Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8760
Peer-review started: May 27, 2016
First decision: July 13, 2016
Revised: July 21, 2016
Accepted: August 5, 2016
Article in press: August 5, 2016
Published online: October 21, 2016
Processing time: 147 Days and 19.2 Hours
To evaluate the immunomodulatory effect of oral administration of PRX-106 in the high-fat diet model.
For 22 wk, C57BL/6 HFD-fed mice received daily oral treatments with BY-2 cells expressing recombinant anti-tumor necrosis factor alpha fusion protein (PRX-106). Mice were followed for serum liver enzyme and triglyceride levels, liver histology and intrahepatic and systemic FACS.
The orally administered non-absorbable PRX-106 was biologically active. Altered distribution of CD4+CD25+FoxP3+ between the liver and spleen and an increase in the intrasplenic-to-intrahepatic CD4+CD25+FoxP3+ ratio and a decrease in the intrasplenic-to-intrahepatic CD8+CD25+FoxP3+ ratio were observed. An increase in intrahepatic NKT cells and a decrease in the intrasplenic-to-intrahepatic NKT ratio were noted. Assessment of the CD4-to-CD8 ratios showed sequestration of CD8+ lymphocytes in the liver. These effects were associated with a decrease in serum triglyceride levels, decrease in the aspartate aminotransferase levels, serum glucose levels, and HOMA-IR score. A decrease in hepatic triglycerides content was observed in the high dose-treated mice.
Orally administered PRX-106 shows biological activity and exerts an immunomodulatory effect, alleviating liver damage. The data suggest that PRX-106 may provide an oral immunotherapy for nonalcoholic steatohepatitis.
Core tip: The BY-2 plant cell-expressed recombinant anti-tumor necrosis factor alpha (TNF) fusion protein (PRX-106) that consists of the soluble form of the human TNF receptor fused to the Fc component of a human IgG1 domain was orally administered in high-fat diet model. Altered distribution of CD4+CD25+FoxP3+ and a decrease in the intrasplenic-to-intrahepatic CD8+CD25+FoxP3+ ratio were observed. These effects were associated with a decrease in serum triglyceride levels, decrease in the aspartate aminotransferase levels, serum glucose levels, and HOMA-IR score. A decrease in hepatic triglycerides content was observed in the high dose-treated mice. The data suggest that PRX-106 may provide an oral immunotherapy for nonalcoholic steatohepatitis.
- Citation: Ilan Y, Ben Ya'acov A, Shabbat Y, Gingis-Velitski S, Almon E, Shaaltiel Y. Oral administration of a non-absorbable plant cell-expressed recombinant anti-TNF fusion protein induces immunomodulatory effects and alleviates nonalcoholic steatohepatitis. World J Gastroenterol 2016; 22(39): 8760-8769
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8760.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8760
A chronic inflammatory state and dysfunction of metabolic-inflammatory signaling are involved in the development of various aspects of non-alcoholic steatohepatitis (NASH)[1,2]. Several downstream signaling pathways, which provide the crosstalk between inflammatory and metabolic signaling, have been identified. The pro-inflammatory axis consisting of the nuclear transcription factor NF-kappa B and its upstream kinase IKK-beta is one pathway responsible for nutritionally induced inflammation[3]. c-Jun N-terminal kinase and I kappa beta kinase (I kappa K) have been identified as mediators of insulin resistance (IR)[4,5]. These pathways are activated along with other signals by tumor necrosis factor alpha (TNF-α)[6].
The importance of TNF in human and animal fatty liver diseases, which were induced both by genetic manipulation and by overnutrition, has been demonstrated[7]. Neutralization of TNF-α activity improves IR and fatty liver disease in animals. Adiponectin is a potent TNF-neutralizing and anti-inflammatory adipocytokine. Both in vitro and in vivo studies showed its importance in counteracting inflammation and IR[8,9]. Some of the anti-inflammatory effects of adiponectin are mediated by suppression of TNF synthesis and the promotion of anti-inflammatory cytokines, including IL-10 and the IL-1 receptor antagonist[7].
The NLRP6 and NLRP3 inflammasomes and the effector protein IL-18 negatively regulate NASH progression[10]. Inflammasome deficiency-associated changes in the configuration of the gut microbiota are associated with exacerbated hepatic steatosis and inflammation through influx of TLR4 and TLR9 agonists into the portal circulation, leading to the enhanced hepatic TNF-α expression that drives NASH progression[10]. A recent meta-analysis showed a difference in the TNF-α-238 genotype distribution between nonalcoholic fatty liver disease (NAFLD) patients and controls, suggesting that a polymorphism at position-238 is a risk factor for NAFLD[11]. Increased gut permeability along with bacterial translocation (BT) and increased lipopolysaccharide (LPS) levels have been described in patients with NASH[12,13]. BT at mesenteric lymph nodes leads to lymphocyte activation[14]. The TNF-alpha mRNA expression in liver tissue is significantly higher in patients with NASH and correlates with the increase in the plasma levels of LPS binding protein.
The development of biological agents that target TNF have markedly changed the therapeutic approach to inflammatory diseases[15]. Pentoxifylline, an anti-TNF-α agent was shown to reduce aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and to improve liver histological scores in patients with NAFLD/NASH[16]. Parenteral administration of recombinant anti-TNF proteins lowers disease activity and, in some patients, induces remission. A report described a NASH patient who experienced rapid normalization of liver biochemistry during treatment for an associated rheumatoid arthritis with the humanized anti-TNF-alpha antibody adalimumab[17].
Etanercept is a recombinant, dimeric, soluble tumor necrosis factor receptor fusion protein that blocks only soluble TNF but not membrane-bound TNF. Parenteral administration of etanercept is being used for rheumatoid arthritis, juvenile rheumatoid arthritis, psoriatic arthritis, psoriasis, and ankylosing spondylitis[15,18]. Parenterally administered TNF antagonists are generally well tolerated but carry a risk of side effects. Areas of concern include opportunistic and non-opportunistic infections, vaccination, neurological complications, hepatotoxicity, hematological side effects, malignancies, infusion reactions and autoimmunity. Contraindications, such as heart failure and acute infectious diseases, are also of concern[19]. The immunosuppressive capacity of these agents necessitates a rigorous long-term safety follow-up, and the potential risks of their use should always be taken into consideration[19,20].
Oral delivery of therapeutic proteins is a major goal when developing new therapeutic modalities. The BY-2 plant cell-expressed recombinant anti-TNF fusion protein (PRX-106) consisting of the soluble form of the human TNF receptor (TNFR) fused to the Fc component of a human IgG1 domain can be orally administered, and PRX-106 has an amino acid sequence that is identical to that of Enbrel™.
The aim of the present study was to determine the immunomodulatory effect of oral administration of plant cells expressing PRX-106 in an animal model of NASH.
Male C57BL/6 mice (11-12-wk-old) were obtained from Harlan Laboratories (Jerusalem, Israel) and maintained in the Animal Core of the Hadassah-Hebrew University Medical School. Mice were administered standard laboratory chow and water ad libitum and kept on a 12 h light/dark cycle. Animal experiments were carried out according to the guidelines of the Hebrew University-Hadassah Institutional Committee for the Care and Use of Laboratory Animals and with the committee’s approval. Mice were fed a high-fat diet (HFD, Harlan, TD88137; 42% of the calories are from fat) from day 0 until their sacrifice at 24 wk.
Four groups of C57BL/6 mice, n = 10 each, were orally fed three times a week for 24 wk with one of the following at a volume of 35 μL: phosphate-buffered saline (PBS, group A), BY-2 cells at 28.8 mg of BY- (mock cells, group B), 2.88 mg (0.5 μg TNF) of BY+ (group C), or 2.88 mg (10 μg anti-TNF) of BY+ (group D). Fresh preparations were made before each administration.
The immunomodulatory effect of PRX-106 was determined by FACS analysis and serum cytokines.
Isolation of splenocytes and hepatic lymphocytes: Spleens and livers were kept in RPMI-1640 supplemented with FCS. Spleens were crushed through a 70 μm nylon cell strainer[21] and centrifuged (1250 rpm for 7 min) to remove debris. Red blood cells were lysed with 1 mL of cold 155 mmol/L ammonium chloride lysis buffer and immediately centrifuged (1250 rpm for 3 min). The splenocytes were then washed and suspended in 1 mL of RPMI + FCS. Any remaining connective tissue was removed. The viability, as assessed using trypan blue staining, was above 90%. For the isolation of hepatic lymphocytes, livers were first crushed through a stainless mesh (size 60, Sigma), and the cell suspension was placed in a 50 mL tube for 5 min so that the cell debris could form a pellet. The cell suspension was slowly underlaid with 10 mL of Lymphoprep (Ficoll, Axis-Shield PoC AS, Oslo, Norway) in a 50 mL tube. The tubes were then centrifuged at 1800 rpm for 18 min. Cells at the interface were collected and transferred to a new tube that was centrifuged again at 1800 rpm for 10 min. The resulting pellet of hepatocyte-depleted lymphocytes was suspended in a final volume of 250 μL, approximately 1 × 106 intrahepatic lymphocytes were recovered per mouse liver.
Flow cytometry: Flow cytometry was performed on splenocytes and hepatic lymphocytes, which were suspended in 1 mL of FACS buffer (PBS + 1% BSA + 0.1% sodium azide). The cells were stained with the antibodies for CD8, CD4, CD25, Foxp3, CD3, and NK 1.1. Flow cytometry was performed using LSR-II, and and FSC express software.
Cytokine measurement: Serum TNF-α levels were measured in each animal using commercial kits, according to the manufacturer’s instructions (Quantikine, R&D Systems, Minneapolis, MN, United States).
Liver enzymes: Serum was obtained from individual mice. The serum AST and ALT levels were determined using an automatic analyzer.
Triglyceride levels in the serum were measured using standard kits.
Fasting serum glucose and insulin levels were measured on day 1 and at week 24.
Glucose tolerance test: Glucose tolerance test (GTT) was performed on all animals in all groups at weeks 8 and 24.
Histological examination of the liver: Paraffin-embedded liver sections were prepared from each mouse. The livers were cut into 4-5 μm thin slices and stained with hematoxylin-eosin. A blinded pathologist examined the tissues using light microscopy to score for morphological and histopathological changes that are characteristic of NAS. Trichome staining was used to evaluate liver fibrosis. The maximal score for steatosis (= 3) was assigned for greater than 66%. The maximal score for lobular inflammation (= 3) was assigned for > 4 foci/× 200, and hepatocyte ballooning (= 2) was assigned for many cells/prominent ballooning. The maximal NAS score is a simple arithmetic combination of all three features (3 + 3 + 2 = 8).
Hepatic triglycerides content: Accumulation of intracellular triglycerides (TGs) within the liver was quantified using a modification of the Folch method. TGs were extracted from aliquots of snap-frozen livers and then assayed. Triglyceride determination was performed spectrophotometrically using a GPO-Trinder kit (Sigma, Rehovot, Israel), and the levels were normalized to the protein content in the homogenate.
Statistical analysis was performed using Student’s t test. A P value less than 0.05 was considered significant.
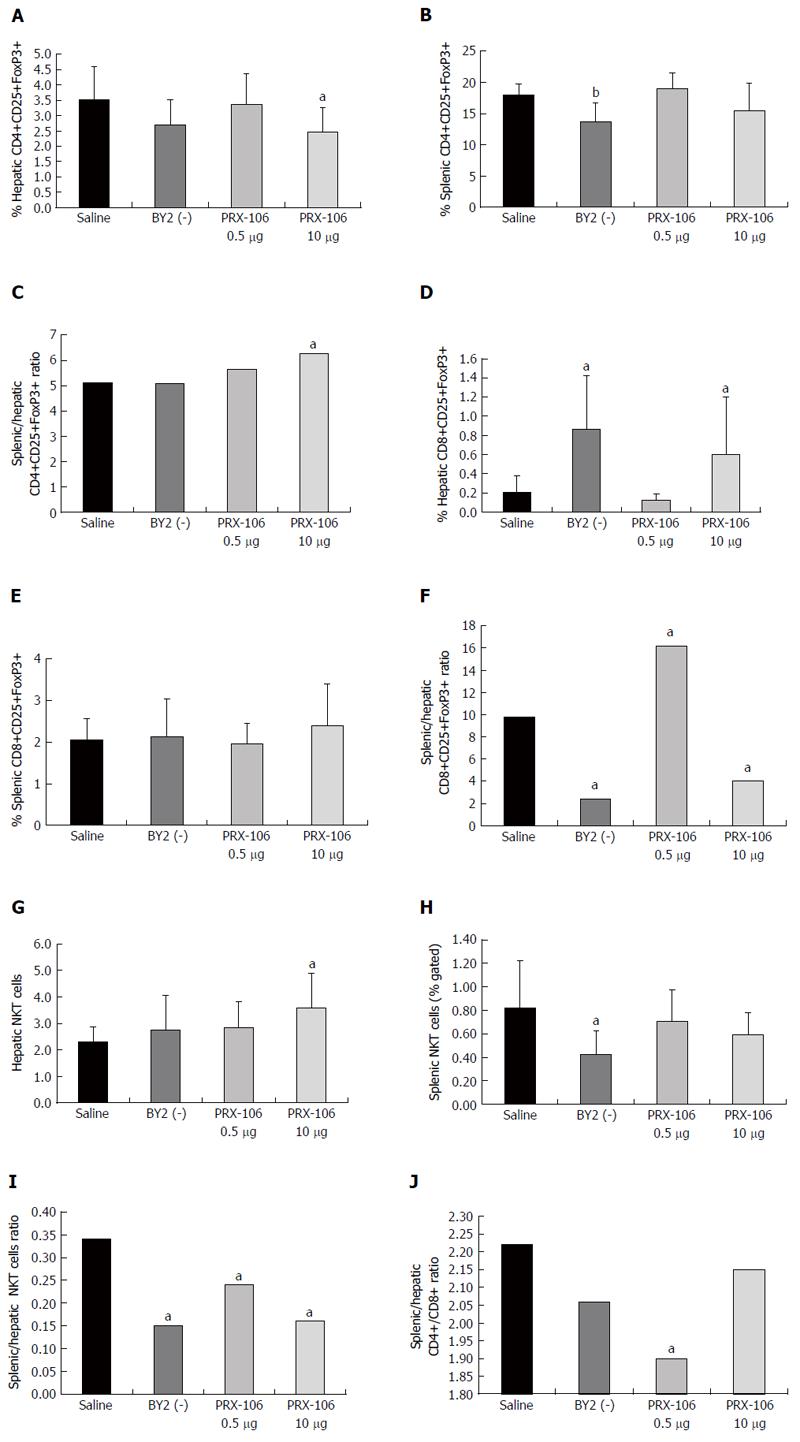
Oral administration of PRX-106 altered the distribution of CD4+CD25+FoxP3+ Tregs. Figure 1A shows the significant decrease in intrahepatic Tregs in the high dose-treated mice from the 3.53% value in the controls to 2.48% (P < 0.05). Figure 1B shows the significant effect on intrasplenic Tregs only for BY2 (-) treated mice from 18.4% to 13.73% (P < 0.01). A reduction trend was noted for the high dose-treated group (15.49%). The intrasplenic-to-intrahepatic CD4+CD25+FoxP3+ Treg ratio increased significantly in the high dose PRX-106-treated mice, as seen in Figure 1C, from 5.12 in controls to 6.25 (P < 0.05). The data indicate an effect of PRX106 on the redistribution of CD4+CD25+FoxP3+ Tregs.
Oral administration of PRX-106 also altered the distribution of CD8+CD25+FoxP3+ Tregs. Figure 1D shows a significant increase in intrahepatic CD8+CD25+FoxP3+ Tregs in the BY2 (-) and high dose-treated mice. The levels increased to 0.6% and 0.86%, respectively, compared with 0.21% in the controls (P < 0.05). Figure 1E shows no statistically significant effect on intrasplenic CD8+CD25+FoxP3+ Tregs. The intrasplenic-to-intrahepatic CD8+CD25+FoxP3+ Treg ratio was significantly altered by the treatments, as shown in Figure 1F. The ratio was 9.8 in the controls and decreased to 2.4 and 4 in the BY2 (-)- and high dose-treated mice while increasing to 16.2 in the low dose-treated mice (P < 0.005 compared with controls).
Oral administration of PRX-106 altered the distribution of NKT (CD3+NK1.1+) lymphocytes. Figure 1G shows an increase in intrahepatic NKT cells in the high dose-treated mice, with levels increasing 3.58% compared 2.31% in controls (P < 0.05). Figure 1H shows a significant decrease in the intrasplenic NKT cells in the BY2(-)-treated mice, in which the levels decreased to 0.42% compared with 0.82% in controls (P < 0.05). The intrasplenic-to-intrahepatic NKT ratio significantly decreased in all treated groups, as shown in Figure 1I. The levels decreased to 0.15, 0.24 and 0.16 for the BY2(-)-treated and low dose and high dose PRX106-treated mice, respectively, compared with 0.35 in the controls (P < 0.05 vs controls).
To determine the effect of treatment on lymphocyte trapping in the liver, the CD4/CD8 lymphocyte ratio was calculated. The splenic CD4/CD8 ratio was 1.62, 1.48, 1.56 and 1.46 for the controls, BY2 (-)-treated and low and high dose PRX106-treated mice, respectively. The hepatic CD4/CD8 ratio was 0.73, 0.72, 0.82, and 0.68 for the controls, BY2(-)-treated and low and high dose PRX106-treated mice, respectively. Figure 1J shows the ratio between the splenic and hepatic CD4/CD8 ratios. For all treated groups, a decrease in the ratio was found: 2.06, 1.9, and 2.15 for the BY2(-)-treated and the low and high dose PRX106-treated mice compared with 2.2 for the controls (P < 0.005 for low dose PRX106 compared with controls). The data suggest that the treatment is associated with sequestration of CD8+ lymphocytes in the liver.
Oral administration of PRX-106 was associated with a mild increase in serum TNF-α levels. The levels increased to 11.53, 13.85, and 10.25 pg/mL for BY2 (-)-treated and low and high dose PRX106-treated mice, respectively, compared with 7.07 pg/mL in the controls (P < 0.05 for low dose PRX106 compared with controls). The data support the notion that the observed anti-inflammatory effect is not associated with a reduction in TNF-α levels.
During the 24 wk of the experiment, the weight doubled for the mice in all groups. The average weight gain was 82.5%, 83.3%, 81.8%, and 89.1% for the controls, BY2(-)-treated and low and high dose PRX106-treated mice, respectively (P = NS). The data support the notion that the beneficial effect on the liver, glucose, and lipid metabolism was independent of weight.
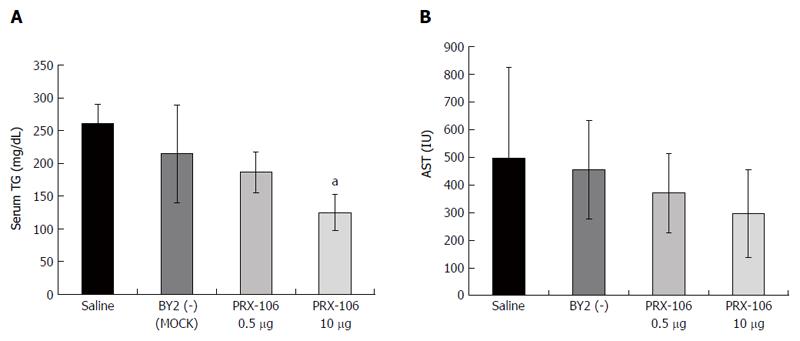
Both dosages of PRX-106 exerted a beneficial effect on the serum TGs at week 24, as shown in Figure 2A. The serum triglyceride levels decreased to 186 and 124 mg/dL for the low and high PRX106-treated groups compared with 260 mg/dL for untreated controls (P < 0.01 for high dose vs controls). Figure 2B shows that oral administration of the high dose of PRX-106 decreased the AST levels at week 24. The AST levels were 297 compared with 496 IU for the high dose PRX106 vs the controls, respectively (P = 0.06). No significant effects on the ALT levels were observed.
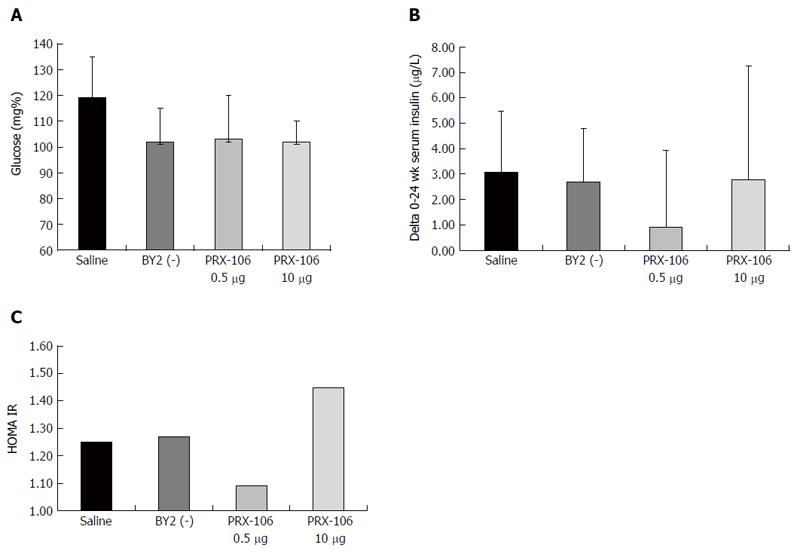
A beneficial effect of oral PRX106 on fasting glucose levels was noted. At week 5, a significant decrease in serum glucose levels was observed for the lower dose-treated PRX 106 with levels of 88, 76 and 86 mg% for the BY2(-)-treated and low and high dose PRX106-treated mice, respectively, compared with 104 mg% in the controls (P < 0.05 for low dose vs controls). Figure 3A shows the beneficial effect of the treatments at the end of trial, with a reduction in the glucose levels to 102, 103, and 102 mg% for the BY2(-)-treated and low and high dose PRX106-treated mice, respectively, compared with 119 mg% in controls. Figure 3B shows the beneficial effect of the treatment on the delta of the increase in fasting insulin levels between week 0 and 24. A trend for a reduction in the average increase in insulin levels between weeks 0 and 24 was noted in all treated mice. The average change in insulin levels was 2.69, 0.92, and 2.77 pg/mL for BY2 (-)-treated and low and high dose PRX106-treated mice, respectively, compared with 3.06 pg/mL in controls. Figure 3C shows the effect of oral PRX106 on the HOMA-IR. A reduction trend for a reduction to 1.09 was observed for mice in the low dose PRX106-treated group compared with the 1.25 value in the controls. No significant differences were noted between groups in the oral GTT performed at weeks 7 and 22 of the study.
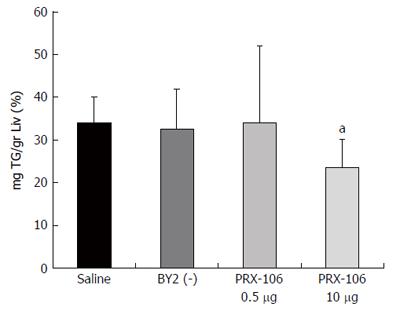
A decrease in hepatic TG content was observed in the high dose-treated mice, as shown in Figure 4. The triglyceride content was reduced to 23.54% per g liver for the high dose PRX-106-treated mice compared with 33.98% for the controls (P = 0.03).
Orally administered plant cells expressing recombinant anti-TNF fusion protein show biological activity and exert an immunomodulatory effect, alleviating the liver damage in the HFD model.
The pathogenesis of NASH involves a number of immune mechanisms[22]. Immunomodulatory treatments have been suggested to play a role in alleviating the disease[1]. The results of the present study show that oral administration of non-absorbable PRX-106 exerted an immunomodulatory effect. Oral administration of PRX-106 was associated with an increase in the intrasplenic-to-intrahepatic CD4+CD25+FoxP3+ ratio, suggesting that the drug generates a signal in the gut that promotes regulatory cells in the periphery, which can account for its anti-inflammatory effects[23]. Resident Treg cells in adipose tissue modulate metabolism and glucose homeostasis[24]. Interactions among leptin, Treg cells and adipose tissue are potential targets for therapeutic interventions[25]. The Treg frequency is lower in TNF-α transgenic mice than in wild-type mice[26]. In humans, anti-TNFα therapy has a clinical effect associated with promotion of Treg number and function. Anti-TNFα therapy increased the Treg proportion and suppressed effector T cells in patients with arthritis[27].
Oral administration of PRX-106 also altered the distribution of CD8+CD25+FoxP3+ Tregs. CD8+CD25+FoxP3+ Tregs were suggested to be important in the induction of the systemic anti-inflammatory effects mediated by several immunomodulatory agents[28,29] and to suppress inflammation in several immune-mediated disorders[30]. The data suggest a dose dependency of the observed immune system effect, which may underlie some of the differences noted in the clinical effects.
Oral administration of PRX-106 altered the distribution of NKT (CD3+NK1.1+) lymphocytes. The intrasplenic-to-intrahepatic NKT ratio significantly decreased in all treated groups. NKT cells play a regulatory role that helps to prevent diet-induced obesity and metabolic dysfunction[31]. A reduction in the number and an altered function of intrahepatic NKT lymphocytes have been reported in leptin-deficient ob/ob mice, a murine model for NASH[32-35]. NKT cells were suggested to be important in the regulation of immune-mediated disorders[36,37], and alteration of their distribution was shown to be relevant in immunomodulation[38-42]. Our results further support a regulatory role for NKT cells in the crosstalk between metabolism and the immune system[31].
The ratio between the splenic and hepatic CD4/CD8 ratios decreased for all treated groups, supporting the notion that the treatment is associated with sequestration of CD8+ lymphocytes in the liver. The liver is a site at which apoptotic CD8+ cells accumulate during the clearance phase of peripheral immune responses[43]. It serves as a “graveyard” for T cells activated in the periphery[44]. The liver was shown to be an important site for CD8+ accumulation during tolerance induction in a process that was independent of NK cells[45]. Our results support the ability of PRX-106 to promote CD8 lymphocytes in the liver during an active systemic inflammatory process.
Oral administration of PRX-106 was associated with a mild increase in serum TNF-α levels, suggesting that the anti-inflammatory effects are independent of this cytokine. PRX-106 is not absorbed, and its immunomodulatory effect is therefore associated with biological activity in the gut. A similar effect was reported for the oral administration of the non-absorbable anti-CD3[46-49] and delayed release 6 mercaptopurine[50], all of which generate a similar signal in the gut which alters the systemic immune system.
Oral administration of PRX106 was associated with a reduction in the serum triglyceride, glucose, insulin, and AST levels. A decrease in hepatic TG content was observed in the high dose-treated mice. Taken together, our data suggest that the profound immunomodulatory effect of oral PRX106 is associated with improvement in the metabolic syndrome in the HFD model.
Etanercept is a TNFR2-Fc fusion protein that blocks only soluble TNF but not membrane-bound TNF[51]. Parenteral administration of this compound has been successfully used in the treatment of rheumatoid arthritis and several other immunomodulatory treatments. The data of the present study support an immunomodulatory effect of orally administered PRX-106. Several immunomodulatory agents exert a different effect on the systemic immune system when administered orally compared with during parenteral administration[52-56]. Their local effect on the gut is different than when the drug is administered parentally. These compounds use the inherit ability of the immune system of the gut to systemically promote Tregs[54,55] and to exert potent anti-inflammatory effects[54,55]. These effects are not associated with generalized immune suppression.
Adjuvants were suggested to be important for augmenting the effect of orally administered immunomodulatory agents[48,49,56]. The plant cell wall, which is composed of cellulose, serves to protect the active molecule from acid-base changes in the stomach. The data of the present study suggest that the plant cell wall also serves as an immune adjuvant in the gut. Oral administration of mock cells, BY2 (-), which included vehicle alone, did exert some immunomodulatory effects.
Previous studies showed that oral administration of BY-2 cells expressing PRX-106 alleviated immune-mediated liver injury in the Concanavalin A immune-mediated hepatitis model[57]. Similarly, in the TNBS colitis model, oral administration of BY-2 plant cells expressing PRX-106 resulted in a decrease in the weight loss associated with immune-mediated colitis, along with improvement in bowel histology[58].
In summary, orally administered plant cells expressing recombinant anti-TNF fusion protein show biological activity when administered orally. It alters the systemic immune environment and affects both the intrahepatic and splenic regulatory T lymphocyte and NKT cell subsets. These changes were associated with the observed beneficial effects on the metabolic syndrome in the HFD model. As a non-absorbable agent, PRX106 may serve as a potent immunomodulatory agent that lacks immunosuppressive properties.
The BY-2 plant cell-expressed recombinant anti-TNF fusion protein (PRX-106) that consists of the soluble form of the human TNF receptor (TNFR) fused to the Fc component of a human IgG1 domain was orally administered. The aim of the study was to evaluate the immunomodulatory effect of oral administration of PRX-106 in the high-fat diet (HFD) model of non-alcoholic steatohepatitis (NASH).
PRX-106 consisting of the soluble form of the human TNFR fused to the Fc component of a human IgG1 domain can be orally administered, and PRX-106 has an amino acid sequence that is identical to that of Enbrel™.
For 22 wk, C57BL/6 HFD-fed mice received daily oral treatments with BY-2 cells expressing PRX-106. Orally administered PRX-106 shows biological activity and exerts an immunomodulatory effect, alleviating liver damage. The data suggest that PRX-106 may provide an oral immunotherapy for NASH. The orally administered non-absorbable PRX-106 was biologically active. Altered distribution of CD4+CD25+FoxP3+ between the liver and spleen and an increase in the intrasplenic-to-intrahepatic CD4+CD25+FoxP3+ ratio and a decrease in the intrasplenic-to-intrahepatic CD8+CD25+FoxP3+ ratio were observed. An increase in intrahepatic NKT cells and a decrease in the intrasplenic-to-intrahepatic NKT ratio were noted. Assessment of the CD4-to-CD8 ratios showed sequestration of CD8+ lymphocytes in the liver. These effects were associated with a decrease in serum triglyceride levels, decrease in the aspartate aminotransferase levels, serum glucose levels, and HOMA-IR score. A decrease in hepatic TG content was observed in the high dose-treated mice.
PRX-106 is biologically active when administered orally. It alters the systemic immune environment and affects both the intrahepatic and splenic regulatory T lymphocyte and NKT cell subsets. These changes were associated with the observed beneficial effects on the metabolic syndrome in the HFD model of NASH. As a non-absorbable agent, PRX106 may serve as a safe and potent immunomodulatory agent that lacks immunosuppressive properties.
A chronic inflammatory state and dysfunction of metabolic-inflammatory signaling are involved in the development of various aspects of NASH. Oral delivery of therapeutic proteins is a major goal when developing new therapeutic modalities.
This is a pre-clinical trial that shows the potential beneficial effect of oral administration of PRX-106 in a model for NASH. Further clinical trials are required for showing its effects in patients with type 2 diabetes and NASH.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Peltec S, Sherif ZA, Strom SC S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Ilan Y. Immune therapy for nonalcoholic steatohepatitis: are we there yet? J Clin Gastroenterol. 2013;47:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 6376] [Article Influence: 354.2] [Reference Citation Analysis (1)] |
| 3. | Cai D. NFkappaB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle. 2009;8:2542-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Sztajnkrycer MJ, Bond GR. Chronic acetaminophen overdosing in children: risk assessment and management. Curr Opin Pediatr. 2001;13:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond). 2008;32 Suppl 7:S52-S54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S53-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 473] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Andrade-Oliveira V, Câmara NO, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. 2015;2015:681612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Gu P, Xu A. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord. 2013;14:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1875] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 11. | Wang JK, Feng ZW, Li YC, Li QY, Tao XY. Association of tumor necrosis factor-α gene promoter polymorphism at sites -308 and -238 with non-alcoholic fatty liver disease: a meta-analysis. J Gastroenterol Hepatol. 2012;27:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol. 2006;12:1493-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Úbeda M, Muñoz L, Borrero MJ, Díaz D, Francés R, Monserrat J, Lario M, Lledó L, Such J, Álvarez-Mon M. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology. 2010;52:2086-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Wiedmann MW, Mössner J, Baerwald C, Pierer M. TNF alpha inhibition as treatment modality for certain rheumatologic and gastrointestinal diseases. Endocr Metab Immune Disord Drug Targets. 2009;9:295-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Schramm C, Schneider A, Marx A, Lohse AW. Adalimumab could suppress the activity of non alcoholic steatohepatitis (NASH). Z Gastroenterol. 2008;46:1369-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Hoy SM, Scott LJ. Etanercept: a review of its use in the management of ankylosing spondylitis and psoriatic arthritis. Drugs. 2007;67:2609-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:167-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Rongioletti F, Burlando M, Parodi A. Adverse effects of biological agents in the treatment of psoriasis. Am J Clin Dermatol. 2010;11 Suppl 1:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Falcone M, Facciotti F, Ghidoli N, Monti P, Olivieri S, Zaccagnino L, Bonifacio E, Casorati G, Sanvito F, Sarvetnick N. Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. J Immunol. 2004;172:5908-5916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Valenti L, Fracanzani AL, Fargion S. The immunopathogenesis of alcoholic and nonalcoholic steatohepatitis: two triggers for one disease? Semin Immunopathol. 2009;31:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol. 2014;5:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol. 2011;23:282-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med. 2010;16:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Biton J, Semerano L, Delavallée L, Lemeiter D, Laborie M, Grouard-Vogel G, Boissier MC, Bessis N. Interplay between TNF and regulatory T cells in a TNF-driven murine model of arthritis. J Immunol. 2011;186:3899-3910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Huang Z, Yang B, Shi Y, Cai B, Li Y, Feng W, Fu Y, Luo L, Wang L. Anti-TNF-α therapy improves Treg and suppresses Teff in patients with rheumatoid arthritis. Cell Immunol. 2012;279:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Tsai YG, Lee CY, Lin TY, Lin CY. CD8+ Treg cells associated with decreasing disease activity after intravenous methylprednisolone pulse therapy in lupus nephritis with heavy proteinuria. PLoS One. 2014;9:e81344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Notley CA, McCann FE, Inglis JJ, Williams RO. ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ Treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis Rheum. 2010;62:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Correale J, Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol. 2010;67:625-638. [PubMed] |
| 31. | Martin-Murphy BV, You Q, Wang H, De La Houssaye BA, Reilly TP, Friedman JE, Ju C. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS One. 2014;9:e80949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Krzych U, Schwenk R, Guebre-Xabier M, Sun P, Palmer D, White K, Chalom I. The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites. Immunol Rev. 2000;174:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498-5503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 459] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 36. | Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 638] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 37. | Zakka LR, Fradkov E, Keskin DB, Tabansky I, Stern JN, Ahmed AR. The role of natural killer cells in autoimmune blistering diseases. Autoimmunity. 2012;45:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Adar T, Ben Ya’acov A, Lalazar G, Lichtenstein Y, Nahman D, Mizrahi M, Wong V, Muller B, Rawlin G, Ilan Y. Oral administration of immunoglobulin G-enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cells. Clin Exp Immunol. 2012;167:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | El Haj M, Ben Ya’acov A, Lalazar G, Ilan Y. Potential role of NKT regulatory cell ligands for the treatment of immune mediated colitis. World J Gastroenterol. 2007;13:5799-5804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Menachem Y, Trop S, Kolker O, Shibolet O, Alper R, Nagler A, Ilan Y. Adoptive transfer of NK 1.1+ lymphocytes in immune-mediated colitis: a pro-inflammatory or a tolerizing subgroup of cells? Microbes Infect. 2005;7:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Shibolet O, Kalish Y, Klein A, Alper R, Zolotarov L, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Adoptive transfer of ex vivo immune-programmed NKT lymphocytes alleviates immune-mediated colitis. J Leukoc Biol. 2004;75:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Trop S, Nagler A, Ilan Y. Role of NK1.1+ and AsGm-1+ cells in oral immunoregulation of experimental colitis. Inflamm Bowel Dis. 2003;9:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Holz LE, McCaughan GW, Benseler V, Bertolino P, Bowen DG. Liver tolerance and the manipulation of immune outcomes. Inflamm Allergy Drug Targets. 2008;7:6-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Shibolet O, Alper R, Zolotarov L, Trop S, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. The role of intrahepatic CD8+ T cell trapping and NK1.1+ cells in liver-mediated immune regulation. Clin Immunol. 2004;111:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya’acov A, Shabat Y, Nimer A, Hemed N, Zolotarovya L, Lichtenstein Y. Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial. J Clin Immunol. 2015;35:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Halota W, Ferenci P, Kozielewicz D, Dybowska D, Lisovoder N, Samira S, Shalit I, Ellis R, Ilan Y. Oral anti-CD3 immunotherapy for HCV-nonresponders is safe, promotes regulatory T cells and decreases viral load and liver enzyme levels: results of a phase-2a placebo-controlled trial. J Viral Hepat. 2015;22:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA. 2010;107:9765-9770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 49. | Ilan Y, Zigmond E, Lalazar G, Dembinsky A, Ben Ya’acov A, Hemed N, Kasis I, Axelrod E, Zolotarov L, Klein A. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J Clin Immunol. 2010;30:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Israeli E, Goldin E, Fishman S, Konikoff F, Lavy A, Chowers Y, Melzer E, Lahat A, Mahamid M, Shirin H. Oral administration of non-absorbable delayed release 6-mercaptopurine is locally active in the gut, exerts a systemic immune effect and alleviates Crohn’s disease with low rate of side effects: results of double blind Phase II clinical trial. Clin Exp Immunol. 2015;181:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut. 2007;56:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 53. | Wu HY, Maron R, Tukpah AM, Weiner HL. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J Immunol. 2010;185:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Mizrahi M, Ilan Y. The gut mucosa as a site for induction of regulatory T-cells. Curr Pharm Des. 2009;15:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Castro-Sánchez P, Martín-Villa JM. Gut immune system and oral tolerance. Br J Nutr. 2013;109 Suppl 2:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Ilan Y. Oral tolerance: can we make it work? Hum Immunol. 2009;70:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Shaaltiel Y, BYa A, Shabat Y, Zolotarov L, Gingis-Velitski S, Almon E, Aviezer D, Ilan Y. Oral administration of a plant cell expressed recombinant anti-TNF fusion protein in biologically active in the gut and alleviates immune mediated hepatitis. Hepatology. 2013;751 Suppl 58:564A. |
| 58. | Shaaltiel Y, BYaA , Shabbat Y, Zolotarov L, Gingis-Velitski S, Almon E, Aviezer D, Ilan Y. A novel method for anti-TNF based-oral immunotherapy: Oral administration of a plant cell-expressed recombinant anti-TNF fusion protein for treating of Crohn’s disease. Gastroenterology. 2014;5 Suppl 1:S901. [DOI] [Full Text] |