Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8178
Peer-review started: July 22, 2016
First decision: August 29, 2016
Revised: September 1, 2016
Accepted: September 8, 2016
Article in press: September 8, 2016
Published online: September 28, 2016
Processing time: 66 Days and 15.1 Hours
To analyze retrospectively a 5-year experience of human hepatocyte isolation from resected liver tissues with benign disease.
We established a method of modified four-step retrograde perfusion to isolate primary human hepatocytes. Samples were collected from the resected livers of patients with intrahepatic duct calculi (n = 7) and liver hemangioma (n = 17). Only the samples weighing ≥ 15 g were considered suitable for hepatocyte isolation. By using the standard trypan blue exclusion technique, hepatocyte viability and yield were immediately determined after isolation.
Twenty-four liver specimens, weighing 15-42 g, were immediately taken from the margin of the removed samples and transferred to the laboratory for hepatocyte isolation. Warm ischemia time was 5-35 min and cold ischemia time was 15-45 min. For the 7 samples of intrahepatic duct calculi, the method resulted in a hepatocyte yield of 3.49 ± 2.31 × 106 hepatocytes/g liver, with 76.4% ± 10.7% viability. The 17 samples of liver hemangioma had significantly higher yield of cells (5.4 ± 1.71 × 106 cells/g vs 3.49 ± 2.31 × 106 cells/g, P < 0.05) than the samples of intrahepatic duct calculi. However, there seems to be no clear difference in cell viability (80.3% ± 9.67% vs 76.4% ± 10.7%, P > 0.05). We obtained a cell yield of 5.31 ± 1.87 × 106 hepatocytes/g liver when the samples weighed > 20 g. However, for the tissues weighing ≤ 20 g, a reduction in yield was found (3.08 ± 1.86 × 106 cells/g vs 5.31 ± 1.87 × 106 cells/g, P < 0.05).
Benign diseased livers are valuable sources for large-number hepatocyte isolation. Our study represents the largest number of primary human hepatocytes isolated from resected specimens from patients with benign liver disease. We evaluated the effect of donor liver characteristics on cell isolation, and we found that samples of liver hemangioma can provide better results than intrahepatic duct calculi, in terms of cell yield. Furthermore, the size of the tissues can affect the outcome of hepatocyte isolation.
Core tip: We retrospectively analyzed a 5-year experience of human hepatocyte isolation from surgically-resected normal tissues and established an efficient technique for a special kind of liver samples for large-scale human hepatocyte isolation. Our study represents the largest number of primary human hepatocytes isolated from resected specimens from patients with benign liver disease. We evaluated the effect of donor liver characteristics on cell isolation, and we found that samples of liver hemangioma can provide better results than intrahepatic duct calculi, in terms of cell yield. Furthermore, the size of the tissues can affect the outcome of hepatocyte isolation.
- Citation: Meng FY, Liu L, Liu J, Li CY, Wang JP, Yang FH, Chen ZS, Zhou P. Hepatocyte isolation from resected benign tissues: Results of a 5-year experience. World J Gastroenterol 2016; 22(36): 8178-8186
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8178.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8178
Demands for primary human hepatocytes for basic research and therapeutic applications are continuously increasing[1-3]. Large quantities of primary human hepatocytes can be isolated mainly from two sources: discarded liver transplants and liver specimens obtained during partial hepatectomy[4,5]. Compared to organs from discarded liver transplants, the latter are easily accessible and more frequently available. Furthermore, resected liver donations are usually planned and often occur during normal working hours. Nevertheless, surgically-resected tissue is of varying quality, which can affect the yield and viability of isolated hepatocytes[6]. It is important to standardize the use of the surgical specimens to maximize the availability of high-quality hepatocytes.
Some authors have previously suggested protocols for human hepatocyte isolation from surgically-resected liver tissues[5,7,8]. However, the majority of these groups have used tissues from malignant tumors[9]. Often, totally healthy tissues are needed for clinical application of hepatocyte transplantation, artificial liver, and hepatocyte immortalization[10,11]. There are still no systematic studies in the literature that have investigated resected specimens from patients with benign liver disease for large-scale hepatocyte isolation. Therefore, available data about the effect of the donor liver on isolated hepatocyte yield are scarce.
To address these issues, we retrospectively analyzed a 5-year experience of human hepatocyte isolation from surgically-resected normal tissues and evaluated the effect of donor liver characteristics on hepatocyte isolation outcome. Our study, presented herein, represents the largest number of primary human hepatocytes isolated from resected specimens from patients with benign liver disease. We established an efficient technique for a special kind of liver samples for large-scale human hepatocyte isolation.
The aim of our study was to rescue resected healthy liver tissues that would have been otherwise discarded, for hepatocyte isolation. We also compared two different kinds of liver samples and their results in different cell yield and viability.
We chose patients with benign liver disease, intrahepatic duct calculi and liver hemangioma, treated in our hospital. Following our hospital’s institutional and ethical guidelines, and after obtaining tissue donors’ consent, samples weighing ≥ 15 g were collected from 24 patients undergoing partial hepatectomy, including 7 liver intrahepatic duct calculi and 17 liver hemangioma (Table 1). To avoid unnecessary damage to the hepatocytes, continuous clamping was applied in all 24 cases, with a short intraoperative warm ischemia time (WIT) (5-35 min). After the liver tissues (15-42 g) were resected from the abdominal cavity, they were immediately placed into ice-cold Ringer’s lactate solution, under sterile conditions. The tissues were transferred directly to the laboratory for hepatocyte isolation, with a limited cold ischemic time (15-45 min).
| No. | Sex | Age (yr) | Blood group | Disease | Tissue weight (g) | Warm ischemia time (min) | Hepatocyte (× 105/g) | Viability (%) |
| 1 | Male | 48 | O+ | Hemangiomas | 15 | 30 | 28.00 | 62 |
| 2 | Male | 52 | A+ | Hemangiomas | 18 | 25 | 20.56 | 73 |
| 3 | Female | 23 | A+ | Calculus | 21 | 25 | 12.86 | 69 |
| 4 | Male | 55 | O+ | Hemangiomas | 26 | 30 | 18.08 | 65 |
| 5 | Female | 45 | A+ | Hemangiomas | 27 | 25 | 51.85 | 76 |
| 6 | Female | 36 | B+ | Hemangiomas | 32 | 15 | 65.63 | 85 |
| 7 | Male | 47 | AB+ | Calculus | 38 | 10 | 71.05 | 92 |
| 8 | Male | 42 | A+ | Hemangiomas | 42 | 10 | 54.76 | 86 |
| 9 | Male | 52 | A+ | Hemangiomas | 20 | 5 | 63.30 | 87 |
| 10 | Male | 63 | O+ | Hemangiomas | 33 | 25 | 72.11 | 93 |
| 11 | Female | 46 | O+ | Hemangiomas | 27 | 20 | 43.70 | 68 |
| 12 | Male | 57 | A+ | Hemangiomas | 30 | 35 | 55.21 | 75 |
| 13 | Male | 44 | B+ | Calculus | 25 | 15 | 63.81 | 84 |
| 14 | Male | 61 | A+ | Calculus | 17 | 5 | 25.20 | 86 |
| 15 | Female | 57 | A+ | Hemangiomas | 32 | 7 | 62.50 | 89 |
| 16 | Male | 27 | A+ | Hemangiomas | 27 | 15 | 52.65 | 76 |
| 17 | Female | 55 | B+ | Calculus | 23 | 7 | 32.07 | 66 |
| 18 | Male | 61 | O+ | Hemangiomas | 31 | 25 | 68.80 | 90 |
| 19 | Male | 37 | B+ | Hemangiomas | 37 | 21 | 72.22 | 92 |
| 20 | Male | 65 | B+ | Calculus | 20 | 15 | 17.07 | 72 |
| 21 | Male | 79 | O+ | Calculus | 26 | 20 | 22.51 | 66 |
| 22 | Male | 47 | O+ | Hemangiomas | 35 | 5 | 67.72 | 89 |
| 23 | Male | 51 | B+ | Hemangiomas | 24 | 12 | 59.27 | 77 |
| 24 | Male | 67 | O+ | Hemangiomas | 30 | 17 | 62.25 | 82 |
To ensure flow of appropriate buffers, a peristaltic pump with adjustable speed was used in our perfusion system. This system was formed by mounting a peristaltic pump with two silicone tubings immersed in a variable-sized water tank to accommodate the liver tissues. The water bath temperature was adjusted and maintained at 37 °C for liver undergoing perfusion and digestion. The pump speed was set at constant flow.
The method used several reagents that had been prepared in advance (Table 2). The reagents were mixed thoroughly and filter solutions were prepared using 0.22-μm filters, after which the pH value was adjusted to 7.4 and the solutions were stored at 4 °C.
| Resolution | Ingredient | Concentration (g/L) |
| PB (Perfusion buffer) | Double-distilled water (3000 mL) | |
| NaCl (27 g) | 9 | |
| KCl (1.26 g) | 0.42 | |
| NaHCO3 (6.3 g) | 2.1 | |
| Glucose (2.7 g) | 0.9 | |
| Hepes (14.34 g) | 4.78 | |
| PBE (Perfusion buffer with EDTA) | PB (1000 mL) | |
| EDTA (0.37 g) | 0.37 | |
| PBD (Perfusion buffer with dispase) | PB (500 mL) | |
| Dispase II (Sigma) (4.2 g) | 8.4 | |
| PBC (Perfusion buffer with collagenase) | PB (500 mL) | |
| Collagenase IV (Sigma) (0.25 g) | 0.5 | |
| CaCl2•2H2O (0.275 g) | 0.55 | |
| WB (Washing buffer) | Double-distilled water (2500 mL) | |
| NaCl (17.5 g) | 7 | |
| KCl (1.15 g) | 0.46 | |
| CaCl2•2H2O (0.325 g) | 0.13 | |
| Hepes (5.95 g) | 2.38 | |
| Bovine serum albumin (2.5 g) | 1.0 | |
| WBD (Washing buffer with DNase) | WB (Washing buffer) (1500 mL) | |
| MgCl2•6H2O (0.15 g) | 0.1 | |
| MgSO4•7H2O (0.15 g) | 0.1 | |
| DNase I (Sigma) (0.15 g) | 0.1 |
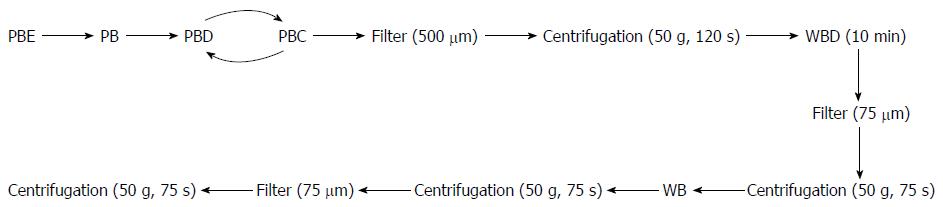
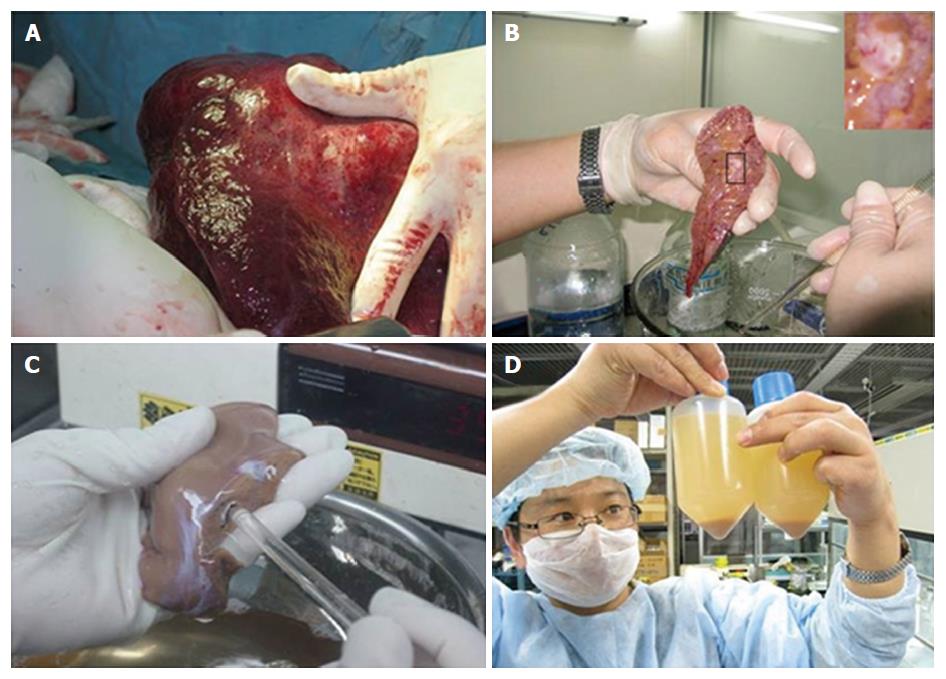
We adopted a rigorous and stringent isolation protocol for all liver tissues. Precise time between tissue resection and isolation commencement was recorded. Liver wedges were usually obtained from segments II/III and were carefully cut and weighed. Primary human hepatocytes were isolated under strict sterile conditions using a modified four-step retrograde perfusion technique (Figure 1). To eliminate interpersonal variability, all of the subsequent isolation procedures were carried out by single individual (Meng FY).
The liver samples were cannulated into the main blood vessel on the cut surface. The liver tissue was flushed with ice-cold perfusion buffer with EDTA (PBE) via blood vessels on the cut surface. This step was important to remove excess blood and help to determine the vessels that would offer optimal perfusion subsequently. The chosen vessel was cannulated with a suitable pipette tip, and flushed with PBE at 37 °C. In some cases, there might be more than one cut surface, or there might be a cut or tear on the outer capsule of the liver tissue (Glisson’s capsule). These were required to be sewed-up in advance to ensure optimal perfusion of the tissue.
After flushing with perfusion buffer to clear the PBE, the tissue was then continuously perfused with a pre-warmed digestion buffer solution (perfusion buffer with dispase and perfusion buffer with collagenase). After sufficient digestion, the liver Glisson’s capsule was mechanically disrupted by using an operating knife blade. The isolated hepatocytes were released into the medium by gentle shaking, leaving behind the connective tissue and any undigested material. The resultant hepatocyte suspension was divided equally into sterile centrifuge bottles. The suspension was then filtered through a 500-μm nylon mesh and centrifuged at 50 × g for 2 min at 4 °C. We regularly applied a 10-min cell incubation step by using wash buffer solution containing DNase I (WBD). Cell clumps were broken up and damaged cells were digested. The suspension was then filtered (75 s), and the resultant cells were harvested by low-speed centrifugation at 50 × g for 75 s. This was followed by washes in cold wash buffer solution, filtration (60 s) and another centrifugation step (50 × g, 75 s, 4 °C). Finally, the resultant hepatocyte clumps were resuspended in cold William’s Medium E (Sigma). Hepatocyte yield and viability were immediately determined using the standard trypan blue exclusion technique after isolation/purification.
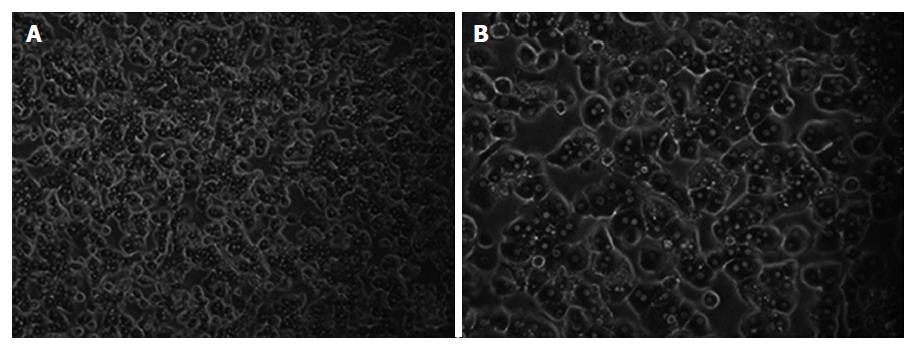
Culture medium was William’s E Medium supplemented with 100 μg/mL streptomycin, 100 mU/mL penicillin and 10% fetal bovine serum (FBS). Freshly isolated hepatocytes were seeded in culture flasks at a concentration of 4 × 105 to 5 × 105/mL. The culture medium was changed every 24 h. The morphology of the cultured cells was assessed throughout the entire culture period using light microscopy. Phase-contrast microscopy pictures were taken with a Nikon Diaphot inverted microscope.
The type of liver tissues used and the hepatocytes isolated are shown in Table 1. The liver tissue donors had intrahepatic duct calculi (n = 7) and liver hemangioma (n = 17). To improve cell availability, we investigated the influence of WIT and cold ischemia time and liver donor characteristics on the outcome of freshly isolated hepatocytes from surgically-resected liver tissues. All patients (15 males and 9 females) were negative for hepatitis C virus, hepatitis B virus surface antigen, and human immunodeficiency virus. All of the patients had normal liver function tests prior to surgery. The mean donor age was 50.7 years (range: 23-79 years). Blood group was O+ in 8 cases, A+ in 9, AB+ in 1, and B+ in 6 (Table 1). Data from our work revealed that patient sex, age and blood group had no correlation with cell yield and viability.
Liver wedges were prepared as shown in Figure 2. Representative images of isolated primary human hepatocytes, in culture for the first week, are shown in Figure 3. Similar morphological changes in the cultured cells were observed during the first week. The cells maintained normal morphology for at least 1 wk during culture in William’s E Medium.
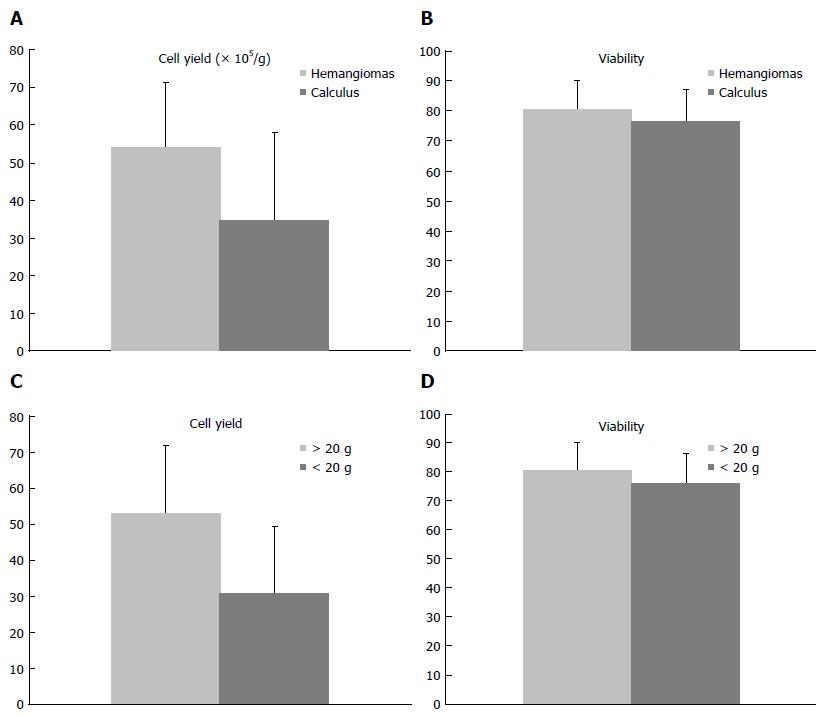
We processed and isolated human hepatocytes from 24 liver wedges. The WIT, i.e., the interval between clamping and bathing in ice-cold solution, averaged 17.5 ± 8.8 min (range: 5-35 min) (Table 1). The cold ischemia time, i.e., the interval between liver resection and perfusion, averaged 19.3 ± 3.3 min (range: 15-45 min). For the 7 samples of intrahepatic duct calculi, the method resulted in a hepatocyte yield of 3.49 ± 2.31 × 106 hepatocytes/g liver, with 76.4% ± 10.7% viability. However, for the 17 samples of liver hemangioma, we got better results for the hepatocyte yield (5.4 ± 1.71 × 106 cells/g vs 3.49 ± 2.31 × 106 cells/g, P < 0.05) compared to the samples of intrahepatic duct calculi (Figure 4A). However, there seemed to be no clear difference in cell viability (80.3% ± 9.67% vs 76.4% ± 10.7%, P > 0.05) (Figure 4B). In our study, we obtained a cell yield of 5.31 ± 1.87 × 106 hepatocytes/g liver when the samples weighed > 20 g. However, for the tissues that weighed ≤ 20 g, a reduction in yields was found (3.08 ± 1.86 × 106 cells/g vs 5.31 ± 1.87 × 106 cells/g, P < 0.05) (Figure 4C). In addition, no difference in cell viability was observed (80.0% ± 9.85% vs 76.0% ± 10.5%, P > 0.05) (Figure 4D).
All of the cultured primary hepatocytes demonstrated albumin synthesis in the first week (Table 3). Serum albumin concentrations were determined using immunonephelometry (Array; Beckman Instruments, Galway, Ireland). The isolated hepatocytes showed significantly increased albumin synthesis after 2 d of culture. When human hepatocytes were cultured in William’s E Medium supplemented with 10% FBS, the cells maintained their polygonal shape until day 5 (Figure 3).
| Time | Albumin (g/L) |
| 24 h | 0.79 ± 0.31 |
| 7 d | 1.36 ± 0.42 |
| 10 d | 1.09 ± 0.21 |
Hepatocyte isolation is a time-consuming and costly procedure[12,13]. However, surgical specimens are of varying quality, which can affect the cell yield and viability. It is important to identify surgically-resected tissues with the best expectations concerning cell quality and yield[14-16]. The aim of the present study was to analyze retrospectively the influence of the donor liver characteristics on the outcome of primary hepatocyte isolation from surgically-resected liver tissue. Once such a standardized method has been established, isolated hepatocytes will be easily accessible and more frequently available.
Hepatocyte isolation started in the mid-1960s[17]. After that, many innovative techniques were introduced to improve the results[8,18,19]. However, most of the innovative techniques have been applied exclusively to tissues obtained from resected liver tumors or from whole organ donors[20]. We established a modified four-step collagenase retrograde perfusion technique for isolation of hepatocytes from non-diseased liver tissue removed at surgical resection.
The modified method, compared to the traditional method, can improve results, allowing isolation of a large number of hepatocytes of high quality. After hepatocyte isolation, a 10-min incubation step using DNase I (WBD) was used. Cell clumps break up and damaged cells are digested. Our technique resulted in a hepatocyte yield of 4.85 ± 2.05 × 106 cells/g liver. The viability of the isolated hepatocytes, using the trypan blue exclusion technique, was 79.17% ± 9.90%.
Intrahepatic duct calculi and liver hemangioma are two common diseases among the local population. Tissues from patients undergoing partial hepatectomy are the most frequently available sources for hepatocyte isolation. However, not all of these tissues can be used to isolate large numbers of hepatocytes. Hepatic fibrosis often occurs in patients with intrahepatic duct calculi, who generally do not support successful cell isolation.
Different with other experiences reported in the literature[21], our results showed that the 17 samples of liver hemangioma had significantly higher yield of cells (5.4 ± 1.71 × 106 cells/g) than the samples of intrahepatic duct calculi (3.49 ± 2.31 × 106 cells/g, P < 0.05). However, there seemed to be no clear difference in cell viability (80.3% ± 9.67% vs 76.4% ± 10.7%, P > 0.05).
Cytotoxic bile acids accumulate in the hepatocytes during cholestasis, which is thought to induce hepatocyte necrosis and contribute to development of liver cirrhosis[22,23]. This could explain the lower cell yield observed in hepatocytes isolated from cholestatic livers[24-26]. In contrast, Iqbal et al[27] observed no significant difference in cell yield and viability in hepatocytes isolated from resected cirrhotic livers, as compared to non-cirrhotic livers. Further investigation should be made to evaluate the usage of resected cirrhotic livers for cell isolation.
Compared to resected livers from patients with intrahepatic duct calculi, we more frequently obtained a reliable source of normal liver tissue from patients with hemangioma. Furthermore, we found that left lateral sector segments were usually suitable to obtain normal resected tissues, with proportionate volumes, after which the lobular blood vessels can be easily exposed for catheterization. Of the total 24 samples, 19 were obtained from left hemi-hepatectomy. The fragments of hepatic tissue were cut from the periphery of the discarded material, surrounded by the hepatic capsule. It is important that the liver lobe should be incised with a single cut, and then the lobular blood vessels can be exposed for catheterization. We consider that the first perfusion step to drain off blood in the vessels is critical, because it can significantly affect the outcome of the subsequent collagenase digestion.
Among the general features of the patient, blood group, age and sex, did not have any influence on the yield or viability of the isolated human hepatocytes. In contrast, several investigations have described a decrease in hepatocyte viability or yield with an increase in patient age[8,28]. This may have resulted from the distribution of diseases among the different age groups, which can significantly affect the outcome of hepatocyte isolation[8]. Patients aged > 50 years had malignant diseases mainly. In our study, all of the specimens were obtained from patients with benign liver disease. Without the interfering factors, we observed that patient age had no correlation with cell yield and viability.
WIT can affect the outcome of hepatocyte isolation. It is reported that porta hepatis clamping during liver resection results in a low yield of isolated hepatocytes[26]. There is a negative correlation between WIT (< 30 min) and low cell yield[26]. It is also reported that intermittent clamping is more damaging to the liver than continuous clamping[29]. As for cold ischemia time, it is reported that ≤ 24 h has no effect on hepatocyte yield and viability[28,30,31]. As shown in our study, when resected liver tissue is used for hepatocyte isolation, the WIT and cold ischemia time are always short. So, WIT and cold ischemia time seem to have no obvious effect on the outcome of hepatocyte isolation from surgically-resected tissues.
The size of the tissue can affect the outcome of hepatocyte isolation. Alexandre et al[26] concluded that the percentage of digested liver decreased when tissue weights were > 100 g. In our study, we found a reduction in yields when the tissue weighed ≤ 20 g (Table 2). One reason is that small tissue samples always have several cut surfaces, without an integrated hepatic capsule. Another reason is that it is difficult to find a visible vessel orifice on the cut surface to perfuse collagenase solutions. In contrast to the large tissue samples, small samples always have a longer isolation process with insufficient digestion. This suggests that the four-step perfusion technique for tissues ≤ 20 g should be modified.
One possible method to improve the isolation outcome of small tissue samples is to glue the section surfaces in order to avoid leakage of the perfusate. However, some studies have reported that the use of glue tends to decrease the yield of viable cells[26]. Another possible method to increase the hepatocyte yield is to separate the viable cells using a Percoll centrifugation technique. We need to consider whether Percoll centrifugation is acceptable for the large quantity of hepatocytes required for clinical application. Due to the small numbers of tissues in our study, further investigations are needed to evaluate the optimal procedure for small resected specimens.
In conclusion, benign diseased livers appear to be a valuable source of a large number of isolated human hepatocytes. We consider that patient age has no correlation with cell yield and viability. Mild cirrhotic livers should not be arbitrarily excluded from cell isolation. We recommend, for optimal isolation, to use liver specimens weighing > 20 g and to avoid the use of liver specimens with severe fibrosis.
The authors thank Dr. Naoya Kobayashi from Okayama University Graduate School of Medicine and Dentistry, Japan, for his contributions to this work.
There are still no systematic studies investigating resected specimens from patients with benign liver disease for large-scale hepatocyte isolation. Therefore, available data about the effect of the donor liver on isolated hepatocyte yield are scarce.
The authors established an efficient technique for a special kind of liver samples for large-scale human hepatocyte isolation. They retrospectively analyzed a 5-year experience of human hepatocyte isolation from surgically-resected normal tissues and evaluated the effect of donor liver characteristics on the hepatocyte isolation outcome.
The authors consider that patient age has no correlation with cell yield and viability. Mild cirrhotic livers should not be arbitrarily excluded from cell isolation. They recommend, for optimal isolation, to use liver specimens weighing > 20 g and to avoid the use of liver specimens with severe fibrosis.
Benign diseased livers appear to be a valuable source of a large number of isolated human hepatocytes.
WIT (warm ischemia time), the interval between clamping and bathing in ice-cold solution. Cold ischemia time, the interval between storing in ice-cold solution and starting isolation.
The manuscript is well written. The study, conducted for 5 years, is well described and the results are clear and explained the target of the authors research in a good manner.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Conti B S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Lee JS, Shin J, Park HM, Kim YG, Kim BG, Oh JW, Cho SW. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1162] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 3. | Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater. 2013;8:014106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture & amp; cryopreservation. Semin Cell Dev Biol. 2002;13:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Baccarani U, Sanna A, Cariani A, Sainz-Barriga M, Adani GL, Zambito AM, Piccolo G, Risaliti A, Nanni-Costa A, Ridolfi L. Isolation of human hepatocytes from livers rejected for liver transplantation on a national basis: results of a 2-year experience. Liver Transpl. 2003;9:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Sahi J, Hamilton G, Sinz M, Barros S, Huang SM, Lesko LJ, LeCluyse EL. Effect of troglitazone on cytochrome P450 enzymes in primary cultures of human and rat hepatocytes. Xenobiotica. 2000;30:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Serralta A, Donato MT, Orbis F, Castell JV, Mir J, Gómez-Lechón MJ. Functionality of cultured human hepatocytes from elective samples, cadaveric grafts and hepatectomies. Toxicol In Vitro. 2003;17:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Vondran FW, Katenz E, Schwartlander R, Morgul MH, Raschzok N, Gong X, Cheng X, Kehr D, Sauer IM. Isolation of primary human hepatocytes after partial hepatectomy: criteria for identification of the most promising liver specimen. Artif Organs. 2008;32:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Schmidt M, Pei L. Synthetic toxicology: where engineering meets biology and toxicology. Toxicol Sci. 2011;120 Suppl 1:S204-S224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, Miyazaki M, Cai J, Tanaka N, Fox IJ. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287:1258-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Soto-Gutiérrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Meng FY, Zhou P. [Latest advances in immortalized human hepatocytes]. Zhonghua Ganzangbing Zazhi. 2009;17:395-397. [PubMed] |
| 13. | Meng FY, Chen ZS, Han M, Hu XP, Zhou P. An improved purification approach with high cell viability and low cell loss for cryopreserved hepatocytes. Cryobiology. 2010;60:238-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Meng FY, Chen ZS, Han M, Hu XP, He XX, Liu Y, He WT, Huang W, Guo H, Zhou P. Porcine hepatocyte isolation and reversible immortalization mediated by retroviral transfer and site-specific recombination. World J Gastroenterol. 2010;16:1660-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Meng FY, Liu L, Yang FH, Li CY, Liu J, Zhou P. Reversible immortalization of human hepatocytes mediated by retroviral transfer and site-specific recombination. World J Gastroenterol. 2014;20:13119-13126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Bao J, Fisher JE, Lillegard JB, Wang W, Amiot B, Yu Y, Dietz AB, Nahmias Y, Nyberg SL. Serum-free medium and mesenchymal stromal cells enhance functionality and stabilize integrity of rat hepatocyte spheroids. Cell Transplant. 2013;22:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Howard RB, Christensen AK, Gibbs FA, Pesch LA. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967;35:675-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Müller P, Aurich H, Wenkel R, Schäffner I, Wolff I, Walldorf J, Fleig WE, Christ B. Serum-free cryopreservation of porcine hepatocytes. Cell Tissue Res. 2004;317:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 3877] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 20. | Kobayashi N. Artificial cells for the development of cell therapy. Cell Transplant. 2008;17:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Fontaine M, Schloo B, Jenkins R, Uyama S, Hansen L, Vacanti JP. Human hepatocyte isolation and transplantation into an athymic rat, using prevascularized cell polymer constructs. J Pediatr Surg. 1995;30:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kaplan MM. Primary biliary cirrhosis--a first step in prolonging survival. N Engl J Med. 1994;330:1386-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Rolo AP, Palmeira CM, Wallace KB. Interactions of combined bile acids on hepatocyte viability: cytoprotection or synergism. Toxicol Lett. 2002;126:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Imamura H, Kawasaki S, Shiga J, Bandai Y, Sanjo K, Idezuki Y. Quantitative evaluation of parenchymal liver cell volume and total hepatocyte number in cirrhotic patients. Hepatology. 1991;14:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kawasaki S, Imamura H, Bandai Y, Sanjo K, Idezuki Y. Direct evidence for the intact hepatocyte theory in patients with liver cirrhosis. Gastroenterology. 1992;102:1351-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Alexandre E, Cahn M, Abadie-Viollon C, Meyer N, Heyd B, Mantion G, Cinqualbre J, David P, Jaeck D, Richert L. Influence of pre-, intra- and post-operative parameters of donor liver on the outcome of isolated human hepatocytes. Cell Tissue Bank. 2002;3:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Iqbal S, Elcombe CR, Elias E. Maintenance of mixed-function oxidase and conjugation enzyme activities in hepatocyte cultures prepared from normal and diseased human liver. J Hepatol. 1991;12:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Lloyd TD, Orr S, Patel R, Crees G, Chavda S, Vadyar H, Berry DP, Sherlock D, Dennison AR. Effect of patient, operative and isolation factors on subsequent yield and viability of human hepatocytes for research use. Cell Tissue Bank. 2004;5:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | van Wagensveld BA, van Gulik TM, Gelderblom HC, Scheepers JJ, Bosma A, Endert E, Obertop H, Gouma DJ. Continuous or intermittent vascular clamping during hemihepatectomy in pigs: hyaluronic acid kinetics in the assessment of early microvascular liver damage. Eur J Surg. 2000;166:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Serrar H, El-Kadi A, Du Souich P, Haddad P. Cytochrome P-450 content and activity after cold storage of rat hepatocytes in university of wisconsin and sodium-lactobionate-sucrose solutions. Liver Transpl Surg. 1999;5:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Fisher RL, Gandolfi AJ, Brendel K. Human liver quality is a dominant factor in the outcome of in vitro studies. Cell Biol Toxicol. 2001;17:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |