Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9427
Peer-review started: July 1, 2016
First decision: August 8, 2016
Revised: August 18, 2016
Accepted: September 6, 2016
Article in press: September 6, 2016
Published online: November 14, 2016
Processing time: 135 Days and 0.2 Hours
To clarify the prevalence of occult hepatitis B virus (HBV) infection (OBI) and the association between OBI and liver disease progression, defined as development of liver cirrhosis or hepatocellular carcinoma (HCC), worsening of Child-Pugh class, or mortality in cases of chronic hepatitis C virus (HCV) infection.
This prospective cohort study enrolled 174 patients with chronic HCV infection (chronic hepatitis, n = 83; cirrhosis, n = 47; HCC, n = 44), and evaluated disease progression during a mean follow-up of 38.7 mo. OBI was defined as HBV DNA positivity in 2 or more different viral genomic regions by nested polymerase chain reaction using 4 sets of primers in the S, C, P and X open reading frame of the HBV genome.
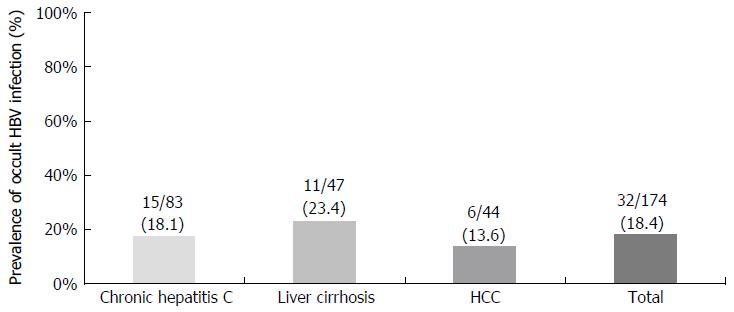
The overall OBI prevalence in chronic HCV patients at enrollment was 18.4%, with 16.9%, 25.5% and 13.6% in the chronic hepatitis C, liver cirrhosis and HCC groups, respectively (P = 0.845). During follow-up, 52 patients showed disease progression, which was independently associated with aspartate aminotransferase > 40 IU/L, Child-Pugh score and sustained virologic response (SVR), but not with OBI positivity. In 136 patients who were not in the SVR state during the study period, OBI positivity was associated with neither disease progression, nor HCC development.
The prevalence of OBI in chronic HCV patients was 18.4%, and OBI was not associated with disease progression in South Koreans.
Core tip: Whether occult hepatitis B virus (HBV) infection affects the outcomes of chronic hepatitis C virus infection is controversial. This prospective observational study aimed to clarify the association between occult HBV infection and liver disease progression defined as development of liver cirrhosis, worsening of Child-Pugh class, hepatocellular carcinoma or mortality in patients with chronic hepatitis C infection in South Korea.
- Citation: Cho J, Lee SS, Choi YS, Jeon Y, Chung JW, Baeg JY, Si WK, Jang ES, Kim JW, Jeong SH. Occult hepatitis B virus infection is not associated with disease progression of chronic hepatitis C virus infection. World J Gastroenterol 2016; 22(42): 9427-9436
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9427
Prevalence of hepatitis C virus (HCV) infection involves about 3% of the global population, or approximately 170 million people[1]. Chronic HCV infection can lead to liver cirrhosis or hepatocellular carcinoma (HCC) in 20%-30% of cases[2-4]. The HCV life cycle occurs exclusively in the cell cytoplasm, and the HCV RNA genome does not integrate into the host genome[5,6]. Therefore, the pathogenic mechanisms of HCV include complex interaction of HCV proteins and host proteins, inducing chronic inflammation, inhibition of apoptosis, stimulation of cell proliferation and fibrosis, leading to genetic alteration of hepatocytes, liver cirrhosis and ultimately HCC[7]. In contrast, hepatitis B virus (HBV) has a direct oncogenic effect by integration of HBV DNA into the host genome, causing insertional mutagenesis, in addition to the indirect effects by HBV protein-host interaction.
HCV and HBV have similar transmission routes, and coinfection with HCV and HBV can increase the risk of HCC, compared to HCV monoinfection[8]. Even if serum hepatitis B surface antigen (HBsAg) is negative, HBV DNA can exist in the liver or blood of people in an occult HBV infection (OBI) state. The pathogenic role of OBI in the development of cirrhosis or HCC among patients with chronic HCV infection is still extremely controversial[9-12]. Moreover, the prevalence of OBI in chronic HCV-infected patients shows a wide range of variation in different global regions[13,14]. There are no data on the prevalence or effect of OBI in chronic HCV infection in South Korea, an HBV endemic region.
This study aimed to clarify the prevalence of OBI in the blood of patients with chronic HCV infection, and to estimate the association between OBI and liver disease progression defined as development of liver cirrhosis, decompensation (worsening of Child-Pugh class), HCC or mortality among the subjects by prospective observation. We also investigated the HBV genotype on the HBsAg-coding open reading frame of HBV gene (S-ORF) in OBI-positive patients.
This prospective cohort study included 174 consecutively enrolled patients with HBsAg-negative, chronic HCV infection in Seoul National University Bundang Hospital between November 2005 and May 2014. Chronic HCV infection was defined as HCV RNA positivity > 6 mo with HBsAg negativity. Among them, 83 patients were given diagnoses of chronic hepatitis C, 47 patients of liver cirrhosis, and 44 patients of HCC. The diagnostic criteria for liver cirrhosis included the presence of portal hypertension manifested as splenomegaly, thrombocytopenia < 100000/mm3, ascites, varices, or hepatic encephalopathy and imaging findings compatible with liver cirrhosis. The diagnosis of HCC was based on histological examination or typical radiographic image findings consisting of arterial enhancement and venous wash-out of hepatic nodules on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI)[15]. HBsAg-positive patients or human immunodeficiency virus coinfected patients were excluded. Informed consent was obtained from all patients, and the study protocol was approved by the Institutional Review Board of the hospital.
At enrollment, data on the demographics, socioeconomic status, alcohol consumption, and smoking habits were obtained using a standard questionnaire. In addition, laboratory tests, ultrasonography or CT examination of the liver was performed for all patients at baseline. Those collected data were entered into the electronic case report form at the Korean HCV cohort study group homepage (available from URL: http://www.hcvcohort.or.kr/).
All patients were monitored for clinical status and given laboratory tests and imaging examinations, including ultrasonography, CT or MRI, every 3-12 mo. A total of 72 patients were treated with Pegylated interferon alpha (peg-IFNα) and ribavirin for 24-48 wk according to HCV genotype before the study enrollment (n = 24) and during the study period (n = 48). Survival and mortality, including cause of death, were confirmed using examination of the final medical records, telephone calls to participants or their family members, and death certificate data obtained from the Korean Statistical Information Service[16]. The disease progression was defined as: (1) occurrence of liver cirrhosis, HCC, decompensation (worsening of Child-Pugh class), or mortality in patients with chronic hepatitis; (2) occurrence of HCC, decompensation, or mortality in patients with cirrhosis; and (3) occurrence of mortality in patients with HCC. Time to disease progression was defined as the interval between the date of enrollment and the date of occurrence of HCC, liver cirrhosis, worsening of Child Pugh class, death, last observation, or September 30, 2015.
Serum or plasma samples were obtained from 174 patients and stored at -70 °C. HBV DNA was extracted from 400 μL of serum or plasma using a QIAamp DNA Blood Mini Kit, (Qiagen Inc., Hilden, Germany) according to the manufacturer’s instructions. DNA was eluted from the spin column in 50 μL of elution buffer. Nested polymerase chain reaction (PCR) was conducted with AccuPower HotStart PCR PreMix (Catalog No. k-5051; Bioneer Inc., Seoul, Republic of Korea) using 4 sets of primers to detect S, C, P and X regions of the HBV genome (Supplementary Table 1). According to the Taormina Expert Meeting Statements[17], the presence of OBI was defined as proved positivity in 2 or more different viral genomic regions by nested PCR. AM6 plasmid purchased from the Korea Cell Line Bank (positive control) and serum obtained from normal healthy persons or distilled water (negative control) was used. The first round of PCR was performed in a final volume of 20 μL at 94 °C for 2 min followed by 35 cycles of 94 °C for 30 s, 52-65 °C for 30 s (52 °C for S, 58 °C for C, 58 °C for P, 65 °C for X) and 72 °C for 30 s, and a final extension step of 72 °C for 5 min. Using 5 μL of the first PCR product, the second round PCR was performed under the same conditions as the first round of PCR except for the annealing step temperature (58 °C for S, 52 °C for C, 52 °C for P, 65 °C for X). By nested PCR with sets of 10-fold serially diluted AM6 templates, the detection limit was estimated as 54.5 copies/μL of AM6 plasmid.
| Variable | Total (n = 174) | Chronic hepatitis (n = 83) | Liver cirrhosis (n = 47) | HCC (n = 44) |
| Mean age, yrbc | 66.5 ± 9.9 | 65.6 ± 9.8 | 65.3 ± 10.1 | 69.7 ± 9.3 |
| Male sex | 105 (60.3) | 54 (65.1) | 24 (51.1) | 27 (61.4) |
| Body mass index (kg/m2) | 23.3 ± 3.0 | 23.6 ± 3.0 | 23.0 ± 3.2 | 23.0 ± 2.8 |
| Ex or current smoker (n = 173) | 51 (29.5) | 26 (31.3) | 10 (21.7) | 15 (34.1) |
| Alcohol intake (social or heavy) (n = 173) | 80 (46.2) | 42 (50.6) | 17 (37.0) | 21 (47.8) |
| Anti-HBc (n = 100) | 75 (75.0) | 35 (71.4) | 18 (85.7) | 22 (73.3) |
| Hemoglobin (g/dL)c | 13.5 ± 1.8 | 14.1 ± 1.5 | 13.5 ± 1.9 | 12.4 ± 1.9 |
| Platelet (× 103/μL)ab | 151.3 ± 87.4 | 188.0 ± 55.7 | 117.5 ± 120.2 | 118.3 ± 66.2 |
| Albumin (g/dL)ab | 4.0 ± 0.5 | 4.2 ± 0.2 | 3.8 ± 0.5 | 3.7 ± 0.5 |
| Total bilirubin (mg/dL)a | 1.0 ± 0.6 | 1.0 ± 1.7 | 1.2 ± 0.6 | 1.1 ± 0.8 |
| ALP (IU/L)ab | 100.3 ± 47.3 | 89.2 ± 30.9 | 102.0 ± 37.0 | 119.3 ± 71.4 |
| AST (IU/L)ab | 75.8 ± 103.3 | 76.0 ± 141.9 | 72.6 ± 47.8 | 78.8 ± 45.3 |
| ALT (IU/L) | 77.2 ± 138.5 | 89.7 ± 193.2 | 68.0 ± 59.3 | 63.3 ± 41.0 |
| Creatinine (mg/dL)a | 0.9 ± 0.4 | 1.0 ± 0.5 | 0.8 ± 0.2 | 0.9 ± 0.2 |
| Prothrombin time (INR)ab | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| AFP > 20 ng/mL (n = 171)ab | 30 (17.5) | 5 (6.2) | 9 (19.6) | 16 (36.4) |
| Child-Pugh class (A/B/C)ab | ||||
| A | 160 (92.0) | 83 (100) | 40 (85.1) | 37 (84.1) |
| B | 12 (6.9) | 0 | 5 (10.6) | 7 (15.9) |
| C | 2 (1.1) | 0 | 2 (4.3) | 0 |
| MELD score (n = 167)ab | 8.5 ± 2.3 | 8.0 ± 2.3 | 9.3 ± 2.2 | 8.9 ± 2.1 |
| HCV genotype (1/2) (n = 135) | 60/75 | 32/35 | 16/26 | 12/14 (46.2%/53.8%) |
| (44.4%/55.6%) | (47.8%/52.2%) | (38.1%/61.9%) | ||
| Antiviral treatment | ||||
| No antiviral treatment | 102 (58.7) | 45 (54.2) | 25 (53.2) | 32 (72.7) |
| Treatment without SVR | 34 (19.5) | 14 (16.9) | 11 (23.4) | 9 (20.5) |
| Treatment with SVRbc | 38 (21.8) | 24 (28.9) | 11 (23.4) | 3 (6.8) |
For 5 OBI-positive samples, the entire S region (the preS1, preS2 and S region) of the HBV genome was amplified by nested PCR using different sets of primers. The PCR products were purified and sequenced to identify the HBV genotype. The condition for the first and second round of PCR was 95 °C for 5 min, followed by 30 cycles of 95 °C 60 s, 52 °C 45 s, and 72 °C 90 s with a final extension of 72 °C for 5 min. The PCR products were analyzed by electrophoresis on 1% agarose gels, which were stained with Gel Red, and visualized on a UV transilluminator. The PCR products were sequenced by a commercial sequencing company (Bioneer Inc.). HBV DNA sequences were aligned using Clustal W (http://www.clustal.org), and phylogenetic trees were constructed using the neighbor-joining method. Bootstrap values are indicated for the major nodes as a percentage of the data obtained from 1000 re-samplings. MEGA version 6.0.6 was used for phylogenetic tree construction and mutation analysis. We determined HBV genotypes by phylogenetic analysis based on 14 reference strains obtained from GenBank (accession numbers AY641558.1, AF286594.1, AY247032.1, AY641559.1, D16667.1, D50519.1, AF305422.1, M57663.2, X70185.1, AB100695.1, D00329.1, AB074755.1, X02496.1, AB554024.1).
Categorical variables were compared with the chi-square test or 2-tailed Fisher’s exact test, and continuous variables were compared with the Mann-Whitney test or Kruskal-Wallis test. The cumulative probabilities of disease progression and HCC were analyzed using the Kaplan-Meier method. Predictors associated with disease progression and HCC were determined by the Cox proportional regression model. Risk was expressed by hazard ratio and 95%CI. A P value of < 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using PASW software (version 22; IBM SPSS Statistics for Windows, Armonk, NY, United States).
The baseline characteristics of the 174 study patients with chronic HCV infection are summarized in Table 1, showing the mean age to be 66.5 years, male sex for 60.3%, and past or current alcohol users of 46.2%. They included 83 patients with chronic hepatitis, 47 patients with liver cirrhosis and 44 patients with HCC. The HCV genotype 1 and 2 were detected in 60 patients (44.4%) and 75 patients (55.6%), respectively. Antiviral therapy with peg-IFNα and ribavirin combination regimen was undertaken in 72 patients before enrollment or during follow-up, and overall sustained virologic response (SVR) rate (defined as undetectable serum HCV RNA at 24 wk after the end of the treatment) was 52.8%. The anti-hepatitis B core immunoglobulin G test was performed in 100 patients, for which 75 patients showed positive results.
The positive detection rate of plasma or serum HBV DNA was 18.4% (32 among 174 total patients) defined as at least 2 positive results on nested PCR among 4 different sets covering 4 ORFs of HBV genomes. The positive rate was not different among 3 different diagnostic categories at enrollment: 14 of 83 (16.9%) in chronic hepatitis C, 12 of 47 (25.5%) in liver cirrhosis, and 6 of 44 (13.6%) in HCC (Figure 1). Therefore, the prevalence of OBI did not parallel the severity of liver disease at study enrollment.
To investigate the clinical factors that might be associated with OBI positivity, we compared various variables including age, sex, body mass index, laboratory results, model for end-stage liver disease score, Child-Pugh score and HCV genotypes between the patients with and without OBI. However, there were no significant differences between OBI-positive and OBI-negative patients, as shown in Table 2.
| Variable | OBI (+) (n = 32) | OBI (-) (n = 142) | P value |
| Mean age, yr | 67.1 ± 9.6 | 66.4 ± 9.9 | 0.952 |
| Male sex | 19 (59.4) | 86 (60.6) | 0.527 |
| Body mass index (kg/m2) | 23.9 ± 3.2 | 23.2 ± 3.0 | 0.180 |
| Anti-HBc (n = 100) | 6 (66.7) | 69 (75.8) | 0.399 |
| Anti-HBs (n = 157) | 14 (48.3) | 72 (56.3) | 0.283 |
| Hemoglobin (g/mL) | 13.3 ± 1.3 | 13.5 ± 1.9 | 0.364 |
| Platelet (× 103/mL) | 150.5 ± 79.5 | 151.5 ± 89.3 | 0.978 |
| Albumin (g/dL) | 3.9 ± 0.5 | 4.0 ± 0.4 | 0.593 |
| Total bilirubin (mg/dL) | 1.1 ± 0.8 | 1.0 ± 0.5 | 0.118 |
| AST (IU/L) | 62.7 ± 40.3 | 78.8 ± 112.6 | 0.546 |
| ALT (IU/L) | 54.7 ± 43.5 | 82.2 ± 151.6 | 0.148 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.4 | 0.779 |
| PT-INR | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.459 |
| MELD score | 8.3 ± 1.9 | 8.6 ± 2.4 | 0.744 |
| HCV genotype (1/2) | 12/15 (44.4%/55.6%) | 48/60 (44.4%/55.6%) | 0.587 |
| AFP > 20 ng/mL | 6 (19.4) | 23 (16.5) | 0.441 |
| Child-Pugh class | 0.436 | ||
| A | 28 (87.5) | 132 (93.0) | |
| B | 4 (12.5) | 8 (5.6) | |
| C | 0 | 2 (1.3) | |
| Antiviral treatment | |||
| No antiviral treatment | 21 (65.6) | 81 (57.0) | 0.430 |
| Treatment without SVR | 5 (19.2) | 29 (26.4) | 0.616 |
| Treatment with SVR | 6 (18.8) | 32 (22.5) | 0.814 |
| Disease progression | 7 (21.9) | 45 (31.7) | 0.392 |
| Development of HCC | 3 (11.5) | 11 (10.6) | 1.000 |
| Follow-up period (mo) | 42.5 ± 34.7 | 36.3 ± 26.9 | 0.555 |
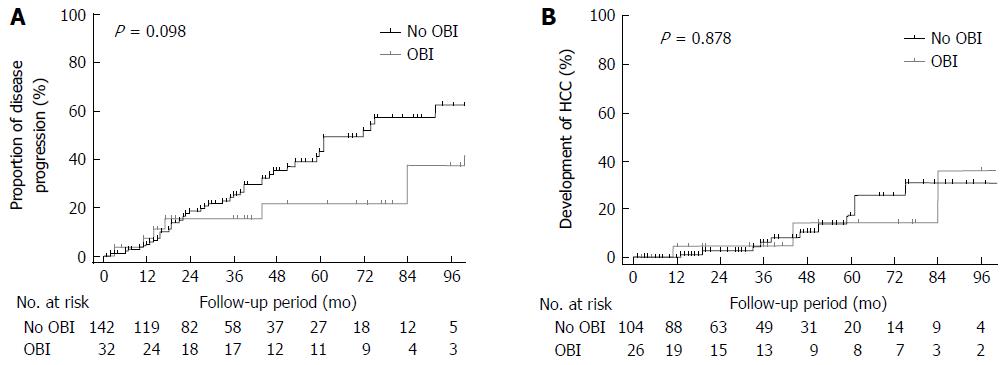
During the mean follow-up duration of 37.4 mo, 52 patients showed composite disease progression: 12 patients developed liver cirrhosis from chronic hepatitis; 13 patients developed decompensated cirrhosis (worsening of Child-Pugh class); 14 patients developed HCC; and 13 patients died. As shown in Table 3, OBI positivity was not a significant factor associated with disease progression in either univariate or multivariate analysis. However, AST > 40 IU/L, increased Child-Pugh score at enrollment, and SVR were significantly associated with disease progression in multivariate analysis. Moreover, there was no significant difference in development of disease progression between the patients with or without OBI (Figure 2A).
| Variable | HR | P value | Adjusted HR | P value |
| OBI | 0.494 (0.210-1.164) | 0.107 | 0.510 (0.208-1.251) | 0.141 |
| Age (per year) | 1.020 (0.990-1.050) | 0.194 | ||
| Male sex | 0.935 (0.534-1.636) | 0.814 | ||
| Anti-HBc positivity | 2.291 (0.878-5.976) | 0.090 | ||
| AST > 40 IU/L | 3.730 (1.740-7.996) | 0.001 | 3.419 (1.117-10.463) | 0.031 |
| ALT > 40 IU/L | 2.454 (1.273-4.730) | 0.007 | 0.737 (0.297-1.826) | 0.510 |
| GGT > 70 IU/L | 2.736 (1.571-4.765) | < 0.001 | ||
| AFP > 20 ng/mL | 2.247 (1.238-4.079) | 0.008 | 1.370 (0.721-2.600) | 0.336 |
| Child-Pugh score | 2.136 (1.628-2.802) | < 0.001 | 1.716 (1.230-2.394) | 0.001 |
| (per unit) | ||||
| MELD score | 1.111 (1.009-1.224) | 0.032 | 0.987 (0.860-1.134) | 0.856 |
| (per unit) | ||||
| SVR | 0.263 (0.104-0.663) | 0.005 | 0.317 (0.121-0.828) | 0.019 |
| HCV genotype 1 | 1.465 (0.780-2.753) | 0.231 |
Of the 130 patients without HCC at enrollment, there was no significant difference in HCC development between the patients with or without OBI (Figure 2B). On multivariate analysis, Child-Pugh score was an independent factor for HCC development (Table 4).
| Variable | HR | P value | Adjusted HR | P value |
| OBI | 0.904 (0.251-3.264) | 0.878 | 0.860 (0.209-3.535) | 0.835 |
| Age (per year) | 1.016 (0.962-1.073) | 0.571 | ||
| Male sex | 1.531 (0.536-4.374) | 0.427 | ||
| AST > 40 IU/L | 6.120 (1.351-27.729) | 0.019 | 3.383 (0.664-17.226) | 0.142 |
| ALT > 40 IU/L | 3.573 (0.976-13.075) | 0.054 | ||
| Creatinine (per unit) | 0.051 (0.003-0.892) | 0.042 | 0.075 (0.003-2.200) | 0.133 |
| AFP > 20 ng/mL | 3.381 (1.129-10.121) | 0.029 | 1.706 (0.512-5.678) | 0.384 |
| PT-INR (per unit) | 10.081 (1.036-77.802) | 0.027 | ||
| Child-Pugh score | 3.065 (1.741-5.399) | < 0.001 | 2.818 (1.547-5.135) | 0.001 |
| (per unit) | ||||
| MELD score | 1.057 (0.862-1.296) | 0.597 | ||
| (per unit) | ||||
| SVR | 0.289 (0.064-1.302) | 0.106 | ||
| HCV genotype 1 | 2.049 (0.644-6.517) | 0.224 |
After exclusion of 34 patients who achieved SVR during the study period, OBI was not a significant factor associated with either disease progression, nor HCC development (Supplementary Figure 1).
Anti-HBs and anti-HBc were both evaluated in 87 patients. OBI positivity was 2.9% (1/34) in anti-HBc (+) and anti-HBs (+) patients, 9.4% (3/32) in anti-HBc (+) and anti-HBs (-) patients, 11.1% in anti-HBc (-) and anti-HBs (+) patients, and 15.4% in anti-HBc (-) and anti-HBs (-) patients. Therefore, positivity for anti-HBc alone did not represent OBI positivity.
In the 32 OBI-positive patients, 5 samples were available for full S-ORF sequence analysis including preS1, preS2 and S gene. The phylogenetic analysis showed that all 5 samples were HBV genotype C2, which was same as the HBV genotype reported in almost all patients with chronic HBV infection in South Korea.
In this study, the prevalence of OBI was 18.4% in the blood of HBsAg-negative patients with chronic HCV infection in South Korea by nested PCR, in whom OBI prevalence was not higher in the HCC group (13.6%) than in the chronic hepatitis group (18.1%) or liver cirrhosis group (23.4%). Moreover, neither positive OBI nor presence of anti-HBc was significantly associated with disease progression or HCC development on multivariate analysis. The HBV genotype detected in all 5 OBI-positive patients was genotype C2, which was the same as almost all of the detected genotypes in chronic hepatitis B patients in Korea.
The prevalence of OBI in the liver tissue or serum of anti-HCV-positive patients has been reported variously, both in prospective and retrospective studies. The prevalence of OBI detected in liver tissue was reported as 38.8% in 326 Italian patients with chronic HCV infection[18], 57% in 100 Portuguese patients[19], and 15.0% in 167 Japanese patients as determined by nested PCR[13], and 50% in 44 patients with HCV-related advanced cirrhosis in the United States as determined by real-time PCR[20].
