Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8820
Peer-review started: June 27, 2016
First decision: August 8, 2016
Revised: August 21, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: October 21, 2016
Processing time: 117 Days and 16.1 Hours
To compare the aspiration needle (AN) and core biopsy needle (PC) in endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of abdominal masses.
Consecutive patients referred for EUS-FNA were included in this prospective single-center trial. Each patient underwent a puncture of the lesion with both standard 22-gauge (G) AN (Echo Tip Ultra; Cook Medical, Bloomington, Indiana, United States) and the novel 22G PC (EchoTip ProCore; Cook Medical, Bloomington, Indiana, United States) in a randomized fashion; histology was attempted in the PC group only. The main study endpoint was the overall diagnostic accuracy, including the contribution of histology to the final diagnosis. Secondary outcome measures included material adequacy, number of needle passes, and complications.
Fifty six consecutive patients (29 men; mean age 68 years) with pancreatic lesions (n = 38), lymphadenopathy (n = 13), submucosal tumors (n = 4), or others lesions (n = 1) underwent EUS-FNA using both of the needles in a randomized order. AN and PC reached similar overall results for diagnostic accuracy (AN: 88.9 vs PC: 96.1, P = 0.25), specimen adequacy (AN: 96.4% vs PC: 91.1%, P = 0.38), mean number of passes (AN: 1.5 vs PC: 1.7, P = 0.14), mean cellularity score (AN: 1.7 vs PC: 1.1, P = 0.058), and complications (none). A diagnosis on the basis of histology was achieved in the PC group in 36 (64.3%) patients, and in 2 of those as the sole modality. In patients with available histology the mean cellularity score was higher for AN (AN: 1.7 vs PC: 1.0, P = 0.034); no other differences were of statistical significance.
Both needles achieved high overall diagnostic yields and similar performance characteristics for cytological diagnosis; histological analysis was only possible in 2/3 of cases with the new needle.
Core tip: Endoscopic ultrasound-guided fine needle aspiration and cytological analysis of the obtained material represents an established modality for diagnosis of intra- and paramural lesions. Recently developed fenestrated needles enable specimen acquisition for histological analysis aiming to improve diagnostic accuracy. We prospectively compared the 22 gauge standard aspiration needle with the same-diameter novel core biopsy needle in sampling of abdominal masses. Both needles yielded similar overall diagnostic accuracy, while no significant differences were evident regarding sample adequacy for the analysis, quality, and cellularity of specimens, number of needle passes, feasibility, and complications. The diagnostic contribution of histology with the novel needle was limited.
- Citation: Sterlacci W, Sioulas AD, Veits L, Gönüllü P, Schachschal G, Groth S, Anders M, Kontos CK, Topalidis T, Hinsch A, Vieth M, Rösch T, Denzer UW. 22-gauge core vs 22-gauge aspiration needle for endoscopic ultrasound-guided sampling of abdominal masses. World J Gastroenterol 2016; 22(39): 8820-8830
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8820.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8820
Endoscopic ultrasound (EUS) has become widely-used for diagnostic purposes regarding lesions arising from the pancreas, upper gastrointestinal tract, as well as adjacent structures, such as the liver and lymph nodes. In this setting, EUS-guided fine needle aspiration (EUS-FNA) is currently considered a technique with an excellent safety profile with reported sensitivity of 60% to 95% and overall diagnostic accuracy ranging from 60% to 90%[1-3].
However, EUS-FNA performance is dependent on numerous factors, including those related to target lesion (location, size, characteristics), technical details (type of needle used, aspiration/biopsy method, number of passes, material processing), and involved personnel (endoscopist expertise, presence of on-site experienced cytopathologist)[4-7]. Usually, cytology is the basis of EUS-FNA based tissue diagnosis. However, cytological specimens obtained by means of standard EUS-FNA are of limited value in diagnosing entities like gastrointestinal stromal tumors, lymphomas, and autoimmune pancreatitis that mostly require additional immunocytochemistry and/or flow cytometry (e.g., lymphomas) or tissue processing and immunohistochemical evaluation for an accurate classification[8-10]. Furthermore, individualized tumor therapy may mandate a more detailed immunohistological analysis in the future that may not be sufficiently covered by cytology.
To overcome these limitations of cytology and to reliably retrieve samples suitable for histopathological analysis, several new needles have been developed in recent years, with variable success[10-12]; most recently, a novel needle (EchoTip ProCore; Cook Medical, Bloomington, Indiana, United States) has been developed that features a hollowed-out reverse bevel. This side fenestration promises to enable the acquisition of core biopsy specimens with preserved architecture and thus improve diagnostic yield. Fulfilling those expectations, recent published results on the performance of ProCore needles demonstrate high diagnostic accuracy rates from 82% to 96%[13-17].
We hypothetized that this novel needle is superior in diagnostic performance, given its advanced technical characteristics. Therefore, we conducted this randomized study in order to prospectively compare the standard 22-gauge (G) aspiration needle (AN) with the 22G EchoTip ProCore needle (PC) in terms of diagnostic accuracy, adequacy of the obtained material for evaluation, histocytological quality of the samples, technical feasibility, and related complications.
This single-center prospective study was conducted at the Department of Interdisciplinary Endoscopy of the University Hospital Hamburg-Eppendorf in Germany from August 2011 to November 2013. The study protocol was approved by the Institutional Clinical Research Ethics Committee (study number: PV 3835) and was registered at Clinicaltrials.gov (ID: NCT02181140). All of the enrolled patients provided written informed consent for the procedure and study participation.
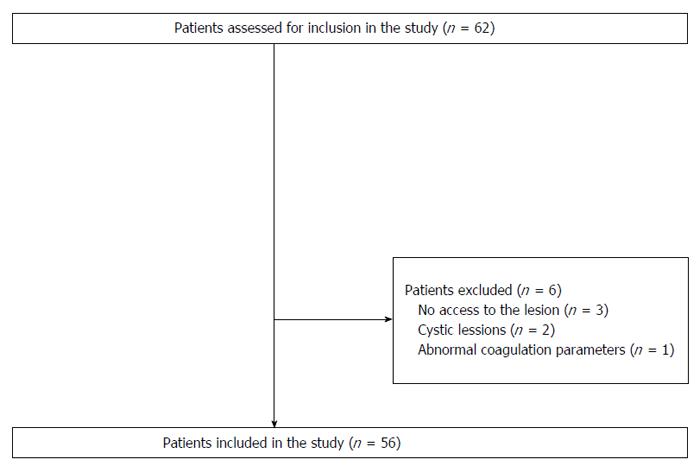
A total of 56 patients, between 18 and 85 years old, were enrolled in the study. All of the patients with an indication for EUS-FNA for the assessment of pancreatic lesions, paramural mass lesions, or subephitelial tumors (SET) were included. Exclusion criteria were: (1) very difficult or impossible access to the target site (i.e., post-operative anatomic alterations, interpositioned vessels); (2) cystic lesions without solid tissue; (3) coagulopathy (Quick time < 40% or platelets-PLTs < 40000/mm3) or ongoing anticoagulant medications except ASS; and (4) poor performance status (Eastern Cooperative Oncology Group-ECOG IV).
For each eligible patient, the following data were recorded: basic characteristics [age, gender, body mass index (BMI)], symptoms (pain, jaundice, weight loss), lesion location, laboratory data [complete blood count-CBC, international normalized ratio (INR)], and available imaging studies prior to EUS.
During the study, the following parameters were recorded: location, size, and echogenicity of the lesions, dose of the propofol administered for sedation, number of passes for each needle, specimen adequacy for evaluation, cellularity and cytological/histological quality of the material, cytohistological analysis result, and complications within 24 h after the intervention.
Follow-up was performed by telephone interviews, hospital visits, and chart reviews until the patient’s death or termination of the study. Inquiries included symptoms, laboratory and/or imaging tests, and subsequent interventions, namely repeated tissue acquisition and surgery.
All of the procedures were carried out by 4 experienced endoscopists, assisted by one endoscopy nurse. A linear array echoendoscope (GF-UCT 180, Olympus Europa, Hamburg, Germany) connected to a processor featuring a color Doppler function (Aloka Alpha 7, Hitachi Medical Corporation, Tokyo, Japan) was used in all of the examinations. The patients remained in the left lateral position under conscious sedation by means of intravenous propofol administration. Oxygen via the nasal cannula and recording of the vital signs were continuously provided. No on-site cytopathologist was available.
Following careful scope manipulations, the endoscopist obtained visualization of the target lesion and, using the color Doppler function, excluded vessel interposition along the puncture route. The lesion parameters, including exact location, size, and echogenicity, were assessed and recorded. Subsequently, the puncture of the mass by both the standard 22G aspiration needle (AN; Echo Tip Ultra; Cook Medical, Bloomington, Indiana, United States) and the novel 22G core biopsy needle (PC; EchoTip ProCore; Cook Medical, Bloomington, Indiana, United States) was performed in a randomized order as determined by a computer-generated randomization assignment that took place prior to the procedure. The PC has a 5.2F shaft, a core trap sized 2 mm, and a reverse-bevel length of 5.9 mm. The same sampling technique was used for both needles to avoid technical biases. In detail, after the needle had successfully entered the lesion, its stylet was removed and suction was applied using a 10 mL syringe. During each puncture, the needle was moved back and forth between 10 and 20 times and, at the end, it was withdrawn from the mass after suction was released. The number of passes depended on the examiner’s estimation of the yielded material with a maximum 3 passes being attempted with each device according to the specifications of the ethics committee.
Following each pass, the further processing of the acquired material was performed by the endosonographer, given the lack of an on-site cytopathologist. This was done, in detail, as following:
In the case of the standard AN, the specimens were smeared onto glass slides by stylet’s reintroduction or by air flushing into the needle. Cytological evaluation was subsequently undertaken after air dried fixation and staining with the Giemsa method.
Regarding the PC, the material was completely flushed out with saline solution. When small-core biopsy cylinders (defined as whitish pieces of tissue with apparent bulk, which did not consist of blood and, therefore, dissolved in saline solution) were identified, they were retrieved by syringe suction and subsequently placed into formalin for histological analysis. The remaining material was used for the preparation of cytological smears. Core biopsy cylinders were subsequently cut to obtain hematoxylin-eosin stained sections. Cytological smears were fixed in ethanol and stained with Papanikolaou method.
The biopsy material was evaluated due to diagnostic and quality parameters by a study cytopathologist who was blinded to the type of the needle used.
The cytological and histological findings by EUS-FNA were classified as positive for malignancy if an unequivocal diagnosis of malignancy was made. It is noteworthy that gastrointestinal stromal tumors, neuroendocrine tumors (NET), and intraductal papillary mucinous neoplasia cases were included in the group of malignancies for the analysis of diagnostic parameters. The acquired material was regarded as adequate for cytological/histological analysis using the cytopathology quality scoring as described below.
The main outcome parameters used for comparisons between the standard aspiration and ProCore needles were: (1) the rate of correct diagnosis of the obtained material and related diagnostic discrimination values (sensitivity, specificity, diagnostic accuracy). The gold standard criteria for diagnosis were considered as one or more of the following: definite EUS-FNA (see above), surgical resection, or clinical follow-up exceeding 12 mo. In the PC group, the cytology and histology results were considered together for the overall diagnosis; in the AN group, only cytology was used for diagnosis; and (2) the percentage of cases in which the collected specimen was regarded by the cytopathologist as adequate for cytological/histological examination defined as a cytology/histology quality score 1-3 (s. below).
Secondary endpoints included comparisons of several performance parameters, such as the following: (1) the number of needle passes needed to achieve a gain of a macroscopically optimal sample; (2) the quality of the cytological/histological specimens was rated with scores from 0 to 3 [0, non-representative; 1, representation questionable (poorly preserved, crush artifacts, overlapping cell groups); 2, representation limited (scant amount of diagnostic cells); and 3, representative], as modified from Payne et al[18]; (3) the cellularity of specimens was expressed by a score in a scale from 0 to 2 (0, poorly preserved (cellularity not reliably assessable); 1, low cellularity; 2, high cellularity); and (4) the rate of procedure-related complications. These included bleeding, perforation, acute pancreatitis, hemobilia, or death; patients were specifically followed for 24 h after the intervention.
Sample size was based on an inferiority design comparing both needles in diagnostic accuracy. Assuming the PC to be superior (sensitivity: 75% vs 60%), 53 evaluable patients were required (power 80%, alpha 0.05) to show superiority of the core needle.
Descriptive statistics for continuous variables are presented as means or medians with standard deviations (SD) or range, respectively. Categorical variables are reported as absolute values and percentages. Sensitivity, specificity, and diagnostic accuracy are calculated according to their definitions. Of note, the analyses only considered cases with adequate material.
Differences between the performance of AN and PC regarding the sample adequacy for evaluation as well as the rates of correct diagnosis were assessed using the McNemar’s test. Comparisons between the number of needle passes, cellularity score, quality of cytological/histological material score and the complication rates were assessed using the non-parametric Wilcoxon rank sum test. The level of statistical significance for all the tests was defined at a probability value of less than 0.05 (P < 0.05). Datasets were compiled by using Microsoft Excel and all the statistical analyses were performed with IBM SPSS Statistics V22.0 software (SPSS Inc., Chicago, IL, United States).
A total of 56 patients were finally included in the study (Figure 1); patient demographics and baseline characteristics are presented in detail in Table 1. About 2/3 of the lesions were in the pancreas; lesion parameters are shown in Table 2. Confirmation of the final diagnoses was performed on the basis of surgery (n = 26), definite EUS-FNA result (n = 16), clinical follow-up for more than 12 mo (n = 6), or by more than one means (n = 8). The mean propofol dose administered for sedation was 482 mg.
| Parameter | Value |
| No. of patients | 56 |
| Age (yr), mean (SD) | 68 (12) |
| Sex, n (%) | |
| Male | 29 (51.8) |
| Female | 27 (48.2) |
| BMI (kg.m-2), mean (SD) | 25.6 (3.6) |
| Presenting symptom(s) (% ) | |
| Pain | 22.4 |
| Weight loss | 28.3 |
| Jaundice | 19.6 |
| Parameter | n (%) |
| Location | |
| Pancreas | 38 (67.9) |
| Lymph nodes | 13 (23.2) |
| SMT | 4 (7.1) |
| Other | 1 (1.8) |
| Diameter (mm), mean (SD) | 33 (12) |
| Echogenicity on EUS1 | |
| Hyper-/hypo-/iso-echoic | 7 (12.7)/44 (80)/2 (3.6) |
| Non-homogeneous | 2 (3.6) |
| Final diagnosis | |
| Pancreatic adenocarcinoma | 25 (44.6) |
| Pancreatic NET | 7 (12.5) |
| Lymph node metastasis | 6 (10.7) |
| Inflammatory lymph node | 5 (8.9) |
| GIST | 3 (5.4) |
| Chronic pancreatitis | 2 (3.6) |
| Pancreatic metastasis2 | 2 (3.6) |
| Cholangiocarcinoma | 1 (1.8) |
| Pancreatic lymphoma | 1 (1.8) |
| Lymphoma | 1 (1.8) |
| Leiomyoma | 1 (1.8) |
| IPMN | 1 (1.8) |
| Lymphoma renal infiltration | 1 (1.8) |
| Gold standard method | |
| Surgery | 26 (46.4) |
| Definite EUS-FNA | 16 (28.6) |
| Clinical follow-up (> 12 mo) | 6 (10.7) |
| Combination | 8 (14.3) |
Technically successful advancement of the AN into the target lesion and sample collection was achieved in all of the cases. Inadequate for cytological analysis material was obtained in 2 cases, including pancreatic NET (n = 1) and gastric antrum SET (n = 1). A wrong diagnosis was reached in 6 patients. Missed cases comprised pancreatic adenocarcinoma (n = 2), inflammatory lymph nodes (n = 2), IPMN (n = 1), and pancreatic lymphoma (n = 1). No procedure-related complications were captured. Detailed performance characteristics of AN are shown in Table 3.
| Characteristic | Type of needle (all cases/histology cases) | ||
| AN (n = 56/36) | PC (n = 56/36) | P value | |
| Needle passes, mean (SD) | 1.5 (0.6)/1.5 (0.7) | 1.7 (0.6)/1.7 (0.6) | 0.14/0.16 |
| Cellularity, mean (SD) | 1.7 (0.6)/1.7 (0.6) | 1.1 (0.3)/1 (0) | 0.058/0.0342 |
| Cytologic/histologic quality, median (range) | 2.6 (0-3)/ 3 (0-3) | 2.4 (0-3)/3 (0-3) | 0.083/0.49 |
| Adequacy for diagnosis, n (%) | 54 (96.4)/35 (97.2) | 51 (91.1)/36 (100) | 0.38/0.99 |
| Correct diagnosis1, n (%) | 48/54 (88.9)/30/35 (85.7) | 49/51 (96.1)/34/36 (94.4) | 0.25/0.25 |
Technical success of EUS-FNA using the PC was universal (100%) and yielded adequate specimens (36 histological and 15 cytological) in all but 5 (pancreatic adenocarcinoma; n = 4, gastric antrum SET; n = 1) cases. A correct diagnosis, compared to the gold standard, was achieved in 49 evaluable patients. Missed cases included pancreatic adenocarcinoma (n = 1) and pancreatic lymphoma (n = 1). No adverse events were encountered (Table 3).
Adequate for interpretation material was obtained by the usage of at least one needle in 55/56 cases (98.2%). The gastric antrum SET case (n = 1) allowed no sufficient specimen collection in general. At least one device correctly diagnosed 53 of the 55 eligible for the assessment individuals (96.4%) with the exception of pancreatic adenocarcinoma (n = 1) and pancreatic lymphoma (n = 1). The collection of adequate material by both needles was achieved in 50/56 cases (89.3%). In 47 of them (94%), the cytological/histological analysis results were in agreement, while in only 2 of these patients (4.4%) both specimens yielded an incorrect diagnosis (missed cases; pancreatic adenocarcinoma, n = 1 and pancreatic lymphoma, n = 1) .
As shown in Table 3, there was no statistically significant difference between the AN and the PC in terms of the adequacy of specimens for the evaluation, mean number of passes, mean cellularity score, median specimen cytological or histological quality score, rates of correct diagnosis, technical success (100% in both) or complications (none in both).
In the 36 patients in whom histological material was obtained with PC, AN achieved better mean cellularity score (P = 0.034). Differences regarding the adequacy of specimens for evaluation, mean number of passes, median specimen quality score, rates of correct diagnosis, technical success, and complications were of no statistical importance (Table 3).
Table 4 summarizes the results of the two needles and provides separate data on pancreatic mass and lymph node subgroups.
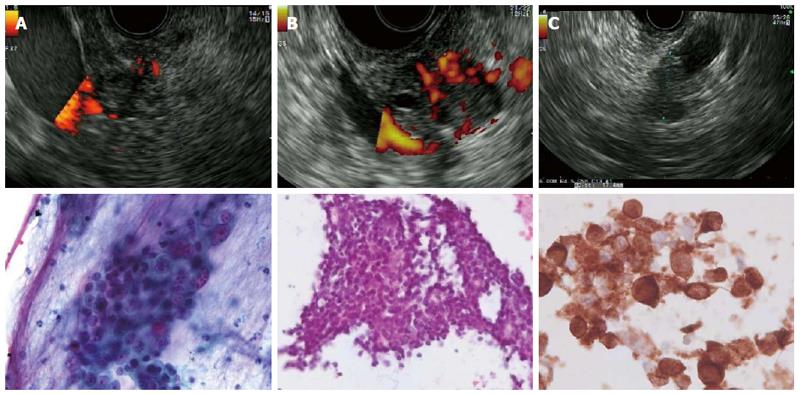
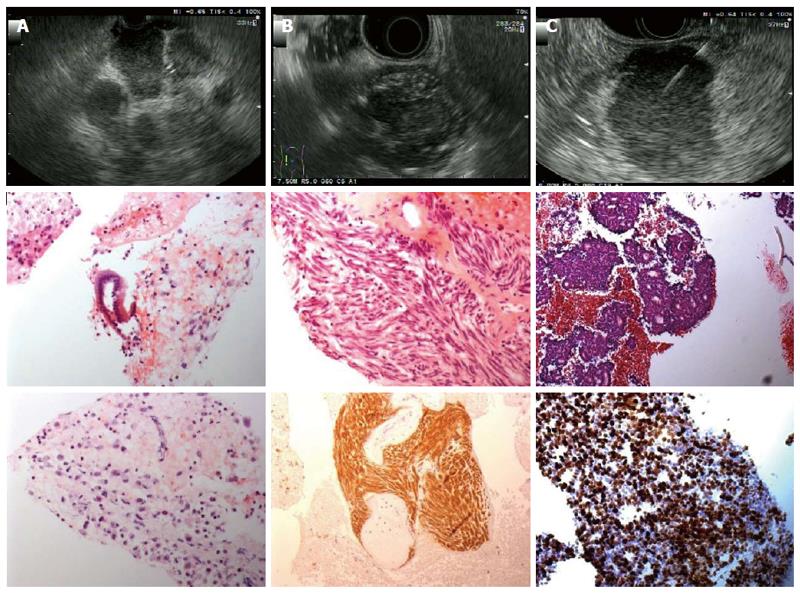
Figures 2 and 3 present various lesions as shown during EUS, including their appearance in the cytological/histological analysis.
EUS-FNA has been established as a safe and efficient technique for the diagnosis of solid lesions accessible from the upper gastrointestinal tract. Many prospective and retrospective series have reported reliable sensitivity, specificity, and overall diagnostic accuracy, mostly depending on the methodological factors, including on-site cytopathological evaluation, type of needle used, and number of needle passes[19,20]. In almost all of these studies, the diagnosis rested on cytological analysis. This appears to be well established; however, cytological analysis suffers from several principal limitations such as subjective interpretation with the lack of a standardized second-opinion process as is usual in histopathology, different categories with regards to the certainty of diagnosis (“suspicious” results are mostly counted positive in the respective studies), and further limitations concerning tissue characterization. In this setting, the complementary to routine cytology use of one or more immunocytochemical markers as well as flow cytometry (e.g., in lymphomas) seems to improve diagnostic accuracy. Moreover, molecular genetic analysis (e.g., assay for K-ras or p53 gene mutations by RT-PCR), although currently not a routine component of specimen analysis, increases EUS-FNA sensitivity, especially in patients with small tumors. These techniques may also serve the need for individualized tumor therapy in the future.
Given the limitations of cytology and the additional time and cost for the presence of a cytopathologist, efforts for the acquisition of core tissues suitable for histological analysis have been made leading to a variety of technical modifications beyond increasing needle size. Accordingly, an 18G FNA needle, a 19G FNA needle with a modified suction technique, as well as the Trucut device have been developed; however, studies indicate a somewhat lower efficacy when a transduodenal approach has to be used[21-25]. To overcome this disadvantage, the core tissues collected by EUS-FNA using standard 22G needles have undergone histological assessment[26-30]. The results of the respective studies varied, but showed that adequate specimens for histology were recovered in more than 80% of patients, histology demonstrated good accuracy rates, and combined cytological and histological analysis might be superior to either method alone. With the exception of procedural times and costs, no other significant difference between these two material processing methods was described.
Aiming to procure larger tissue specimens that enable histologic and immunophenotypic characterization, a novel needle device with side fenestration has been recently introduced (EchoTip ProCore). Initially, a 19G version became commercially available, followed by 22G and most recently 25 G. With respect to the 22G PC, the published case series report rates of adequate material for histologic analysis between 52.9% and 88.5% and overall diagnostic accuracy from 82% to 89%[13,15,16]. To enhance its role, a recently published study equals two passes using this reverse-beveled needle with the current gold-standard of FNA with on-site cytopathology assessment[31].
In our randomized study, we directly compared the performance of PC against standard AN (both 22G) on the basis of adequacy of obtained material for subsequent analysis (either cytological or histological) and the ability to provide a correct diagnosis. Although the utilization of both needles appeared technically feasible in all cases, we failed to demonstrate any statistically significant difference between the PC and AN in terms of the adequacy of tissue for the evaluation and rates of correct diagnosis. Moreover, no significant differences were encountered regarding the number of needle passes, specimen cellularity and quality scores and complication rates.
Our results are in line with those of most studies comparing the 22G version of the two needles (Table 5)[32-37] and a recently published meta-analysis of nine comparative studies[38]. On the contrary, Vanbiervliet et al[39] reported in 2014 the significant superiority of the aspiration needle in both overall (cytology and histology) and solely histology adequacy in pancreatic lesions. However, this study is biased in favor of AN since two passes with this needle are compared with only one pass with the PC. In contrast, the PC achieved better adequacy when sampling subepithelial tumors, according to Kim et al[40].
| Ref. | Design | No. of lesions | Target | Needles | Diagnostic yield | Sample adequacy | Comments |
| Witt et al[32] | Retrospective | 18 per needle type | Diverse | PC 22G vs AN 22G | Equivalent | Equivalent | PC: fewer passes needed |
| Strand et al[33] | RCT | 32 punctured by both needles | Pancreas | PC 22G vs AN 22G | AN > PC | Equivalent | Only 2 passes with PC vs 5 with AN, PC technical failure in 16 cases |
| Bang et al[34] | RCT | 28 per needle type | Pancreas | PC 22G vs AN 22G | Equivalent | Equivalent | On-site cytopathologist, needles of different manufactures |
| Lee et al[35] | RCT | 58 per needle type | Pancreas | PC 22/25G vs AN 22/25G | Equivalent | N/A | On-site cytopathologist, PC: fewer passes needed |
| Hucl et al[36] | RCT | 145 punctured by both needles | Diverse | PC 22G vs AN 22G | Equivalent | Equivalent | Only histology, PC: fewer passes needed |
| Mavrogenis et al[37] | RCT | 28 punctured by both needles | Pancreas + LNs | PC 25G vs AN 22G | Equivalent | Equivalent | Different needle gauges, “slow pull” sampling technique |
| Vanbiervliet et al[39] | RCT | 80 punctured by both needles | Pancreas | PC 22G vs AN 22G | Equivalent | Cytology: equivalent | Only 1 pass with PC vs 2 with AN |
| Histology: PC > AN | |||||||
| Kim et al[40] | RCT | 10 with AN, 12 with PC | SET | PC 22G vs AN 22G | PC > AN | PC > AN | Only histology, PC: fewer passes needed |
| Alatawi et al[41] | RCT | 50 per needle type | Pancreas | PC 22G vs AN 22G | Equivalent | Equivalent, cellularity: PC > AN | Equivalent results after 2 passes with PC vs 3 with AN |
The mean number of needle passes needed to achieve a satisfactory specimen did not differ in the present study. This finding opposes that of most similar studies that favor the PC device for fewer passes to achieve an adequate specimen[32,35,36,39,41]. Interestingly, our number of needle passes was much lower than that recommended in several EUS-FNA studies, although it did not lead to substantially lower accuracies[6,42].
The present study has several strengths, including its prospective randomized design, uniform sampling method for both needles, use of needles from the same manufacturer, and head-to-head comparisons that allow for improved statistical validity. Nevertheless, it also bears some limitations. Firstly, there was only one participating center. Secondly, the endoscopists could not be blinded for the needle due to obvious reasons. Thirdly, we enrolled patients with diverse lesion locations. Fourthly, the number of participant is rather small. Fifthly, the lack of an on-site cytopathologist, such as in most of the non-american series and daily routine procedures, may have prevented the optimal initial assessment and processing of the obtained material. Finally, the material obtained with either needle was subject to different preparation procedures - histology can only be attempted with the PC needle and, therefore, possible technical biases may have occurred. On the other hand, the aim of the PC needle is to add histology to the diagnostic armamentarium of EUS-FNA. We could show that this contributed very little to the overall diagnostic accuracy.
Our results may be of limited generalizability given the above mentioned methodological factors. However, they definitely add to the existing body of available literature showing that the two needles do not differ significantly concerning overall technical and diagnostic performance. Keeping that in mind, endoscopists could possibly base the choice of needle type for EUS-FNA on other parameters (i.e., availability, cost, and procedural times).
In conclusion, our study pointed out that, when compared to the standard 22G FNA needle, the reverse-beveled PC of the same gauge yielded a similar overall diagnostic accuracy and performed equally in terms of the sample adequacy for cytological analysis, quality and cellularity of the obtained specimens. This is in line with the majority of published comparative studies. No significant differences regarding the needed number of passes, technical feasibility, and complications were addressed. The overall contribution of histology to the diagnosis with the PC needle was limited.
Athanasios D Sioulas is a scholar of the Hellenic Society of Gastroenterology.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is an established modality for tissue sampling of intra- and paramural lesions, while cytological analysis represents the diagnostic standard of care. The recent development of fenestrated needles facilitates the acquisition of specimens that are also suitable for histological analysis thereby promising the improvement of diagnostic accuracy. The authors aimed to compare the standard aspiration needle (AN) with the novel core biopsy needle (PC) of an identical diameter (22G) in terms of various performance characteristics.
According to these results, both needles performed equally in terms of overall diagnostic accuracy, sample adequacy for analysis, quality and cellularity of specimens, number of needle passes, feasibility, and complications. Interestingly, the histological analysis of the specimens obtained with the PC needle showed no additive contribution to the diagnosis.
The current study adds to the growing body of evidence supporting that both AN and PC demonstrate similar technical and performance characteristics when used for tissue sampling with EUS-FNA.
Keeping the results of they prospective clinical trial in mind, endoscopists could base their choice of needle type for EUS-FNA on other parameters (i.e., availability, cost, and procedural times).
EUS-FNA refers to the acquisition of abnormal tissue samples with specialized needles under endoscopic ultrasound guidance. The diagnostic accuracy of a method represents the association between its result and the disease status of the study participant. The cellularity of a specimen reflects the number of its constituent cells.
This study is well conducted and its conclusions will certainly contribute to the future use of EUS-FNA as a safe and established method of tissue acquisition, as well as make the needle type choice an easier decision.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beg M, Stoos-Veic T, Yusuf MA S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [PubMed] |
| 2. | Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | O’Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Siddiqui AA, Brown LJ, Hong SK, Draganova-Tacheva RA, Korenblit J, Loren DE, Kowalski TE, Solomides C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Hawes RH. The evolution of endoscopic ultrasound: improved imaging, higher accuracy for fine needle aspiration and the reality of endoscopic ultrasound-guided interventions. Curr Opin Gastroenterol. 2010;26:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190. [PubMed] |
| 7. | Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: an efficient approach. Gastrointest Endosc. 2001;54:485-490. [PubMed] |
| 8. | Kopelman Y, Marmor S, Ashkenazi I, Fireman Z. Value of EUS-FNA cytological preparations compared with cell block sections in the diagnosis of pancreatic solid tumours. Cytopathology. 2011;22:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185-190. [PubMed] |
| 13. | Fabbri C, Luigiano C, Maimone A, Tarantino I, Baccarini P, Fornelli A, Liotta R, Polifemo A, Barresi L, Traina M. Endoscopic ultrasound-guided fine-needle biopsy of small solid pancreatic lesions using a 22-gauge needle with side fenestration. Surg Endosc. 2015;29:1586-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Iwashita T, Nakai Y, Samarasena JB, Park DH, Zhang Z, Gu M, Lee JG, Chang KJ. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Larghi A, Iglesias-Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, Abdulkader I, Arcidiacono PG, Costamagna G, Biermann K. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Paik WH, Park Y, Park DH, Hong SM, Lee BU, Choi JH, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of new 22 gauge endoscopic ultrasound core needle using capillary sampling with stylet slow-pull technique for intra-abdominal solid masses. J Clin Gastroenterol. 2015;49:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 18. | Payne M, Staerkel G, Gong Y. Indeterminate diagnosis in fine-needle aspiration of the pancreas: reasons and clinical implications. Diagn Cytopathol. 2009;37:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 20. | Turner BG, Cizginer S, Agarwal D, Yang J, Pitman MB, Brugge WR. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Larghi A, Verna EC, Ricci R, Seerden TC, Galasso D, Carnuccio A, Uchida N, Rindi G, Costamagna G. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: a prospective study. Gastrointest Endosc. 2011;74:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, Hoffman BJ, Wallace MB. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, Soehendra N. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121-127. [PubMed] |
| 26. | Möller K, Papanikolaou IS, Toermer T, Delicha EM, Sarbia M, Schenck U, Koch M, Al-Abadi H, Meining A, Schmidt H. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, Abdulkader I, Larino-Noia J, Antunez J, Forteza J. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O’Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [PubMed] |
| 29. | Papanikolaou IS, Adler A, Wegener K, Al-Abadi H, Dürr A, Koch M, Pohl H, Abou-Rebyeh H, Veltzke-Schlieker W, Wiedenmann B. Prospective pilot evaluation of a new needle prototype for endoscopic ultrasonography-guided fine-needle aspiration: comparison of cytology and histology yield. Eur J Gastroenterol Hepatol. 2008;20:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Brais RJ, Davies SE, O’Donovan M, Simpson BW, Cook N, Darbonne WC, Chilcott S, Lolkema MP, Neesse A, Lockley M. Direct histological processing of EUS biopsies enables rapid molecular biomarker analysis for interventional pancreatic cancer trials. Pancreatology. 2012;12:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Lin M, Hair CD, Green LK, Vela SA, Patel KK, Qureshi WA, Shaib YH. Endoscopic ultrasound-guided fine-needle aspiration with on-site cytopathology versus core biopsy: a comparison of both techniques performed at the same endoscopic session. Endosc Int Open. 2014;2:E220-E223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Witt BL, Adler DG, Hilden K, Layfield LJ. A comparative needle study: EUS-FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Strand DS, Jeffus SK, Sauer BG, Wang AY, Stelow EB, Shami VM. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol. 2014;42:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Cha SW, Cho YD, Park SH. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014;46:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Hucl T, Wee E, Anuradha S, Gupta R, Ramchandani M, Rakesh K, Shrestha R, Reddy DN, Lakhtakia S. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy. 2013;45:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Mavrogenis G, Weynand B, Sibille A, Hassaini H, Deprez P, Gillain C, Warzée P. 25-gauge histology needle versus 22-gauge cytology needle in endoscopic ultrasonography-guided sampling of pancreatic lesions and lymphadenopathy. Endosc Int Open. 2015;3:E63-E68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 39. | Vanbiervliet G, Napoléon B, Saint Paul MC, Sakarovitch C, Wangermez M, Bichard P, Subtil C, Koch S, Grandval P, Gincul R. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: a randomized crossover study. Endoscopy. 2014;46:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Kim GH, Cho YK, Kim EY, Kim HK, Cho JW, Lee TH, Moon JS. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Alatawi A, Beuvon F, Grabar S, Leblanc S, Chaussade S, Terris B, Barret M, Prat F. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 42. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. [PubMed] |