9/31 | 29.0% | 1/18 | 5.6% | 0.070 |
| Early RI ( ≤ 30 d) | 2/49 | 4.1% | 1/31 | 3.2% | 1/18 | 5.6% | 1.000 |
| Late RI (> 30 d) | 8/49 | 16.3% | 8/31 | 25.8% | 0/18 | 0.0% | 0.0201 |
| RI after clinical failure | 5/13 | 38.5% | 2/4 | 50.0% | 3/9 | 33.3% | 1.000 |
| Early RI ( ≤ 30 d) | 0/13 | 38.5% | 2/4 | 50.0% | 3/9 | 33.3% | 1.000 |
| Late RI (> 30 d) | 0/13 | 0.0% | 0/4 | 0.0% | 0/9 | 0.0% | |
| Complications | |||||||
| Major | 3/62 | 4.8% | 2/35 | 5.7% | 1/27 | 3.7% | 1.000 |
| Minor | 4/62 | 6.5% | 1/35 | 2.9% | 3/27 | 11.1% | 0.309 |
| Median survival (d) | 57 (19.75-145.25) | 70 (19.5-213.5) | 48 (26-79) | ||||
Clinical success was achieved in 49 of the 62 cases (79%). Among the 35 patients without carcinomatosis, clinical success was achieved in 31 (88.6%). In the group of patients with PC clinical success rate was significantly lower with only 66.7% of the patients treated successfully (31/35 vs 18/27, P = 0.036) (Table 3). Further analysis of subgroups showed a clinical success rate of 65.2% in patients with PC and ascites. The difference compared to patients without PC was statistically significant as well (31/35 vs 15/23, P = 0.047) (Table 2).
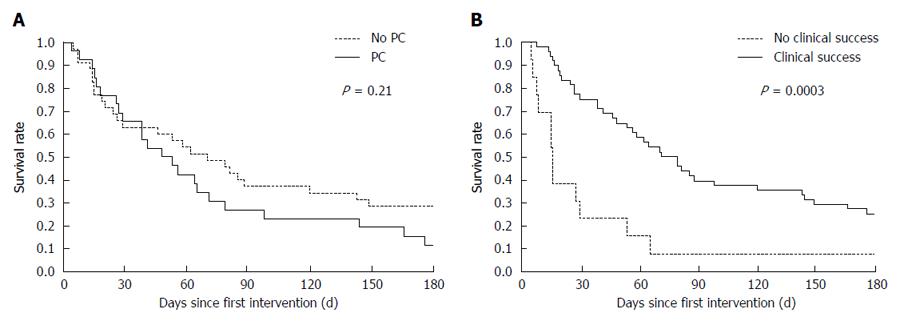
Of the 62 patients, 60 (97%) died during follow-up. Median overall survival was 48 d (26-79 d) in the PC group versus 70 d (19.5-213.5 d) for patients without PC (P = 0.21) (Figure 2A). Whereas the median survival time did not differ significantly according to carcinomatosis status, clinical failure of SEMS placement was associated with shorter survival (Figure 2B). Effectively palliated patients showed a median survival of 75 d compared to only 14.5 d in the group experiencing clinical failure (P = 0.0003).
Ten of 49 (20.4%) patients treated successfully with respect to clinical outcome required further endoscopic intervention (Table 3). Two of these patients experienced early stent failure at respectively 14 and 17 d, while the other eight patients experienced late stent failure at a median of 130.5 d. Eight of ten patients could be palliated successfully by another endoscopic intervention. Six patients were managed by insertion of another SEMS, two by Argon Plasma Coagulation and two by mechanical stent recanalization (data not shown). There were statistically significant more patients experiencing late reintervention after clinical success in the group without PC (8/31 vs 0/18, P = 0.02), whereas no difference could be found with regard to patients experiencing early reintervention (1/31 vs 1/18, P = 1.0) (Table 3). Three patients required more than one reintervention. 63% of all patients were palliated successfully without need for further endoscopic intervention (Figure 1).
Major complications occurred in three patients (4.8%) and were not more frequent in the PC group (P = 1.0) (Table 3). Complications encountered were gastrointestinal bleeding in two patients and severe pain related to hematoma of the stomach wall in one patient. Both cases of hemorrhage were due to diffuse bleeding from the tumor. There was no case of perforation.
Four patients (6.4%) developed minor complications with again no significant difference between the groups (Table 3). Minor complications included intermittent pain (n = 2) treated with analgesics and moderate fever (n = 2). The symptoms were self-limiting in all cases and improved with supportive management.
The present study compared the efficacy of SEMS in patients with malignant GOO and PC to patients without peritoneal disease. Carcinomatosis had been considered a relative contraindication to SEMS placement given the theoretical risk of multifocal obstruction and decreased bowel movement[14-16]. Mendelsohn et al[13] were the first to evaluate the impact of PC on the technical and clinical success of SEMSs for malignant GOO. They found clinical outcomes comparable to patients without peritoneal disease (81% vs 84%). In our study, patients with PC showed a significantly lower clinical success rate compared to those without carcinomatosis (66.7% vs 88.6%, P = 0.036). This is consistent with Korean data, which showed PC to be associated with poor clinical success of SEMS placement in patients with malignant GOO[16-18]. The most common malignancy in these trials was gastric cancer, whereas in western populations the most common underlying disease is pancreatic malignancy[16-18]. Thus, discrepancy in success of SEMS placement between Mendelsohn et al and Korean studies was explained by differences in tumor etiology[16,21].
To our knowledge, this is the first time that an impact of carcinomatosis on clinical success of SEMSs for GOO was shown in a western population. We believe, that discrepancy to Mendelsohn et al[13] is due to differences in patient populations. First, patients in our study had a median age of 70.5 years (63-81 years) compared to a mean age of 64 years in the Mendelsohn study. Second, in the present study, 85% (23/27) of patients with evidence of PC had ascites at the time of stent placement. Mendelsohn describes that 82% of their patients with PC had no or small ascites. This suggests that patients in our study might have had a more advanced peritoneal involvement. Lastly, distribution to groups with or without PC might have been different. Mendelsohn et al identified signs of carcinomatosis in 60% of the patients. Diagnosis based on CT scan in 78% and on previous pathology in 22% of the cases. In the present study, signs of PC were identified in only 43.5% of the patients and diagnosis relied on ultrasound in 40.7%, on CT scan in 33.3% and on pathology in 25.9% of the patients. Ultrasound is excellent in detecting PC with ascites but weaker when ascites is lacking[22,23]. As advanced PC is usually accompanied by ascites and low-volume disease would not have changed our decision to perform stent placement, unsuspicious ultrasound is not generally followed by computed tomography. Routine conduction of CT-scan might result in identification of more patients with early PC without ascites. This could be the reason why Mendelsohn et al[13] identified more patients with PC, despite congruency in baseline characteristics regarding tumor etiology and obstruction sites.
Patient selection might be another reason for differences between both studies. Typically presenting with abdominal pain, severe nausea and vomiting, patients are often in a poor clinical condition[3,4]. Only limited therapeutic options are available and alternatives such as a venting PEG tube or the permanent placement of a nasogastric tube might impair the quality of life[19]. Therefore, we believe that an endoscopic attempt is sometimes justified even if findings seem “borderline suitable” for SEMS placement.
In the present study, technical success rate was high at 96.8%, which is in accordance with recent literature. Clinical success rate was 79% for all patients and 88.6% for those without PC, which is consistent with the current literature[2,10,17,18,24]. As described above, clinical success in patients with carcinomatosis was lower than reported by Mendelsohn et al[13] but in accordance to recent Korean studies[17,18]. Major complications were rare, at a rate of 5%, and did not differ significantly between both patient groups, indicating that SEMS are a safe treatment option for patients with GOO and PC (Table 3).
There were 63% of the patients in both groups that were effectively palliated by SEMS placement and did not need endoscopic reintervention for GOO until death (Figure 1). One of five successful treated patients needed endoscopic reintervention for GOO and 80% of these reinterventions were clinically successful. Jeurnink et al[6] recommended GJJ as the primary treatment option in patients with an expected survival of two months or longer and stent placement for patients, who are expected to live less than two months. As median survival was short (57 d), reinterventions occurred late (median late reintervention 130.5 d) and most reinterventions were clinically successful (8/10 patients) we believe that stent placement was the right therapeutic option for our patients.
There were significantly more late reinterventions (> 30 d) in the group without PC (8/31 vs 0/18, P = 0.02). In fact, none of the patients with PC experienced late stent failure. This might be related to a (though not significant) shorter overall survival first (median 48 d vs 70 d, P = 0.21) and second to recurrence of obstructive symptoms out of reasons other than stent failure. Jeon et al[17] found no difference in stent patency after SEMS placement between patients with and without carcinomatosis. On the other hand, Lee et al[16] assessed obstructive symptom free survival, defined as the period between stent placement and recurrence of obstructive symptoms due to multifocal obstruction, decreased bowel movement and stent failure. They showed presence of carcinomatosis to be an independent predictor of the obstructive symptom-free survival after SEMS placement for malignant GOO. Thus, suffering from decreased bowel movement or multifocal obstruction before occurrence of stent occlusion, patients with PC might not have experienced late reintervention for stent failure.
Implantation of covered stents could decrease the need for reintervention because of stent occlusion, but is associated with a higher risk of stent migration[25,26].
While some previous studies failed to identify any factors that predict survival of patients who underwent SEMS placement for malignant GOO[27], others found evidence that a World Health Organization performance score of 3 or 4 and use of pain medication stronger than tramadol are predictive of shorter survival[28,29]. We did not find a significant difference in overall survival between patients with or without PC (median 48 d vs 70 d, P = 0.21), although our data suggested that patients without PC survived, on average, for longer. Further larger-scale studies are needed to confirm these results. Clinical failure of SEMS placement was a strong predictor for shorter survival (median 14.5 d vs 75 d, P = 0.0003).
The present study has several limitations. First of all, this was a nonrandomized, retrospective, single center study. Due to the retrospective design, we were not able to record ECOG scale and Gastric Outlet Obstruction Scoring System (GOOS) consistently. GOOS, introduced by Adler and Baron[4] in 2002, is an assessment of patients’ level of oral intake. To ensure correct evaluation of effective palliation, we included discharge from hospital without additional parenteral nutrition in our definition of clinical success. As there are other reasons for parenteral nutrition than intolerance of oral intake (e.g., tumor cachexia), we had to accept the risk of misclassification of effectively palliated patients. For our purposes, we were more concerned about the specificity of clinical success, as we wanted to ensure correct evaluation of effective palliation.
Last, all data was collected from patients’ medical records. We did not perform interviews, so we cannot rule out that some patients experiencing stent failure were treated in other hospitals. As our results for reinterventions are consistent with literature and overall survival was short, we do not believe that this applies to a relevant rate of cases[2,13,18,24,29].
Although this study had some limitations, we showed for the first time a significant impact of carcinomatosis on clinical success of SEMS insertion in a western population. In contrast to previous studies, only one type of SEMS was included.
In conclusion, despite differences in clinical success, we believe that SEMS are a reasonable treatment option for patients with GOO and peritoneal disease. There are limited therapeutic options and, although lower than in patients without PC, we reached a clinical success rate of at least 66.7%. Given the fact that alternatives might impair quality of life, that clincal success rate is acceptable and complications are rare, we do not regard carcinomatosis to be a contraindication to enteral stenting. Nevertheless, the lower clinical success rate has to be considered and SEMS placement needs to be critically evaluated in every case of PC and GOO.
Gastric outlet obstruction (GOO) is a frequent complication of advanced gastric and pancreaticobiliary cancer. Over the last decade endoscopic placement of self-expanding metal stents (SEMS) has increasingly been used to treat malignant GOO. Given the risk of multifocal obstruction and reduced gastrointestinal motility, peritoneal carcinomatosis (PC) has been generally considered a relative contraindication to SEMS placement.
At present, there have been few reports to evaluate the effect of PC on success of SEMS for malignant GOO and the existing data is contradictory.
The present study showed a significant impact of carcinomatosis on clinical success of SEMS insertion in a western population. This is consistent with Korean data, which showed PC to be associated with poor clinical success of SEMS placement in patients with malignant GOO.
Given the limited therapeutic options and an acceptable clinical success rate of at least 66.7%, we believe that SEMS are a reasonable treatment option in patients with with PC and GOO.
SEMS, an endoscopically placed endoprosthesis used to bridge gastrointestinal strictures.
It’s an excellent job about SEMS for for the palliation of malignant gastric outlet obstruction in PC patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Lee JI S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Tendler DA. Malignant gastric outlet obstruction: bridging another divide. Am J Gastroenterol. 2002;97:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 290] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 3. | Patton JT, Carter R. Endoscopic stenting for recurrent malignant gastric outlet obstruction. Br J Surg. 1997;84:865-866. [PubMed] |
| 4. | Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (6)] |
| 5. | Huggett MT, Ghaneh P, Pereira SP. Drainage and bypass procedures for palliation of malignant diseases of the upper gastrointestinal tract. Clin Oncol (R Coll Radiol). 2010;22:755-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 359] [Article Influence: 23.9] [Reference Citation Analysis (2)] |
| 7. | Mehta S, Hindmarsh A, Cheong E, Cockburn J, Saada J, Tighe R, Lewis MP, Rhodes M. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004;36:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Weaver DW, Wiencek RG, Bouwman DL, Walt AJ. Gastrojejunostomy: is it helpful for patients with pancreatic cancer? Surgery. 1987;102:608-613. [PubMed] |
| 10. | Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Fiori E, Lamazza A, Burza A, Meucci M, Cavallaro G, Izzo L, Schillaci A, Cangemi V. Malignant intestinal obstruction: useful technical advice in self-expanding metallic stent placement. Anticancer Res. 2004;24:3153-3155. [PubMed] |
| 12. | Tominaga K, Maetani I, Shigoka H, Omuta S, Sato K, Ito S, Saigusa Y, Gomi T, Kohda E. Factors associated with delayed gastric emptying in patients with stent placement for malignant gastric outlet obstruction. Endosc Int Open. 2013;1:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Mendelsohn RB, Gerdes H, Markowitz AJ, DiMaio CJ, Schattner MA. Carcinomatosis is not a contraindication to enteral stenting in selected patients with malignant gastric outlet obstruction. Gastrointest Endosc. 2011;73:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Baron TH, Harewood GC. Enteral self-expandable stents. Gastrointest Endosc. 2003;58:421-433. [PubMed] |
| 15. | Katsanos K, Sabharwal T, Adam A. Stenting of the upper gastrointestinal tract: current status. Cardiovasc Intervent Radiol. 2010;33:690-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Lee JE, Lee K, Hong YS, Kim ER, Lee H, Min BH. Impact of Carcinomatosis on Clinical Outcomes after Self-Expandable Metallic Stent Placement for Malignant Gastric Outlet Obstruction. PLoS One. 2015;10:e0140648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Jeon HH, Park CH, Park JC, Shim CN, Kim S, Lee HJ, Lee H, Shin SK, Lee SK, Lee YC. Carcinomatosis matters: clinical outcomes and prognostic factors for clinical success of stent placement in malignant gastric outlet obstruction. Surg Endosc. 2014;28:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Park CH, Park JC, Kim EH, Chung H, An JY, Kim HI, Shin SK, Lee SK, Cheong JH, Hyung WJ. Impact of carcinomatosis and ascites status on long-term outcomes of palliative treatment for patients with gastric outlet obstruction caused by unresectable gastric cancer: stent placement versus palliative gastrojejunostomy. Gastrointest Endosc. 2015;81:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Sasaki T, Isayama H, Nakai Y, Togawa O, Kogure H, Kawakubo K, Mizuno S, Yashima Y, Ito Y, Yamamoto N. Predictive factors of solid food intake in patients with malignant gastric outlet obstruction receiving self-expandable metallic stents for palliation. Dig Endosc. 2012;24:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Shin YS, Choi CW, Kang DH, Kim HW, Kim SJ, Cho M, Hwang SH, Lee SH. Factors associated with clinical failure of self-expandable metal stent for malignant gastroduodenal obstruction. Scand J Gastroenterol. 2016;51:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kim JH, Song HY, Shin JH, Hu HT, Lee SK, Jung HY, Yook JH. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma. AJR Am J Roentgenol. 2009;193:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Lorenz R, Krestin GP, Schmitz-Rixen T, Arnold G. [The significance of sonography and computed tomography for the diagnosis of the intraperitoneal spread of tumors. Findings in 307 cases of peritoneal carcinosis]. Rofo. 1990;152:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Hanbidge AE, Lynch D, Wilson SR. US of the peritoneum. Radiographics. 2003;23:663-684; discussion 684-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Costamagna G, Tringali A, Spicak J, Mutignani M, Shaw J, Roy A, Johnsson E, De Moura EG, Cheng S, Ponchon T. Treatment of malignant gastroduodenal obstruction with a nitinol self-expanding metal stent: an international prospective multicentre registry. Dig Liver Dis. 2012;44:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kim JW, Jeong JB, Lee KL, Kim BG, Ahn DW, Lee JK, Kim SH. Comparison between uncovered and covered self-expandable metal stent placement in malignant duodenal obstruction. World J Gastroenterol. 2015;21:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Waidmann O, Trojan J, Friedrich-Rust M, Sarrazin C, Bechstein WO, Ulrich F, Zeuzem S, Albert JG. SEMS vs cSEMS in duodenal and small bowel obstruction: high risk of migration in the covered stent group. World J Gastroenterol. 2013;19:6199-6206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Phillips MS, Gosain S, Bonatti H, Friel CM, Ellen K, Northup PG, Kahaleh M. Enteral stents for malignancy: a report of 46 consecutive cases over 10 years, with critical review of complications. J Gastrointest Surg. 2008;12:2045-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | van Hooft JE, Dijkgraaf MG, Timmer R, Siersema PD, Fockens P. Independent predictors of survival in patients with incurable malignant gastric outlet obstruction: a multicenter prospective observational study. Scand J Gastroenterol. 2010;45:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Tringali A, Didden P, Repici A, Spaander M, Bourke MJ, Williams SJ, Spicak J, Drastich P, Mutignani M, Perri V. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc. 2014;79:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (18)] |