The prevalence of OBI in serum/plasma in HCV-related liver disease patients was 5.6% (8/141)[21], 7.8% (11/140)[22], and 5.2% (9/173) as determined by real-time PCR in Japan[23], but it was 43.6% (204/468) in one Japanese study that using nested PCR with only one set of primers covering the X region[24]. The OBI prevalence in chronic HCV patients was 45.7% (42/92) in Morocco[25] and 20% (18/50) in Iran[26], while none of 100 Portuguese patients showed serum OBI[19]. A retrospective study in Taiwan showed that serum OBI prevalence as determined by nested PCR using 3 sets of primers in patients with chronic HCV infection was 14.8% (31 of 210), which did not differ from that of healthy controls (15%, 15/100), and the prevalence of OBI did not parallel the severity of liver disease (14.5% in chronic hepatitis, 8% in liver cirrhosis, and 22% in HCC)[27]. In the present study, the prevalence of OBI in plasma was 18.4% (32 of 174), which did not increase with the severity of liver disease, showing similar results to the Taiwan study consisting of the subjects and detection methods similar to this study.
The variously reported OBI prevalence in patients with chronic HCV infection may be related to the different study subjects, different materials used (liver tissue vs serum), or differences in PCR technology such as nested PCR or real-time PCR using different numbers of primer sets. Our method followed the Taormina Expert Meeting Statements[17], in which the presence of OBI was defined as proved positivity in 2 or more different viral genomic regions by nested PCR and included adequate positive and negative controls. Previous data suggested that the prevalences of OBI detected in liver tissue were higher than those in blood, though reports of head-to-head comparisons of both methodologies are limited[19,28]. Integration of the HBV genome into the host genome is a plausible explanation. In addition, risk factors of HCV infection may affect the prevalence of OBI. As shown in the above United States study, a history of intravenous drug abuse (IVDU) was found in 66% of the subjects, as opposed to 1% of our study population. Because HBV and HCV can share the parenteral transmission route, repeated exposure to HBV or HCV during IVDU may result in relatively high frequency of OBI in those subjects rather than those who were infected through perinatal infection as in Korea.
This study showed that OBI prevalence did not increase according to the severity of liver disease (n = 174), and during a mean prospective follow-up of 38 mo, OBI was not associated with either HCC development or the overall disease progression. However, SVR, Child-Pugh score and AST level were independent factors associated with the disease progression. Even in the patients who did not receive treatment or did not achieve SVR (n = 136), OBI positivity was not associated with either HCC development or disease progression.
The role of OBI in disease progression or development of HCC in patients with chronic HCV infection is still a matter of considerable controversy. Some studies have reported that OBI may contribute to the development of HCC or cirrhosis in chronic HCV-infected patients[9,10,18,21,23,24,29]. In contrast, other reports have shown that OBI is not an important factor in the progression to HCC or cirrhosis[11,12,14,27,28,30,31]. Most previous studies were cross-sectional studies[12,16,18,24], and a meta-analysis including both prospective and retrospective studies reported that OBI contributed to the development of HCC[32]. A prospective study in Italy showed that among 94 patients who were tested for liver OBI and followed for a median 11 years, HCC developed more often in the OBI-positive group (13/37) than in the OBI-negative group (5/57), and OBI-positive patients had shorter cumulative survival rate than OBI-negative patients. Though this study suggests that OBI may lead to HCC development and lower survival, only 94 among a total 326 original study group were followed (follow-up missing rate of 71.2%) and 79 out of 94 patients underwent antiviral treatment with an SVR rate of 33% (26/79). Considering that SVR is a strong independent factor for HCC development or survival, more studies are needed to clarify the significance of OBI in HCV-related liver disease progression.
The possible mechanisms of OBI involvement in hepatocarcinogenesis were HBV-induced accelerated inflammation, HBV genome integration in the host DNA, or promoting effect of HBV proteins in malignant transformation. In a woodchuck model, persistently low level of viremia in liver tissue in the absence of woodchuck HBsAg can lead to liver injury and HCC development[33]. However, there has been no clear evidence supporting the hepatocarcinogenic role of OBI in HCV-related liver disease. Two recently reported independent in vitro studies showed that both HBV and HCV can replicate in the same hepatocyte cells without evidence of viral interference. Therefore, HCV and HBV may interfere with each other by indirect effects of host-viral interactions or host immune response.
Moreover, the epidemiology of HCV or HBV is different according to geographic regions or population. For example, the most common mode of transmission of HBV in Korean people has been perinatal transmission, while HCV infection occurs in later life. In contrast, IVDUs in an HBV non-endemic area may be infected by both viruses simultaneously or repeatedly. Those differences in epidemiology may lead to differences in immunological response to OBI in HCV-infected patients in different regions. In this study, a phylogenetic analysis showed that 5 strains from the occult HBV-infected subjects were all genotype C2, which was detected in nearly 100% of chronic hepatitis B patients in Korea. Because of sample availability, only 5 samples among the 32 patients with OBI were evaluated. Several mechanisms have been considered for occult infections by HBV, such as low HBV DNA and HBsAg levels, mutations in HBV DNA sequence, viral DNA integration in the host genome, infection of peripheral blood mononuclear cells, production of immune complexes containing HBV, altered host immune response, and interference of HCV[34-37].
The present study had several limitations, including being a single center study with relatively small sample size, no availability of liver tissue and no results on quantitative evaluation of HBV DNA.
In conclusion, this study demonstrates that the prevalence of OBI in blood in patients with chronic HCV infection in South Korea was 18.4%, with no significant correlation between OBI positivity and disease progression or HCC. Controversy still exists regarding the role of OBI; therefore, well-designed prospective multicenter studies and experimental studies are warranted.
The authors are indebted to J Patrick Barron, Professor Emeritus, Tokyo Medical University and Adjunct Professor, Seoul National University Bundang Hospital for his editing of this manuscript. The authors are grateful to the devoted research coordinators, Daseul Lee, DaWoonJeong and Hyein Mann from Seoul National University Bundang Hospital. Their active collaboration made it possible to carry out the study.
Whether the occult hepatitis B virus (HBV) infection (OBI) affects the outcomes of chronic hepatitis C virus (HCV) infection is controversial. This study aimed to clarify the prevalence of OBI and the association between OBI and liver disease progression in cases of chronic HCV infection.
The prevalence of OBI in chronic HCV-infected patients has been reported variously in different global regions. The pathogenic role of OBI in the disease progression or development of hepatocellular carcinoma (HCC) in patients with chronic HCV infection is still a matter of considerable controversy. There are no data on the prevalence or effect of OBI in chronic HCV infection in Korea, an HBV endemic region.
The positive detection rate of plasma or serum HBV DNA was 18.4% (32 among 174 patients), defined as at least 2 positive results on nested polymerase chain reaction (PCR) using 4 sets of primers in the S, C, P and X open reading frame of the HBV genome. However, OBI positivity was not a significant factor associated with disease progression. In addition, there was no significant difference in HCC development between the patients with or without OBI.
This study demonstrates that the prevalence of OBI in patients with chronic HCV infection in Korea was 18.4%, with no significant correlation between OBI positivity and disease progression or HCC. Controversy still exists regarding the role of OBI; therefore, well-designed prospective multicenter studies and experimental studies are warranted.
According to the Taormina Expert Meeting Statements, the presence of OBI was defined as proved positivity in 2 or more different viral genomic regions by nested PCR using 4 sets of primers to detect the S, C, P and X regions of the HBV genome. The disease progression was defined as: (1) occurrence of liver cirrhosis, HCC, decompensation (worsening of Child-Pugh class), or mortality in patients with chronic hepatitis; (2) occurrence of HCC, decompensation, or mortality in patients with cirrhosis; and (3) occurrence of mortality in patients with HCC.
This observational study represents an unbiased and well-articulated report on the usefulness of OBI measurement as a prognostic factor for liver disease progression.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Federico L S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Memon MI, Memon MA. Hepatitis C: an epidemiological review. J Viral Hepat. 2002;9:84-100. [PubMed] |
| 2. | van der Poel CL, Cuypers HT, Reesink HW. Hepatitis C virus six years on. Lancet. 1994;344:1475-1479. [PubMed] |
| 3. | Di Bisceglie AM, Goodman ZD, Ishak KG, Hoofnagle JH, Melpolder JJ, Alter HJ. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969-974. [PubMed] |
| 4. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [PubMed] |
| 5. | Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 498] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Fong TL, Shindo M, Feinstone SM, Hoofnagle JH, Di Bisceglie AM. Detection of replicative intermediates of hepatitis C viral RNA in liver and serum of patients with chronic hepatitis C. J Clin Invest. 1991;88:1058-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Kruse RL, Kramer JR, Tyson GL, Duan Z, Chen L, El-Serag HB, Kanwal F. Clinical outcomes of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology. 2014;60:1871-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 475] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [PubMed] |
| 11. | Lok AS, Everhart JE, Di Bisceglie AM, Kim HY, Hussain M, Morgan TR. Occult and previous hepatitis B virus infection are not associated with hepatocellular carcinoma in United States patients with chronic hepatitis C. Hepatology. 2011;54:434-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Jang JY, Jeong SW, Cheon SR, Lee SH, Kim SG, Cheon YK, Kim YS, Cho YD, Kim HS, Jin SY. Clinical significance of occult hepatitis B virus infection in chronic hepatitis C patients. Korean J Hepatol. 2011;17:206-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Obika M, Shinji T, Fujioka S, Terada R, Ryuko H, Lwin AA, Shiraha H, Koide N. Hepatitis B virus DNA in liver tissue and risk for hepatocarcinogenesis in patients with hepatitis C virus-related chronic liver disease. A prospective study. Intervirology. 2008;51:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Adachi S, Shibuya A, Miura Y, Takeuchi A, Nakazawa T, Saigenji K. Impact of occult hepatitis B virus infection and prior hepatitis B virus infection on development of hepatocellular carcinoma in patients with liver cirrhosis due to hepatitis C virus. Scand J Gastroenterol. 2008;43:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kanamori A. Prevalence of low-level hepatitis B viremia in patients with HBV surface antigen-negative hepatocellular carcinoma with and without hepatitis C virus infection in Japan: analysis by COBAS TaqMan real-time PCR. Intervirology. 2007;50:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Statics Korea. Korean Statistical Information Service. Available from: http://www.kosis.kr/ 2015. |
| 17. | Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 18. | Squadrito G, Pollicino T, Cacciola I, Caccamo G, Villari D, La Masa T, Restuccia T, Cucinotta E, Scisca C, Magazzu D. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer. 2006;106:1326-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Cardoso C, Alves AL, Augusto F, Freire R, Quintana C, Gonçalves M, Oliveira AP. Occult hepatitis B infection in Portuguese patients with chronic hepatitis C liver disease: prevalence and clinical significance. Eur J Gastroenterol Hepatol. 2013;25:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Shetty K, Hussain M, Nei L, Reddy KR, Lok AS. Prevalence and significance of occult hepatitis B in a liver transplant population with chronic hepatitis C. Liver Transpl. 2008;14:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Miura Y, Shibuya A, Adachi S, Takeuchi A, Tsuchihashi T, Nakazawa T, Saigenji K. Occult hepatitis B virus infection as a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C in whom viral eradication fails. Hepatol Res. 2008;38:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Hasegawa I, Orito E, Tanaka Y, Hirashima N, Sakakibara K, Sakurai M, Suzuki S, Sugauchi F, Ohno T, Ueda R. Impact of occult hepatitis B virus infection on efficacy and prognosis of interferon-alpha therapy for patients with chronic hepatitis C. Liver Int. 2005;25:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Nakano M, Kawaguchi T, Nakamoto S, Kawaguchi A, Kanda T, Imazeki F, Kuromatsu R, Sumie S, Satani M, Yamada S. Effect of occult hepatitis B virus infection on the early-onset of hepatocellular carcinoma in patients with hepatitis C virus infection. Oncol Rep. 2013;30:2049-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Kitab B, Ezzikouri S, Alaoui R, Nadir S, Badre W, Trepo C, Chemin I, Benjelloun S. Occult HBV infection in Morocco: from chronic hepatitis to hepatocellular carcinoma. Liver Int. 2014;34:e144-e150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Vakili Ghartavol Z, Alavian SM, Amini S, Vahabpour R, Bahramali G, Mostafavi E, Aghasadeghi MR. Prevalence of occult hepatitis B virus in plasma and peripheral blood mononuclear cell compartments of patients with chronic hepatitis C infection in tehran-iran. Hepat Mon. 2013;13:e10134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40:4068-4071. [PubMed] |
| 28. | Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 421] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 29. | Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Filippini P, Piccinino F. HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol. 2001;64:350-355. [PubMed] |
| 30. | Hui CK, Lau E, Wu H, Monto A, Kim M, Luk JM, Lau GK, Wright TL. Fibrosis progression in chronic hepatitis C patients with occult hepatitis B co-infection. J Clin Virol. 2006;35:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Fabris P, Brown D, Tositti G, Bozzola L, Giordani MT, Bevilacqua P, de Lalla F, Webster GJ, Dusheiko G. Occult hepatitis B virus infection does not affect liver histology or response to therapy with interferon alpha and ribavirin in intravenous drug users with chronic hepatitis C. J Clin Virol. 2004;29:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Shi Y, Wu YH, Wu W, Zhang WJ, Yang J, Chen Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int. 2012;32:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Michalak TI, Pardoe IU, Coffin CS, Churchill ND, Freake DS, Smith P, Trelegan CL. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Alencar RS, Gomes MM, Sitnik R, Pinho JR, Malta FM, Mello IM, Mello ES, Bacchella T, Machado MC, Alves VA. Low occurrence of occult hepatitis B virus infection and high frequency of hepatitis C virus genotype 3 in hepatocellular carcinoma in Brazil. Braz J Med Biol Res. 2008;41:235-240. [PubMed] |
| 35. | Kim H, Lee SA, Kim DW, Lee SH, Kim BJ. Naturally occurring mutations in large surface genes related to occult infection of hepatitis B virus genotype C. PLoS One. 2013;8:e54486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Kwei K, Tang X, Lok AS, Sureau C, Garcia T, Li J, Wands J, Tong S. Impaired virion secretion by hepatitis B virus immune escape mutants and its rescue by wild-type envelope proteins or a second-site mutation. J Virol. 2013;87:2352-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Kim H, Lee SA, Won YS, Lee H, Kim BJ. Occult infection related hepatitis B surface antigen variants showing lowered secretion capacity. World J Gastroenterol. 2015;21:1794-1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |