Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9368
Peer-review started: June 27, 2016
First decision: July 29, 2016
Revised: August 13, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: November 14, 2016
Processing time: 138 Days and 14.6 Hours
To investigate the role of interferon regulatory factor 5 (IRF5) in reversing polarization of lung macrophages during severe acute pancreatitis (SAP) in vitro.
A mouse SAP model was established by intraperitoneal (ip) injections of 20 μg/kg body weight caerulein. Pathological changes in the lung were observed by hematoxylin and eosin staining. Lung macrophages were isolated from bronchoalveolar lavage fluid. The quantity and purity of lung macrophages were detected by fluorescence-activated cell sorting and evaluated by real-time polymerase chain reaction (RT-PCR). They were treated with IL-4/IRF5 specific siRNA (IRF5 siRNA) to reverse their polarization and were evaluated by detecting markers expression of M1/M2 using RT-PCR.
SAP associated acute lung injury (ALI) was induced successfully by ip injections of caerulein, which was confirmed by histopathology. Lung macrophages expressed high levels of IRF5 as M1 phenotype during the early acute pancreatitis stages. Reduction of IRF5 expression by IRF5 siRNA reversed the action of macrophages from M1 to M2 phenotype in vitro. The expressions of M1 markers, including IRF5 (S + IRF5 siRNA vs S + PBS, 0.013 ± 0.01 vs 0.054 ± 0.047, P < 0.01), TNF-α (S + IRF5 siRNA vs S + PBS, 0.0003 ± 0.0002 vs 0.019 ± 0.018, P < 0.001), iNOS (S + IRF5 siRNA vs S + PBS, 0.0003 ± 0.0002 vs 0.026 ± 0.018, P < 0.001) and IL-12 (S + IRF5 siRNA vs S + PBS, 0.000005 ± 0.00004 vs 0.024 ± 0.016, P < 0.001), were decreased. In contrast, the expressions of M2 markers, including IL-10 (S + IRF5 siRNA vs S + PBS, 0.060 ± 0.055 vs 0.0230 ± 0.018, P < 0.01) and Arg-1 (S + IRF5 siRNA vs S + PBS, 0.910 ± 0.788 vs 0.0036 ± 0.0025, P < 0.001), were increased. IRF5 siRNA could reverse the lung macrophage polarization more effectively than IL-4.
Treatment with IRF5 siRNA can reverse the pancreatitis-induced activation of lung macrophages from M1 phenotype to M2 phenotype in SAP associated with ALI.
Core tip: We investigated the role of interferon regulatory factor 5 (IRF5) in reversing polarization of lung macrophages during severe acute pancreatitis (SAP). Treatment with IRF5 specific siRNA could reverse the pancreatitis-induced activation of lung macrophages from M1 phenotype to M2 phenotype in vitro. Reduced expression of IRF5 may lead to a new therapeutic approach for SAP associated with acute lung injury.
- Citation: Sun K, He SB, Qu JG, Dang SC, Chen JX, Gong AH, Xie R, Zhang JX. IRF5 regulates lung macrophages M2 polarization during severe acute pancreatitis in vitro. World J Gastroenterol 2016; 22(42): 9368-9377
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9368.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9368
Severe acute pancreatitis (SAP) is a most serious disease especially when multiple remote organs are involved[1,2]. The developing process of this disease is rapid and can result in a series of complications and multiple organ dysfunction (MODS) including the lung, liver, kidney, intestine and stomach[3,4]. MODS is an essential process during SAP. MODS caused nearly half of deaths in SAP patients[5]. Acute lung injury (ALI) is the most common extrapancreatic complication in SAP, which is associated with the high rates of morbidity and mortality. ALI is characterized by accumulation of activated neutrophils and the development of interstitial edema[6,7].
Although accumulation of activated neutrophils in the lungs is characteristic of ALI, macrophages which reside in the pulmonary interstitium and alveoli are considered to be the most critical cells involved in development of the SAP associated with ALI[8-10]. Macrophages are plastic cells displaying versatile functional phenotypes depending on microenvironments. M1 macrophages (M1, the classically activated macrophages) and M2 macrophages (M2, the alternatively activated macrophages) have been defined as the two extremes in a spectrum of macrophage functional phenotypes. M1 macrophages are critical effector cells that kill micro-organisms[11,12]. In contrast, M2 macrophages are involved in the resolution of inflammation[13]. The pro-inflammatory response must be balanced by regulatory and inhibitory effector mechanisms to protect against tissue damage caused by the effects of excessive inflammation[14]. Lung macrophages can be subdivided into alveolar, pleural, interstitial and intravascular macrophages, and alveolar macrophages (AM) considered as the most important cell of the innate immune system[10,15].
Distinct macrophage subtypes are not only characterized by their differences in cytokine release but also by the differential expression of key transcription factors. Krausgruber et al[16] found that transcription factor interferon regulatory factor 5 (IRF5) was a major factor in defining macrophage polarization. They found that IRF5 was the major regulator of pro-inflammatory M1 macrophage polarization. IRF5 directly induces the expression of pro-inflammatory cytokines such as IL-6, IL-12b, and IL-23a while repressing transcription of anti-inflammatory cytokines such as IL-10. Although IRF5 plays an important role in macrophage activation, it has rarely been used to track macrophages in inflammatory disease. In the present study, we used the SAP model induced by intraperitoneal (ip) injection of caerulein, and isolated murine lung macrophages from bronchoalveolar lavage (BAL) fluid to detect IRF5 expression and their polarization in vitro. In addition, we treated lung macrophages with IRF5 specific siRNA (IRF5 siRNA)/IL-4 to reverse macrophage polarization from M1 phenotype to M2 phenotype and compare its effect with IL-4.
C57BL/6 mice, male or female, aged 8-12 wk, were obtained from the Laboratory Animal Center of Jiangsu University, Zhenjiang, Jiangsu, China. The laboratory animal experimental protocol was approved by Institutional Animal Care and Use Committee of Jiangsu University. All experiments followed the laboratory animal care principles. Rats were randomly divided into two groups: (1) SAP group: rats received ip injections of caerulein; and (2) control group: rats received ip injection of an equal volume of PBS. Each group had 15 rats. The RAW264.7 cells (mouse macrophages, M0) were placed in a 12-well plate at 105/mL 24 h before intervention and IFN-γ (100 U/mL) + LPS (5 μg/L) was used to induce M1 phenotype (M1), and IL-4 (10 μg/L) was used to induce M2 phenotype (M2). The three types of macrophages (M0, M1 and M2) were as control compared with lung macrophages.
SAP models were prepared according to the protocol by Tang et al[17]. The mice were deprived of food but allowed access to water 24 h prior to the start of the experiments. SAP was induced in C57BL/6 mice by ip injections of 20 μg/kg body weight (diluting stock solution in saline) caerulein at hourly intervals for a total of seven injections.
For the histological analysis, mouse pancreatic and lung tissue samples were washed thoroughly in PBS, fixed in 10% buffered formalin, and embedded in paraffin, tissue samples were cut into sections. Five μmol/L sections were stained with hematoxylin and eosin using standard procedures and examined by light microscopy. Sections were examined by an experienced morphologist, who was blinded to the sample identity, for tissue injury.
AM was purified according to the method of Small et al[18]. Briefly, anesthetized mice were sacrificed, the lungs from 36 mice were lavaged with RPMI 1640 containing 10% FCS, and BAL was mixed in a 50 mL tube. BAL fluids were then plated, and the AM was allowed to adhere for 2 h at 37 °C, 5% CO2.
BAL cells were incubated for 30 min at 4 °C with rat-anti-F4/80 monoclonal antibody. The cells were analyzed using fluorescence-activated cell sorting (FACS). The BAL cells were seeded on plates for 2 h and non-adherent cells were washed, and the adherent cells were scraped and fixed with 4% paraformaldehyde at 4 °C for 30 min, and then incubated for 2 h with a 1:100 dilution of the CD11b/c antibody and F4/80 antibody (Ebioscience, San Diego, United States) labeled with Alexa Fluor 488 for flow cytometry analysis[19].
Lung macrophages obtained from BAL fluid as well as rats 18 h after SAP induction. One part of polarized lung macrophages was transfected with IRF5 siRNA using a Mouse Macrophage Nucleofector® Kit (LONZA, Anaheim, CA, United States) according to the manufacturer’s instructions. The other part of polarized lung macrophages was incubated with IL-4 (20 ng/mL) for 24 h. Macrophages were collected for further detection after incubation.
Real-time polymerase chain reaction (RT-PCR) was performed with the ABI Prism 7900HT (Applied Biosystems, Foster City, CA, United States) using the SYBR Green PCR Master Mix (Applied Biosystems) according to the protocol in our lab. The PCR primers used were as follows: GAPDH (F: GTATGACTCTACCCACGGCAAGT; R: TTCCCGTTGATGACCAGCTT), TNF-α (F: CCGATTTGCCACTTCATACCA; R: TAGGGCAAGGGCTCTTGATG), IL-10 (F: AGAAGGACCAGCTGGACAACAT; R: CAAGTAACCCTTAAAGTCCTGCAGTA), Arg-1 (F: CCGCAGCATTAAGGAAAGC; R: CCCGTGGTCTCTCACATTG), iNOS (F: CAGCCCTCAGAGTACAACGAT; R: CAGCAGGCACACGCAATGAT), IRF5 (F: GTTGCCTTTGACGGACCTA; R: 5’-GGCCCACTCCAGAACACCT-3’), IL-12 (F: 5’-AAAGGTGCGTTCCTCGTAG; R: CAACAGCATAAGGCCAAGT).
All values were expressed as mean ± SE. Independence t test was used in the comparison of two groups. P values < 0.05 indicate statistical significance. Analyses were performed using SPSS 17.0.
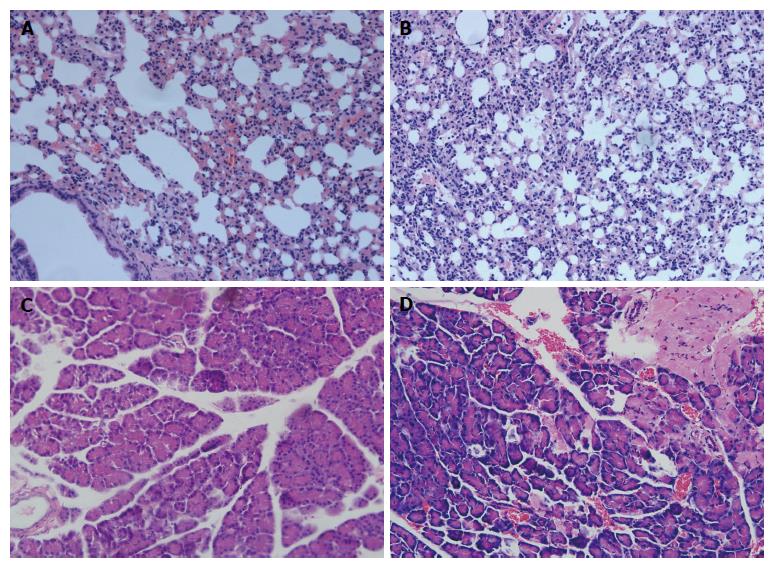
As shown in Figure 1, lung tissue and pancrease tissue from control rats showed a normal structure and no histopathological changes under light microscope (Figure 1A, C). A typical photomicrograph of pancreatic lung injury included lung edema, neutrophil infiltration, necrosis and hemorrhage (Figure 1B). The histological changes of pancreas tissue, such as the infiltration of neutrophils, macrophages, interstitial edema, hemorrhage and focal necrotic areas, were seen in the pancreas tissue of SAP group (Figure 1D). The pathological findings confirmed that we had established the mouse SAP model associated with ALI successfully.
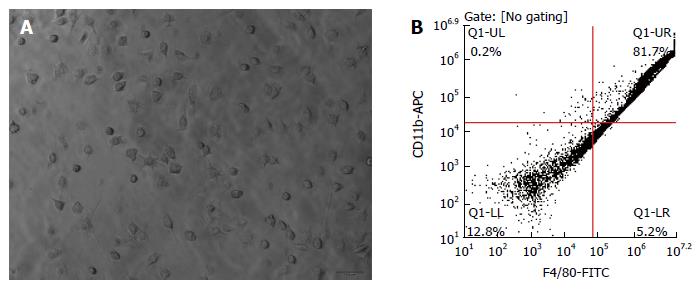
After 24 h primary culture, the lung alveolus macrophages were observed under microscope and examined by FACS for the purity and quantity. The purity of lung alveolus macrophages was approximately 81.7% (Figure 2).
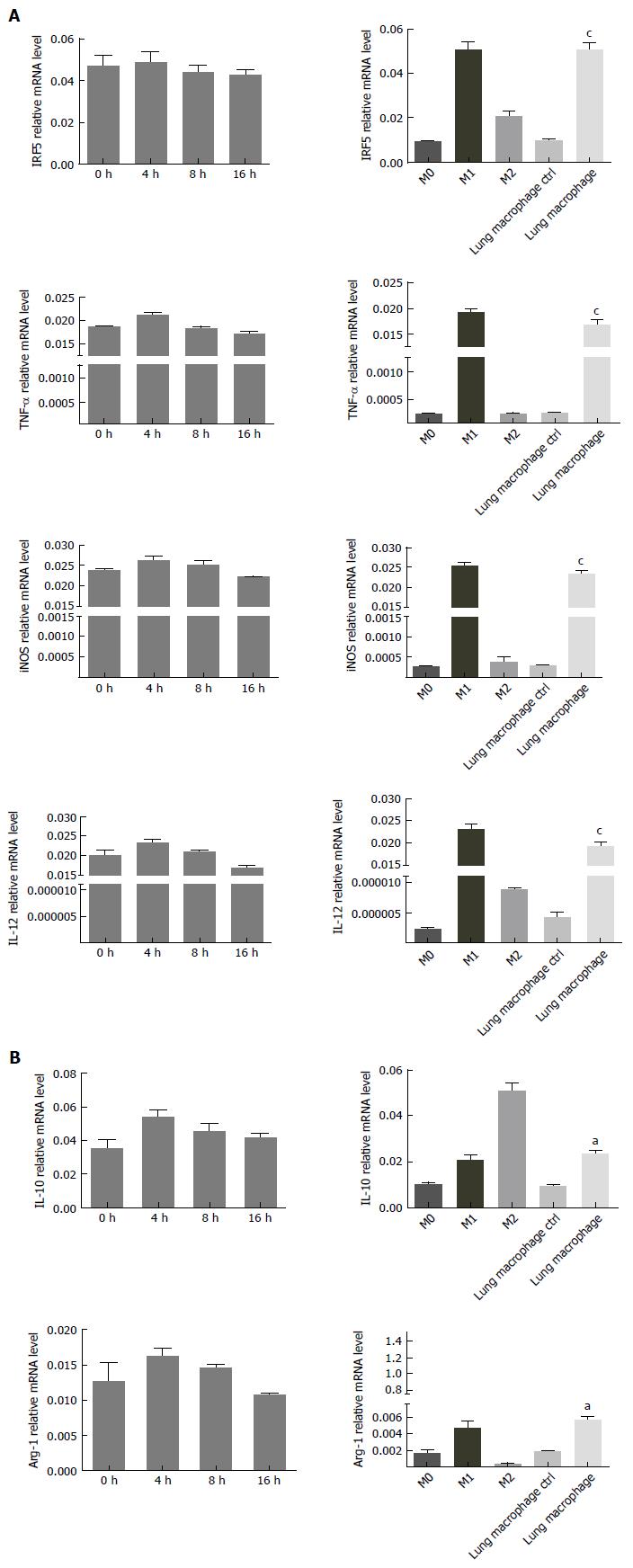
Lung alveolus macrophages were isolated from BAL fluid of the control group and SAP group by FACS. In vitro lung macrophages from SAP group were treated with PBS for 4, 8 and 16 h respectively, and macrophages from control group were treated with PBS for 4 h. The expression of major M1 markers (IRF5, TNF-α, iNOS and IL-12) and M2 markers (IL-10 and Arg-1) were detected by RT-PCR. The polarization of lung macrophages was detected by RT-PCR. As shown in Figure 3, the mRNA levels of lung macrophage markers in SAP group were significantly higher than in the control group (M0) (P < 0.01). In contrast, mRNA levels of lung macrophage markers in SAP group were similar to those in another control group (M1) (P > 0.05). The experimental data indicated a shift to M1 polarization of lung macrophages during the initiation of SAP.
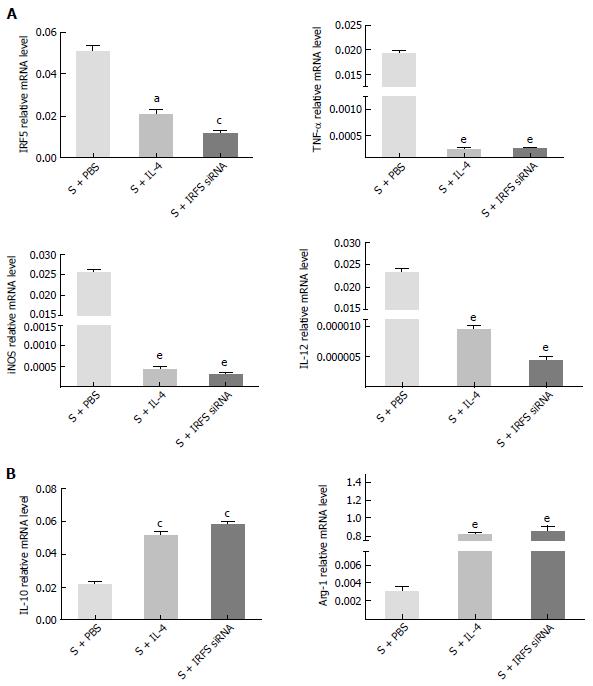
Whether the polarization of lung alveolus macrophages could be reversed from M1 to M2 in vitro was studied. After treatment with PBS/IL-4/IRF5 siRNA, the expression levels of macrophage markers (M1: IRF5, TNF-α, iNOS, IL-12; M2: IL-12, Arg-1) were compared at 4 h. As shown in Figure 4, RT-PCR showed that IL-4/IRF5 siRNA could reduce expression of M1 markers significantly: IRF5 (S + IRF5 siRNA vs S + PBS, 0.013 ± 0.01 vs 0.054 ± 0.047, P < 0.01), TNF-α (S + IRF5 siRNA vs S + PBS, 0.0003 ± 0.0002 vs 0.019 ± 0.018, P < 0.001), iNOS (S + IRF5 siRNA vs S + PBS, 0.0003 ± 0.0002 vs 0.026 ± 0.018, P < 0.001) and IL-12 (S + IRF5 siRNA vs S + PBS, 0.000005 ± 0.00004 vs 0.024 ± 0.016, P < 0.001). In contrast, M2 markers were upregulated by IL-4/IRF5 siRNA treatment: IL-10 (S + IRF5 siRNA vs S + PBS, 0.060 ± 0.055 vs 0.0230 ± 0.018, P < 0.01) and Arg-1 (S + IRF5 siRNA vs S + PBS, 0.910 ± 0.788 vs 0.0036 ± 0.0025, P < 0.001). The results suggested that the polarization of lung macrophages could be reversed by IRF5 siRNA from M1 to M2 as well as by IL-4.
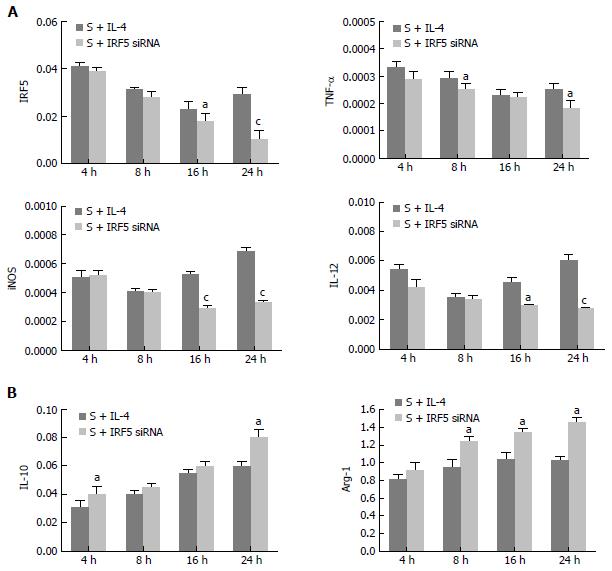
In order to identify a better way to alter the polarization of macrophages in vitro, we compared the expression of M1 and M2 markers after the treatment of IL-4 or IRF5 siRNA. Gene expression in primary culture macrophages from the rats with SAP was determined by real-time PCR. As shown in Figure 5, the effect of treatment with IL-4 and IRF5 siRNA were similar in IRF5 (4 h, 8 h), IL-12 (4 h, 8 h), iNOS (4 h, 8 h), TNF- (4 h,16 h), IL-10 (8 h, 16 h), Arg-1 (4 h) (P > 0.05); but in some groups, the levels of IRF5 (16 h, 24 h), TNF- (8 h, 24 h), iNOS (16 h, 24 h) and IL-12 (16 h, 24 h) were much lower, and Arg-1 (8 h, 16 h, 24 h), IL-10 (4 h, 24 h) were significantly higher after IRF5 siRNA treatment than after IL-4 treatment (P < 0.05). These results indicated that IRF5 siRNA could reverse the lung macrophage polarization more effectively.
SAP can cause SIRS and high morbidity. In SAP, MODS in the early phase is the main cause of high mortality[5,20]. In the early phase, activated macrophages can release humoral mediators which may lead to remote organ injury, including ALI, acute liver injury, acute kidney injury, etc. ALI is one of the most common organ failures in SAP. It is the first cause of patients’ death during the early stage of SAP[21].
A number of investigations have indicated that the severity of SAP and pancreatitis-associated lung injury were regulated by a great deal of inflammatory factors, including inflammatory cells and cytokines[22-24]. Among many inflammatory cells, macrophages are key pro-inflammatory and anti-inflammatory cells, which accumulate in the damaged organ in SAP. Plasticity and functional polarization are hallmarks of macrophages that result in the phenotypic diversity of macrophage populations[25,26]. Thus, targeting selected macrophage pathways is a potential therapeutic strategy for suppressing macrophage-mediated lung injury.
Recently, transcriptional pathways were believed to play an important role in the polarization of macrophage. IRF5 was considered as a major factor in defining macrophage polarization[27,28]. Modulation of IRF5 led to the conversion of one macrophage subset phenotype into the other one[29]. High expression of IRF5 results in polarization of the macrophage phenotype toward M1, however, low expression of IRF5 leads to M2 polarization, which is the anti-inflammatory macrophage phenotype. This indicates a possible broad effect of therapy which targets the induction of IRF5 expression by macrophages[30].
In the present study, a mouse model of SAP associated with ALI was constructed successfully by ip caerulein. We found that lung macrophages were M1 polarized in the early phase of SAP. The M1 state was confirmed by the up-regulated expression of M1 phenotypes and down-regulated expression of M2 phenotypes. And the results conform to the conventional theory that macrophages can induce pro-inflammatory cytokines and amplify the degree of the inflammation during early phase of SAP[31,32].
It has been reported that macrophage polarization could be reversed from M1 phenotype to M2 phenotype[33]. The development and resolution of lung injury are accompanied by alteration of macrophage polarization in the lungs. We hypothesized that lung macrophages which act as M1 phenotype could aggravate inflammation, and M2 could alleviate the inflammation if we reversed the polarization of lung macrophages in SAP by IRF5 siRNA. In the present study, the lung macrophages showed M1 polarization reflected in the high expression of M1 phenotypes (IRF5, TNF-α, iNOS and IL-10). This activation was expected as lung macrophages contact early with mediators released by inflammatory pancreatic tissue. Several studies had reported the plasticity of activated macrophages[34,35]. Furthermore, the M2 macrophage state induced by these mediators can alleviate the inflammatory response associated with SAP. This kind of reversion may alleviate systemic inflammation and promote tissue repair during SAP.
It is interesting to know the capacity of these pancreatitis-activated lung macrophages to be reverted to an anti-inflammatory phenotype from M1 to M2 in vitro. We isolated lung macrophages from BAL fluid of the rats with ALI and the isolated cells were treated by IRF5 siRNA or IL-4. Our data showed that both treatments can reverse macrophage polarization from M1 phenotype to M2 phenotype. After treatment with IRF5 siRNA or IL-4, lung macrophages down- regulated the expression of M1 phenotypes such as IRF5, TNF-α, iNOS and IL-10, which are pro-inflammatory mediators. At the same time, macrophages expressed more M2 phenotypes such as IL-10, which is an important anti-inflammatory factor. Our result indicated that activated lung macrophages obtained from rats with SAP could be reversed to M2 state by IRF5 siRNA or IL-4 treatment.
IL-4 is known to induce the alternative M2 activation of macrophages. M2 macrophage can promote the repair phenotype and counteract the effects of pro-inflammatory cytokines[36]. However, it is reported that IL-4 administration was not enough to reverse the M1 phenotype in lung macrophages therapeutically. The reason could be related to the strongly proinflammatory environment generated in the lung. Because of the long half-life of IRF5 siRNA, it demonstrated its high capability to reverse the polarization of macrophages from M1 phenotype to the M2 phenotype. In our study, we proposed that there may be two reasons for these results: (1) IRF5 mediates inflammatory and immune responses by controlling expression of proinflammatory cytokines downstream of MyD88-dependent Toll-like receptor signaling; and (2) IRF5 regulates the expression of inflammatory gene TNF-α and IL1β directly[37].
In conclusion, treatment with IRF5 siRNA could reverse the pancreatitis-induced activation of lung macrophages from M1 phenotype to M2 phenotype in SAP associated with ALI. Nevertheless, targeting transcription factors for therapeutic aims is still an unknown area. Meanwhile, the molecular mechanism of macrophage polarization is still unknown. In our experiment, silencing the IRF5 regulated a range of inflammatory genes, and this method can reverse the lung macrophage polarization more effectively than IL-4 administration. IRF5 is an attractive target for SAP associated with ALI therapy. However, the molecular mechanisms of IRF5 in macrophage polarization should be further studied.
Severe acute pancreatitis (SAP) is a life threatening disease especially when multiple remote organs and systems are involved in the inflammation. Acute lung injury (ALI) is the most common extrapancreatic complication in SAP. Recently, several reports have demonstrated that macrophages are considered to be the most critical cells involved in development of the acute pancreatitis. M1 macrophages and M2 macrophages have been defined as the two extremes in a spectrum of macrophage functional phenotypes. Interferon regulatory factor 5 (IRF5) was a major factor in defining macrophage polarization. The expression of IRF5 in lung macrophages from an SAP murine model and whether the IRF5 specific siRNA (IRF5 siRNA) could reverse M1 phenotype of macrophages to M2 have not yet been elucidated.
Recently, several reports have demonstrated that macrophages are considered to be the most critical cells involved in development of the acute pancreatitis. Macrophages are plastic cells displaying versatile functional phenotypes depending on microenvironments. Krausgruber et al found that transcription factor IRF5 was a major factor for defining macrophage polarization. IRF5 was the major regulator of pro-inflammatory M1 macrophage polarization.
The authors for the first time investigated the role of IRF5 in reversing polarization of lung macrophages during SAP. Treatment with IRF5 siRNA could reverse the pancreatitis-induced activation of lung macrophages from M1 phenotype to M2 phenotype in vitro.
Lung macrophages adopt a pro-inflammatory activation (M1 phenotype) early during acute pancreatitis. Treatment with IRF5 siRNA could reverse the pancreatitis-induced activation of lung macrophages from M1 to M2 in vitro. The research revealed the function of IRF5 in regulating the polarization of lung macrophages. Reducing the expression of IRF5 may lead to a new therapeutic approach for SAP associated with ALI.
M1 and M2 have been defined as the two extremes in a spectrum of macrophage functional phenotypes. M1 macrophages are critical effector cells that kill microorganisms. In contrast, M2 macrophages are involved in the resolution of inflammation. Distinct macrophage subtypes display differential expression of key transcription factors. IRF5 was the major regulator of pro-inflammatory M1 macrophage polarization.
This is a very well designed, performed and written experimental study. In this study, the authors reported the role of IRF5 in regulating lung macrophages M2 polarization during SAP in vitro. This research reveals a novel function of IRF5 in controlling the polarization of macrophages. Therefore, modification of IRF5 expression may lead to a new therapeutic approach for SAP associated with ALI.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inal V, Peng SY, Ruiz AC S- Editor: Yu J L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Que RS, Cao LP, Ding GP, Hu JA, Mao KJ, Wang GF. Correlation of nitric oxide and other free radicals with the severity of acute pancreatitis and complicated systemic inflammatory response syndrome. Pancreas. 2010;39:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Dang SC, Wang H, Zhang JX, Cui L, Jiang DL, Chen RF, Qu JG, Shen XQ, Chen M, Gu M. Are gastric mucosal macrophages responsible for gastric injury in acute pancreatitis? World J Gastroenterol. 2015;21:2651-2657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Jacob TG, Raghav R, Kumar A, Garg PK, Roy TS. Duration of injury correlates with necrosis in caerulein-induced experimental acute pancreatitis: implications for pathophysiology. Int J Exp Pathol. 2014;95:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Rau BM, Krüger CM, Hasel C, Oliveira V, Rubie C, Beger HG, Schilling MK. Effects of immunosuppressive and immunostimulative treatment on pancreatic injury and mortality in severe acute experimental pancreatitis. Pancreas. 2006;33:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Akbarshahi H, Menzel M, Posaric Bauden M, Rosendahl A, Andersson R. Enrichment of murine CD68+ CCR2+ and CD68+ CD206+ lung macrophages in acute pancreatitis-associated acute lung injury. PLoS One. 2012;7:e42654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Yang ZW, Meng XX, Xu P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19:2513-2520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Xu XW, Yang XM, Bai YH, Zhao YR, Shi GS, Zhang JG, Zheng YH. Treatment with ginkgo biloba extract protects rats against acute pancreatitis-associated lung injury by modulating alveolar macrophage. Prz Gastroenterol. 2014;9:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Xue P, Guo J, Yang XN, Huang W, Xia Q. Changes of neuronal acetylcholine receptor alpha 7 of peritoneal macrophage in experimental acute pancreatitis treated by Chaiqin Chengqi Decoction (). Chin J Integr Med. 2014;20:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Gea-Sorlí S, Guillamat R, Serrano-Mollar A, Closa D. Activation of lung macrophage subpopulations in experimental acute pancreatitis. J Pathol. 2011;223:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1783] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 12. | He L, Marneros AG. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol. 2013;182:2407-2417. [PubMed] |
| 13. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7233] [Cited by in RCA: 6839] [Article Influence: 402.3] [Reference Citation Analysis (0)] |
| 14. | Wang W, Zhou X, Yang F, Sun H, Xu D, Andersson R, Chen B, Zhou M. The effects of 5-fluorouracil on severe acute pancreatitis-inducing apoptosis of macrophages. Pancreas. 2014;43:660-663. [PubMed] |
| 15. | Yang T, Mao YF, Liu SQ, Hou J, Cai ZY, Hu JY, Ni X, Deng XM, Zhu XY. Protective effects of the free radical scavenger edaravone on acute pancreatitis-associated lung injury. Eur J Pharmacol. 2010;630:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1010] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 17. | Tang Y, Han Y, Liu L, Shen W, Zhang H, Wang Y, Cui X, Wang Y, Liu G, Qi R. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromolecules. 2015;16:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol. 2010;184:2048-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Zhao X, Dai J, Xiao X, Wu L, Zeng J, Sheng J, Su J, Chen X, Wang G, Li K. PI3K/Akt signaling pathway modulates influenza virus induced mouse alveolar macrophage polarization to M1/M2b. PLoS One. 2014;9:e104506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Feng YC, Wang M, Zhu F, Qin RY. Study on acute recent stage pancreatitis. World J Gastroenterol. 2014;20:16138-16145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Zhang JX, Dang SC. Ligustrazine alleviates acute lung injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:605-609. [PubMed] |
| 22. | Renzulli P, Jakob SM, Täuber M, Candinas D, Gloor B. Severe acute pancreatitis: case-oriented discussion of interdisciplinary management. Pancreatology. 2005;5:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Elder AS, Saccone GT, Dixon DL. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology. 2012;12:49-56. [PubMed] |
| 24. | Ma P, Yu K, Yu J, Wang W, Ding Y, Chen C, Chen X, Zhao K, Zuo T, He X. Effects of Nicotine and Vagus Nerve in Severe Acute Pancreatitis-Associated Lung Injury in Rats. Pancreas. 2016;45:552-560. [PubMed] |
| 25. | Zechner D, Spitzner M, Müller-Graff T, Vollmar B. Diabetes increases pancreatitis induced systemic inflammation but has little effect on inflammation and cell death in the lung. Int J Exp Pathol. 2014;95:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342-349. [PubMed] |
| 27. | Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243-249. [PubMed] |
| 28. | Dideberg V, Kristjansdottir G, Milani L, Libioulle C, Sigurdsson S, Louis E, Wiman AC, Vermeire S, Rutgeerts P, Belaiche J. An insertion-deletion polymorphism in the interferon regulatory Factor 5 (IRF5) gene confers risk of inflammatory bowel diseases. Hum Mol Genet. 2007;16:3008-3016. [PubMed] |
| 29. | Eames HL, Saliba DG, Krausgruber T, Lanfrancotti A, Ryzhakov G, Udalova IA. KAP1/TRIM28: an inhibitor of IRF5 function in inflammatory macrophages. Immunobiology. 2012;217:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Ding L, Gu H, Gao X, Xiong S, Zheng B. Aurora kinase a regulates m1 macrophage polarization and plays a role in experimental autoimmune encephalomyelitis. Inflammation. 2015;38:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Xu L, Yang F, Lin R, Han C, Liu J, Ding Z. Induction of m2 polarization in primary culture liver macrophages from rats with acute pancreatitis. PLoS One. 2014;9:e108014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Gea-Sorlí S, Closa D. In vitro, but not in vivo, reversibility of peritoneal macrophages activation during experimental acute pancreatitis. BMC Immunol. 2009;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23-35. [PubMed] |
| 34. | Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 808] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 35. | Alasoo K, Martinez FO, Hale C, Gordon S, Powrie F, Dougan G, Mukhopadhyay S, Gaffney DJ. Transcriptional profiling of macrophages derived from monocytes and iPS cells identifies a conserved response to LPS and novel alternative transcription. Sci Rep. 2015;5:12524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Zhang C, Ge CL, Guo RX, He SG. Effect of IL-4 on altered expression of complement activation regulators in rat pancreatic cells during severe acute pancreatitis. World J Gastroenterol. 2005;11:6770-6774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Gharib SA, Johnston LK, Huizar I, Birkland TP, Hanson J, Wang Y, Parks WC, Manicone AM. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J Leukoc Biol. 2014;95:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |