Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8226
Peer-review started: June 15, 2016
First decision: August 8, 2016
Revised: August 11, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: September 28, 2016
Processing time: 106 Days and 16.8 Hours
To perform a systematic review to grade guidelines and present recommendations for clinical management of non-alcoholic fatty liver disease (NAFLD).
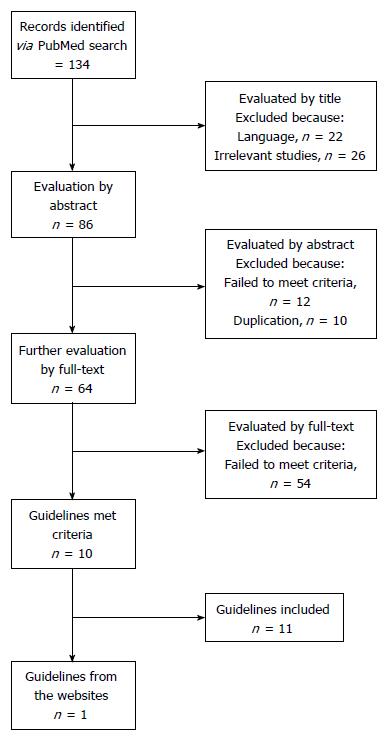
A database search was conducted on PubMed for guidelines published before May 2016, supplemented by reviewing relevant websites. The Appraisal of Guidelines for Research and Evaluation (ARGEE) Instrument II was a tool designed to appraise the methodological rigor and transparency in which a clinical guideline is developed and it is used internationally. It was used to appraise the quality of guidelines in this study. The inclusion criteria include: clinical NAFLD guidelines for adults, published in English, and released by governmental agencies or key organizations.
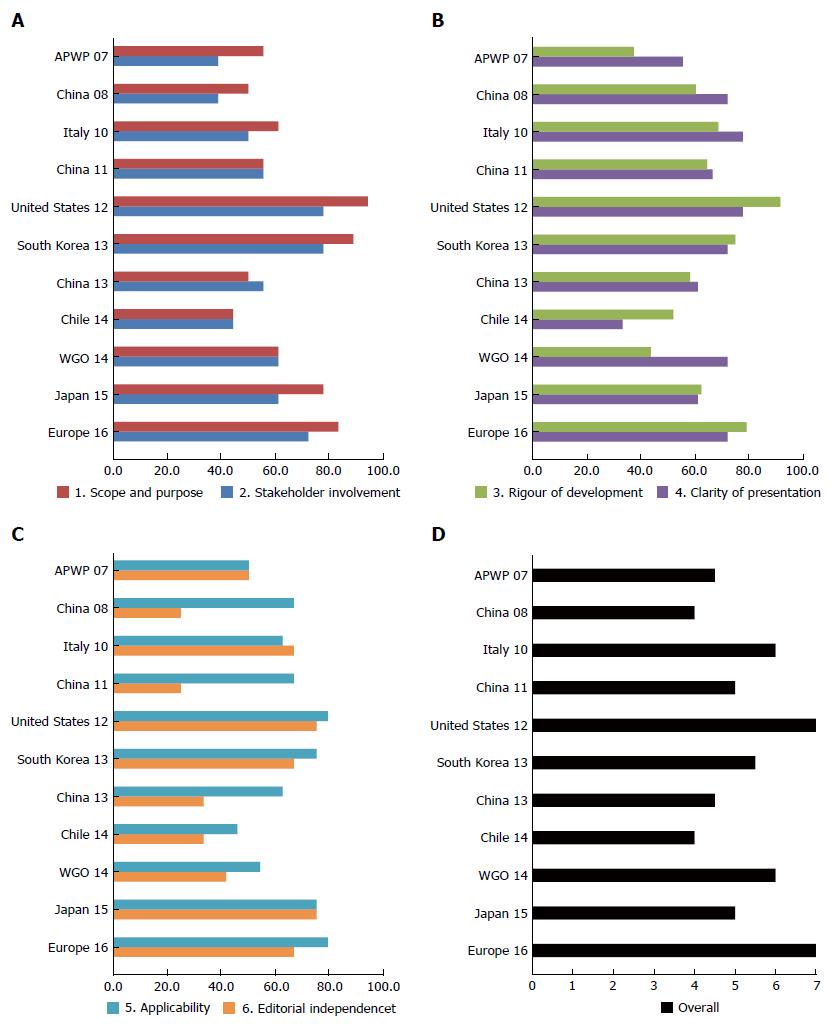
Eleven guidelines were included in this study. Since 2007, guidelines have been released in Asia (3 in China, 1 in South Korea, and 1 in Japan), Europe (1 in Italy), America (1 in United States and 1 in Chile) and three international agencies [European associations joint, Asia-Pacific Working Party and World Gastroenterology Organization (WGO)]. Using the ARGEE II instrument, we found US 2012 and Europe 2016 had the highest scores, especially in the areas of rigor of development and applicability. Additionally, Italy 2010 and Korea 2013 also presented comprehensive content, rigorous procedures and good applicability. And WGO 2014 offered various algorithms for clinical practice. Lastly, a practical algorithm for the clinical management was developed, based on the recommended guidelines.
This is the first systematic review of NAFLD guidelines. It may yield insights for physicians and policy-makers in the development and application of guidelines.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is one of the leading chronic liver diseases globally. A comprehensive study of NAFLD guidelines will be useful for various stakeholders to develop and utilize guidelines. This is the first systematic review to grade NAFLD guidelines and present recommendations for the clinical management of NAFLD. Through systematically evaluating the published guidelines and offering a clinical algorithm, it may yield insights for physicians and policy-makers in the development and application of guidelines.
- Citation: Zhu JZ, Hollis-Hansen K, Wan XY, Fei SJ, Pang XL, Meng FD, Yu CH, Li YM. Clinical guidelines of non-alcoholic fatty liver disease: A systematic review. World J Gastroenterol 2016; 22(36): 8226-8233
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8226.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8226
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of disease ranging from simple hepatic steatosis, to inflammatory non-alcoholic steatohepatitis (NASH) with increasing levels of fibrosis and eventually hepatic cirrhosis[1]. According to the latest guideline released in Europe[2], it is defined by the presence of steatosis in > 5% hepatocytes, in the absence of other causes attributed to hepatic steatosis[3]. Recent advance supports NAFLD as the chronic liver disease component of metabolic syndrome[4].
Younossi et al[5] estimated the global prevalence of imaging-diagnostic NAFLD arrived at 25%, although it varied by region and age. In the United States, it was reported to be between 10% and 30%, which is similar to rates in Europe and Asia[6-8]. It is alarming that the prevalence of NAFLD worldwide is on the rise[9], along with the associated disorders: obesity, insulin resistance, diabetes and metabolic syndrome[8]. New evidence supports NAFLD as a common liver disease presenting across the globe, which warrants the attention of physicians, researchers, and national policy makers. However, gaps between provider knowledge and awareness of clinical practice guidelines exist.
Offering continuing education and developing high-quality national guidelines may help making inroads into the problem of suboptimal NAFLD care. A comprehensive study of the existing guidelines of NAFLD might be useful for helping stakeholders, including physicians, patients, policymakers and governmental bodies to develop and implement guidelines. To our knowledge, this is the first systematic critical appraisal of published guidelines to systematically grade and comprehensively present the evidence-based recommendations for the diagnosis and treatment of NAFLD.
This systematic review was conducted according to the PRISMA guidelines[10].
The database search was conducted on PubMed for guidelines published before May 2016. In the search, we used the following key words and terms: [“fatty liver”(Title)] AND [strategy*(Title) OR guideline*(Title) OR recommendation*(Title) OR management*(Title)].
The literature search was supplemented by searching relevant websites (using the term “fatty liver”), including the following: (1) Australia National Health and Medical Research Council (https://www.nhmrc.gov.au/?); (2) American College of Physicians (https://www.acponline.org/); (3) American Medical Association (http://www.ama-assn.org/ama); (4) Institute for Clinical Systems Improvement (https://www.icsi.org/); (5) Institute of Medicine (http://www.nationalacademies.org/); (6) National Guidelines Clearinghouse (https://www.guideline.gov/); (7) National Institute for Health and Clinical Excellence (https://www.nice.org.uk/); (8) Royal College of Physicians (https://www.rcplondon.ac.uk/); (9) Scottish Intercollegiate Guidelines Network (http://www.sign.ac.uk/); and (10) World Health Organization (http://www.who.int/en/).
The guideline was included in this study, if it met the following criteria: (1) clinical guidelines regarding the diagnosis and management of NAFLD in adults; (2) released by governmental agencies or key health organizations; and (3) published in English. Two investigators independently performed the screen on PubMed and the websites, according to the inclusion criteria. Discrepancies were resolved by the involvement of a third reviewer.
The Appraisal of Guidelines for Research and Evaluation (AGREE) II was a tool designed to appraise the methodological rigor and transparency in which a clinical guideline is developed and it is used internationally. It consists of 23 items grouped in 6 domains, i.e., scope and purpose, stakeholder involvement, rigor of development, clarity and presentation, applicability and editorial independence[11].
As shown in Figure 1, eleven guidelines met the criteria and were included in the final version of this systematic review. Since 2007, five guidelines were released in Asia (3 in China, 1 in Japan and 1 in South Korea), while three guidelines were released in the United States, Italy and Chile, respectively (Table 1). Three guidelines were released by international agencies, i.e. Asia-Pacific Working Party, World Gastroenterology Organization (WGO) and a joint commission of European associations.
| Author(s)/Organization(s) | Published Year | Region/country | Title | Recommendation |
| Chitturi et al[25]. Asia–Pacific Working Party on NAFLD (APWP 07) | 2007 | Asia–Pacific region | Non-alcoholic fatty liver disease in the Asia-Pacific region: Definitions and overview of proposed guidelines | Not recommended |
| Zeng et al[26]. The Chinese National Consensus Workshop on NAFLD (China 08) | 2008 | China | Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases | Not recommended |
| Loria et al[12]. Italian Association for the Study of the Liver (Italy 10) | 2010 | Italy | Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease: A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee | Recommended but modified |
| Fan et al[27]. Chinese Association for the Study of Liver Disease (China 11) | 2011 | China | Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: Update 2010 | Not recommended |
| Chalasani et al[1]. American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association (US 12) | 2012 | United States | The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association | Recommended |
| The Korean Association for the Study of the Liver (South Korea 13)[13] | 2013 | South Korea | KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease | Recommended but modified |
| Gao et al[28]. Study Group of Liver and Metabolism, Chinese Society of Endocrinology (China 13) | 2013 | China | Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: Consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology | Not recommended |
| Arab et al[29]. Chilean Society of Gastroenterology (Chile 14) | 2014 | Chile | Management of nonalcoholic fatty liver disease: An evidence-based clinical practice review | Not recommended |
| LaBrecque et al[14]. World Gastroenterology Organization (WGO 14) | 2014 | World | World Gastroenterology Organization global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis | Not recommended |
| Watanabe et al[30] Japanese Society of Gastroenterology (Japan 15) | 2015 | Japan | Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis | Not recommended |
| European Association for the Study of the Liver, European Association for the Study of Diabetes and European Association for the Study of Obesity (Europe 16)[2] | 2016 | Europe | EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease | Recommended |
Eleven guidelines were appraised according to AGREE II, as presented in Table 1 and Figure 2. We highly recommended the two guidelines, United States 12[1] and Europe 16[2], given the high scores and the authority of the organizations. Additionally, Italy 10[12] and South Korea 13[13] also presented comprehensive content, rigorous procedures and good applicability. Lastly, WGO 14[14] offered a variety of algorithms for clinical practice.
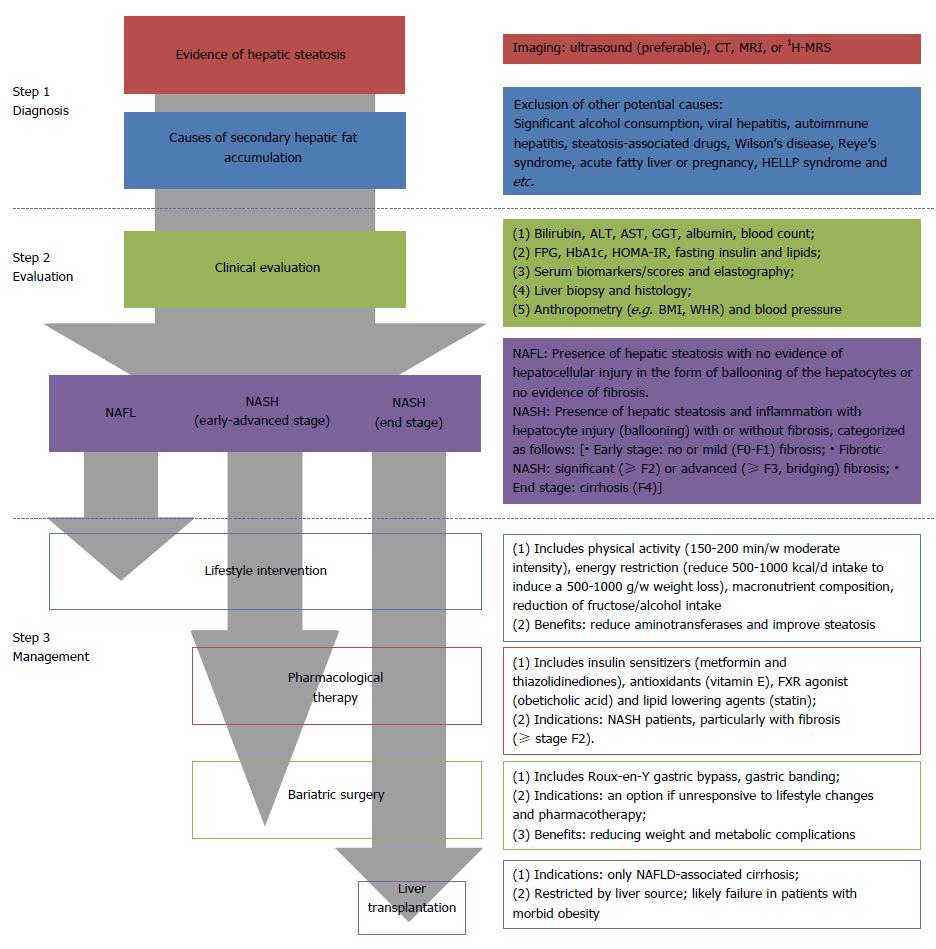
Figure 3 presented a clinical algorithm for the diagnosis and management of NAFLD in adults. Generally, the procedure of clinical practice includes diagnosis, assessment, and management.
This is the first systematic critical appraisal to grade the guidelines and present the evidence-based recommendations for the clinical management of NAFLD. Using the ARGEE II instrument, we found United States 12[1] and Europe 16[2] had the highest scores, especially in the areas of rigor of development and applicability. Additionally, we developed a clinical algorithm for the diagnosis and management of NAFLD in adults.
NAFLD is one of the leading chronic liver diseases in the world[5]. While incidence rates may possibly vary and/or be underreported[15,16], the present situation reinforces the need for a precise and rational system of management for NAFLD. Additionally, the obesity epidemic has led to a rapidly increasing population at risk for NAFLD, and shows no signs of slowing down. Therefore, NAFLD will only become a larger problem in the future if it is not properly prevented and managed now.
Currently, liver biopsy has still been regarded as the gold standard in the diagnostic evaluation of NAFLD[17]. However, a biopsy is an invasive practice, which carries a series of medical risks, e.g. hemorrhage and infection[2]. The non-invasive assessing method that is most suitable for evaluating hepatic steatosis is ultrasound, with a sensitivity of 60%-94% and a specificity of 66%-97%[18], even though it presents less precise in milder degrees of steatosis. Given the widely availability and economic efficiency, ultrasound is recommended as a first-line diagnostic test in most guidelines, rather than liver biopsy and other imaging tools. Additionally, a variety of noninvasive algorithms, based on metabolic and anthropometric tests, have been developed for identifying NAFLD, e.g. the fatty liver index[19] and the hepatic steatosis index[20]. They have been utilized to screen subjects with hepatic steatosis in large epidemiologic studies or predicting potential patients in clinical practice[18]. The development of more accurate and noninvasive diagnostic tools is still a major unmet demand in the clinic.
In terms of treatment, the pathophysiological association between NAFLD and obesity-related diseases, e.g., metabolic syndrome and diabetes, supports structured programs of lifestyle intervention aimed at weight loss, before or in addition to pharmacotherapy[3,21]. The elements of a comprehensive lifestyle approach generally include energy restriction, macronutrient composition, alcohol consumption, coffee drinking and physical activity[2]. Furthermore, published guidelines suggested pharmacotherapy should be exclusively indicated for early-stage NASH with increased risk of advanced NASH[2,22]. The past decade has witnessed some advance in clinical pharmacotherapy trials, e.g., the use of metformin, pioglitazone and vitamin E, however most NASH patients failed to respond to these methods[2,23]. When considering safety and tolerability, no drug has been approved for NAFLD by pharmacological agencies by now, while no specific drug therapy was firmly recommended in the present guidelines[2]. Therefore, it is still imperative to continue research to improve pharmacotherapy for NASH and hepatic fibrosis. Additionally, the role of bariatric surgery in the treatment of NAFLD is still unknown. Current evidence found that NAFLD patients who undergo bariatric surgery require long-term postoperative management, due to an increased risk for fibrosis progression[1,14].
This is the first systematic review of published NAFLD guidelines. Using AGREE II[11], it systematically grades the guidelines and presents the evidence-based recommendations for the clinical management of NAFLD. Additionally, a clinical algorithm for the clinical practice was developed, based on the highly recommended guidelines.
This study has some limitations. To begin with, only the guidelines in English were included in this review. Thus, high-quality guidelines in other languages might have been missed. Second, we chose the AGREE II instrument to evaluate the guidelines, even though there are other appraisals, e.g. Global Rating Scale[24]. Third, guidelines should include information on how to reduce inappropriate practice and improve the efficiency of management. Further, the application of guidelines is crucial in clinical practice. However, we failed to evaluate the acceptance and the application of the guidelines in this review, due to the limited inclusion in the literature included in this review.
In this study, a systematic review was conducted to search and integrate the published guidelines of NAFLD. Furthermore, based on the evaluation of the included guidelines, a clinical algorithm for the diagnosis and management of NAFLD was developed. We hope it will yield insights for physicians and policy-makers in the development and application of guidelines moving forward.
Non-alcoholic fatty liver disease (NAFLD) is one of the leading chronic liver diseases globally. A comprehensive study of NAFLD guidelines will be useful for various stakeholders to develop and utilize guidelines.
New evidence supports NAFLD as a common liver disease presenting across the globe, which warrants the attention of physicians, researchers, and national policy makers. However, gaps between provider knowledge and awareness of clinical practice guidelines exist. Offering continuing education and developing high-quality national guidelines may help making inroads into the problem of suboptimal NAFLD care.
A comprehensive study of the existing guidelines of NAFLD might be useful for helping stakeholders, including physicians, patients, policymakers and governmental bodies to develop and implement guidelines. The authors think, this is the first systematic critical appraisal of published guidelines to systematically grade and comprehensively present the evidence-based recommendations for the diagnosis and treatment of NAFLD.
The systematic review included eleven published NAFLD guidelines in the worldwide. Furthermore, we graded the guidelines, using the AGREE instrument II. Lastly, a practical algorithm for the clinical management was developed, based on the recommended guidelines.
The Appraisal of Guidelines for Research and Evaluation Instrument II was a tool designed to appraise the methodological rigor and transparency in which a clinical guideline is developed and it is used internationally. It consists of 23 items grouped in 6 domains, i.e., scope and purpose, stakeholder involvement, rigor of development, clarity and presentation, applicability and editorial independence.
The authors conducted the first systematic review of published NAFLD guidelines. It may yield insights for physicians and policy-makers in the development and application of guidelines.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abenavoli L, Lee HC, Torabizadeh Z S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 2. | European Association for the Study of the Liver (EASL). Electronic address: easloffice@easloffice.eu; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3173] [Article Influence: 352.6] [Reference Citation Analysis (4)] |
| 3. | Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology. 2016;63:2032-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 562] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7502] [Article Influence: 833.6] [Reference Citation Analysis (0)] |
| 6. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2289] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 8. | Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Zhu JZ, Dai YN, Wang YM, Zhou QY, Yu CH, Li YM. Prevalence of Nonalcoholic Fatty Liver Disease and Economy. Dig Dis Sci. 2015;60:3194-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8863] [Article Influence: 553.9] [Reference Citation Analysis (0)] |
| 11. | Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839-E842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2440] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 12. | Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013;19:325-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Weiß J, Rau M, Geier A. Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int. 2014;111:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 907] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 17. | Spengler EK, Loomba R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin Proc. 2015;90:1233-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 18. | Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 19. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2017] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1059] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 21. | Marchesini G, Mazzotti A. NAFLD incidence and remission: only a matter of weight gain and weight loss? J Hepatol. 2015;62:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 23. | Wilkins T, Tadkod A, Hepburn I, Schade RR. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician. 2013;88:35-42. [PubMed] |
| 24. | Sint Nicolaas J, de Jonge V, de Man RA, ter Borg F, Cahen DL, Moolenaar W, Stolk MF, van Tilburg AJ, Valori RM, van Leerdam ME. The Global Rating Scale in clinical practice: a comprehensive quality assurance programme for endoscopy departments. Dig Liver Dis. 2012;44:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, Mao YM. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Fan JG, Jia JD, Li YM, Wang BY, Lu LG, Shi JP, Chan LY. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18: 163-166). J Dig Dis. 2011;12:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Gao X, Fan JG. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J Diabetes. 2013;5:406-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Arab JP, Candia R, Zapata R, Muñoz C, Arancibia JP, Poniachik J, Soza A, Fuster F, Brahm J, Sanhueza E. Management of nonalcoholic fatty liver disease: an evidence-based clinical practice review. World J Gastroenterol. 2014;20:12182-12201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 30. | Watanabe S, Hashimoto E, Ikejima K, Uto H, Ono M, Sumida Y, Seike M, Takei Y, Takehara T, Tokushige K. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |