Published online Sep 14, 2016. doi: 10.3748/wjg.v22.i34.7718
Peer-review started: March 22, 2016
First decision: May 12, 2016
Revised: July 11, 2016
Accepted: July 31, 2016
Article in press: August 1, 2016
Published online: September 14, 2016
Processing time: 170 Days and 15 Hours
Inflammatory bowel disease (IBD) is a chronic relapsing disease in gastrointestinal tract. Conventional medications lack the efficacy to offer complete remission in IBD therapy, and usually associate with serious side effects. Recent studies indicated that nanoparticle-based nanotherapeutics may offer precise and safe alternative to conventional medications via enhanced targeting, sustained drug release, and decreased adverse effects. Here, we reviewed orally cell-specific nanotherapeutics developed in recent years. In addition, the various obstacles for oral drug delivery are also reviewed in this manuscript. Orally administrated cell-specific nanotherapeutics is expected to become a novel therapeutic approach for IBD treatment.
Core tip: Inflammatory bowel disease includes Crohn’s disease and ulcerative colitis. Nanotherapeutics may outperform conventional medications via the targeted drug delivery, sustained drug release, and decreased adverse effect. The main purpose of this review is to offer an update of efficacy of the orally administrated cell-specific nanotherapeutics that have been developed recently.
- Citation: Si XY, Merlin D, Xiao B. Recent advances in orally administered cell-specific nanotherapeutics for inflammatory bowel disease. World J Gastroenterol 2016; 22(34): 7718-7726
- URL: https://www.wjgnet.com/1007-9327/full/v22/i34/7718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i34.7718
Inflammatory bowel disease (IBD) is a chronic relapsing gastrointestinal (GI) disorder with no permanent cure. It mainly includes Crohn’s disease (CD) and ulcerative colitis (UC), and affects millions of patients worldwide. After 30 years of living with this disease, 8% of CD and 18%-20% of UC patients develop colitis-associated colon cancer, which is the third most common malignancy and one of the leading causes of cancer mortality[1]. Although the etiology of IBD remains largely unknown, a large amount of researches over the last decades demonstrated that the individual’s genetic susceptibility, intestinal microbiota, and immune responses are all involved in the pathogenesis of IBD[2]. Conventionally, IBD is treated by daily administration of high doses of anti-inflammatory or immunosuppressive drugs. Some of these treatments are effective in controlling inflammation. However, their applications have been restricted by problems with long-term efficacy and safety issues. For example, as for corticosteroids, a total daily dose over 20 mg of prednisolone for more than 2 wk is associated with an obvious increased risk of infections[3].
Recently, nanotherapeutics have been recognized as a promising strategy which can potentially revolutionize disease diagnostics and treatments. They offer significant advantages over traditional approaches because of their nanometer scale dimension, targeted drug delivery capacity, controlled drug release, and decreased adverse effects[4,5]. Most importantly, nanotherapeutics have been found to confer similar or even better therapeutic impacts at lower drug concentrations than their conventional counterparts[6]. It was reported that oral administration has been considered as the most convenient approach for colitis therapy-related drug delivery, as it avoids the pain and discomfort associated with injections, minimizes the potential for contamination, and is applicable for a self-medication that can be fully controlled by patients[7]. Accordingly, orally targeted nanotherapeutics have been developed.
The challenges for oral drug delivery are to ensure drug formulations to remain stable in the GI tract, transport adequate amount of active drugs to the specific sites, minimize systemic absorption of the drugs, and lower the risk of adverse side effects[8]. The earliest nanotherapeutics designed for IBD-targeted therapy are based on the physiological features that are particular to colon to trigger drug release[9]. However, physiological conditions can differ among patients and at various stages of IBD, making it very difficult to attain sufficient therapeutic efficiency. Parallel breakthroughs in the understanding of the molecular pathophysiology of IBD and the development of intelligent NPs offer tremendous promise for IBD therapy[10]. In this review, we focus on novel therapeutic approaches using orally targeted nanotherapeutics and their challenges in GI tract.
After oral administration, NP-based nanotherapeutics pass through the esophagus, the stomach, the small intestine and the colon, successively. The pH in the passage ranges from strongly acidic in the stomach (pH 1.5-1.9), to almost neutral in the small intestine, and then slightly acidic (pH 5-7) in the colon[11]. Therefore, NPs have to be stable over a wide pH range. In addition, they also encounter digestive enzymes in stomach (e.g., pancreatic enzymes), bicarbonate and bile salts in the small intestine, as well as abundant microbial population in colon. All of these contents in the GI tract can destabilize NPs and further reduce the effectiveness of their loaded drugs[12,13]. Besides, the semi-solid contents in colonic lumen prevent NPs from diffusing into the inflamed sites.
The mucus on the surface of colon epithelial layer is highly viscoelastic and adhesive, and forms a thick layer (830 ± 110 μm)[14]. It is mainly composed of mucins and lipids, and acts to trap and remove bacteria, viruses, and foreign matters[15]. In healthy colon, there is a continuous mucus which has two layers of sub-structures: the outer is a loosely adherent layer for bacterial adhesion; while the inner is a tightly adherent layer, normally sterile. In colon tissue with IBD, there is a marked increase in bacteria associated with colonic adherent mucus layer[2,16].
Maisel et al[17] developed an unmodified NPs that were mucoadhesive (mucoadhesive particles, MAP), and a PEG-coated NPs which were non-mucoadhesive (mucus-penetrating particles, MPP). In comparison to MAP, MPP tended to penetrate in the GI tract, including colitis tissue. In addition, Ijssennagger et al[18] demonstrated that hydrogen sulfide, mainly produced by sulfate-reducing bacteria, reduced disulfide bonds presented in the mucus network, thereby breaking the mucus barrier. Reduction of disulfide bonds in the gut lumen might represent an exciting method for the penetration of NPs to mucosa.
Inflamed colon is associated with disruption of the intestinal epithelial layer and accumulation of immune cells, leading to the loss of barrier function and increased epithelial permeability[19]. NPs are likely to penetrate into the gaps among epithelial cells, thus increasing the local drug concentration and exerting therapeutic effects there. This phenomenon is called “epithelial enhanced permeability and retention” effect[20,21]. This effect is size-dependent, showing a maximum efficacy in the nano range. Furthermore, in comparison to free drugs in solution or suspension, drug-loaded NPs were shown to accumulate to a greater extent in inflamed tissues and prolong therapeutic effects[20]. Additionally, anti-inflammatory chemical drugs loaded into NPs also showed enhanced cellular uptake by cells in colitis tissues, due to protection of the drugs from efflux systems and mucosal metabolism[22].
NPs generally undergo cellular internalization by paracellular transport or endocytosis into epithelial cells in the GI tract. In the context of cellular uptake by inflamed colon, endocytosis might be the main NP cellular uptake approach. Endocytosis can be triggered by the interaction between the surface moieties of NPs and the specific receptors, which are highly over-expressed on the surface of IBD therapy-related key cells in colitis tissue. Critically, the internalization and transportation of NPs may be enhanced by the specific targeting to these receptors[23].
NPs are entrapped in endosomes after internalization into cells. Subsequently, proton pump, an integral membrane protein, moves protons across the endosome membrane, inducing the pH continuously decrease from 7.2-7.4 to 4.5-5.0[24]. To avoid their degradation in endosome, many therapeutics (e.g., protein, plasmid DNA and siRNA) have to escape from the endosome into the cytosol, where they can associate their targets[25].
To induce efficient endosomal escape, a common strategy is to introduce chemical groups with proton-sponge effect to NPs. Our group previously synthesized a mannosylated bioreducible cationic polymer (PPM) and further spontaneously assembled NPs with siRNA assisted by sodium triphosphate (TPP). In these TPP-PPM/siRNA NPs, the abundant primary and tertiary amine groups in PPM can promote endosomal escape efficiently[26].
After escaping from the endosome, drugs often have to enter certain organelles in order to exert their functions there. Nuclear entry is a prerequisite for some drugs, such as inhibitors of transcription or the cell cycle[27]. For instance, plasmid DNA must be transported into the nucleus. Otherwise, transcription cannot occur[28].
Wang et al[29] synthesized a series of N-terminal stearylated nuclear localization signals, and their further studies showed that such vectors could effectively deliver plasmid DNA into nuclei. The maximum transfection efficiency of these vectors was 80% of that of jetPEITM.
Polyester NPs: Polylactic-co-glycolic acid (PLGA) and polylactic acid (PLA), FDA-approved biodegradable polyesters, have the capacity to encapsulate hydrophilic or hydrophobic drugs to form NPs. Thus, they have been commonly used as drug carrier materials[5,30,31].
Mahajan et al[32] loaded mesalamine into PLGA NPs, and they further administered them once a day to rats with colitis through oral administration or intracolonic administration. It was found that these NPs exerted much higher efficiency in mitigating colitis, in comparison to the free drug in suspension. In addition, the researchers also demonstrated that the mesalamine-loaded PLGA NPs showed selective adherence and enhanced drug accumulation into colitis tissues.
Silicon NPs: Silicon NPs have been widely used as drug vectors since they possess a range of beneficial features for drug delivery, including well-controlled size and size distribution, easily surface functionalization, and negative cytotoxicity, as well as large surface area and pore volume that facilitate drugs to be encapsulated[33].
Moulari et al[34] prepared 5-aminosalicylic acid-loaded silica NPs (around 140 nm), which showed 6-fold better adherence to inflamed colon than tissues from the healthy control mice after oral administration. In trinitrobenzene sulfonic acid (TNBS)-induced colitis mouse model, the NPs tended to accumulate in inflamed sites, and exerted excellent therapeutic efficacy in terms of clinical activity score and myeloperoxidase activity at lower drug doses than those applied in conventional delivery.
pH-sensitive NPs: pH-sensitive NPs take advantage of the pH differences in different regions of the GI tract[35]. The pH in the terminal ileum and colon is generally higher than that in any other regions of the GI tract. One of the simplest ways to modify dosage forms for pH-dependent drug delivery is to coat them with pH-sensitive biocompatible polymers.
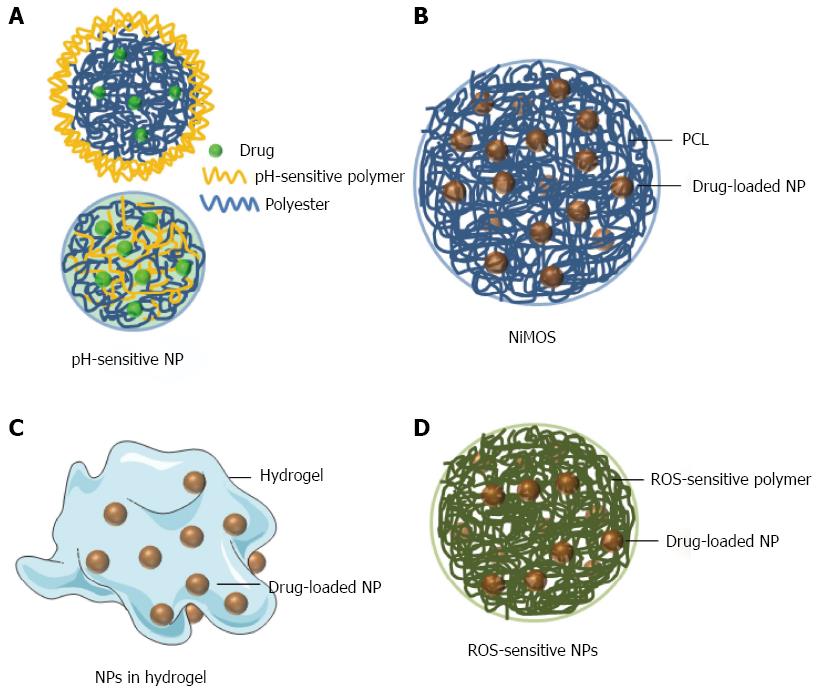
Ali et al[36] showed that budesonide-loaded PLGA NPs that were further coated with Eudragit S100 (Figure 1A upper) significantly alleviated inflammation in the dextran sulfate sodium (DSS)-, TNBS- and oxazolone-induced mouse colitis models. A pH-sensitive NPs can also be produced by mixing a pH-sensitive polymer and a non-pH-sensitive polymer in the NP fabrication process (Figure 1A lower). Beloqui et al[37] found that curcumin-loaded Eudragit S100/PLGA NPs showed obvious pH sensitivity profiles and efficiently decrease the expression/secretion of TNF-α in lipopolysaccharide-activated macrophages. Critically, oral administration of these pH-sensitive NPs significantly reduce neutrophil infiltration and TNF-α secretion in DSS-induced colitis mice. Additionally, histological studies revealed that the treatment mice exhibited almost the same colonic structure morphology as that observed in the healthy control group.
NP-in-microparticle: Recently, Amiji’s group developed a colon-targeted drug formulation named the NP-in-microparticle (MP) oral drug delivery system (NiMOS), as demonstrated in Figure 1B[38]. A multi-compartmental NiMOS drug delivery system consists of gelatin nanoparticles encapsulated within polyε-caprolactone (PCL) MPs[39]. The MP matrix inhibits protein/enzyme degradation, and thus avoids the harsh environment of the GI tract capable of destroying the embedded NPs. When NiMOS reaches the colon, lipases degrade the PCL coating and the encapsulated NPs will be released to colon, which would become available for the uptake by colonic cells[40-43].
Kriegel et al[43] prepared TNF-α siRNA (siTNF)-loaded gelatin NPs, and further encapsulated them in PCL matrix to yield NiMOSs. The animal experiment results based on a DSS-induced colitis mouse model demonstrated that the above NiMOSs remarkably suppressed the expression levels of TNF-α and other pro-inflammatory cytokines (e.g., IL-1β, IFN-γ, and monocyte chemotactic protein-1), and increased the body weight and colon length, in comparison to DSS-treated control mice. These results indicated that siTNF-loaded NiMOS could be a valuable therapeutic option for IBD patients.
NPs in hydrogel: Hydrogels are highly absorbent polymeric networks, and they can contain over 90% water. Chitosan and alginate are biocompatible and biodegradable polysaccharides, and used to prepare oral hydrogel by Merlin’s group[44]. This chitosan/alginate hydrogel was sensitive to the colonic pH and could also be degraded by colonic enzymes. Thus it would collapse when they arrived at colon.
Laroui et al[45] embedded Lysine-proline-valine (KPV)-containing PLA NPs in this hydrogel (Figure 1C). Under its protection, NPs were able to pass through the stomach and upper small intestine, and were released in the inflamed colon. Using this improved NPs in hydrogel system, a 12000-fold lower dose was sufficient to ameliorate mucosal inflammation in mice subjected to acute DSS-induced colitis, in comparison to free KPV in solution.
Reactive oxygen species-sensitive NPs: IBD is accompanied by abnormally high reactive oxygen species (ROS) level in the GI tract, especially in the inflamed areas[46]. The excessive ROS can degrade the extracellular matrix and injure tissues[47]. Based on this fact, ROS-sensitive NPs (Figure 1D) are supposed to specifically release loaded drugs to inflamed colon or scavenge ROS.
Wilson et al[48] synthesized poly(1,4-phenyleneacetone dimethylenethioketal) (PPADT), which is a novel ROS-sensitive polymer. PPADT were further used to encapsulate siTNF/DOTAP complexes to generate thioketal NPs (TKNs). In a DSS-induced colitis mouse model, the mRNA expression levels of TNF-α and several other pro-inflammatory cytokines (IL-1, IL-6 and IFNγ) were significantly decreased and DSS-induced acute colitis was efficiently ameliorated after 5 days of oral administration of TKNs (0.23 mg siRNA/kg body weight)[49].
It was also reported that antioxidant compounds and free radical scavengers improved colitis[50,51]. Vong et al[52] synthesized a novel antioxidative nitroxide radical-containing NP (RNPO) with a diameter of 40 nm. The further mice experiments indicated that the extent of inflammatory reactions and the severity of colitis have been relieved after oral administration of RNPO.
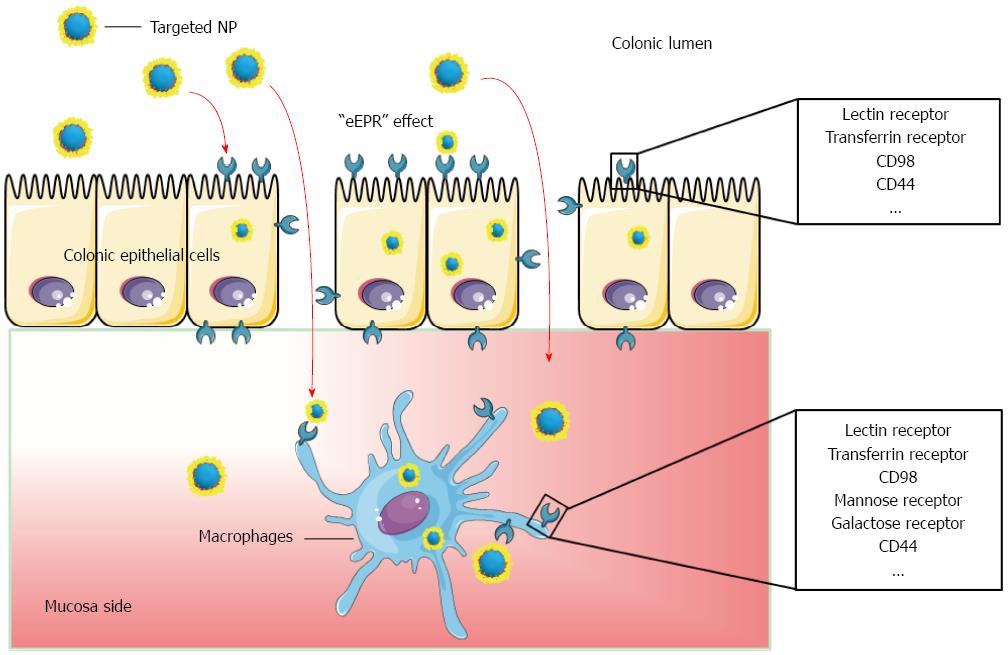
To further reduce side effects and increase the drug concentration at inflamed sites, researchers have sought to induce active targeting[53]. The interactions between targeting ligands on the NPs surface and specific receptors over-expressed at inflamed sites are expected to improve the bioadhesion and the internalization of NPs to specific cells. Various ligand-mediated targeted drug delivery systems are compared in Table 1, and their targeted drug delivery process is shown in Figure 2.
| Ligand | Delivery system | Effect | Ref. |
| Lectin | PLGA | Exhibited a much higher binding and selectivity to inflamed tissue compared to plain NPs | [54] |
| TfR antibodies | Liposomes | Exhibited mucopenetration and a 4-fold increase in uptake by inflamed colon tissues | [58] |
| CD98 antibodies | PEG–urocanic acid-chitosan | Approximately 24% of colonic macrophages were found to have taken up the targeted NPs within 12 h of administration | [66] |
| Mannose | Branched polyethylenimine | 29.5% of the NPs were internalized by colon macrophages | [26] |
| Galactose | Trimethyl chitosan-cysteine | Cellular uptake in activated macrophages was significantly higher for Galactose trimethyl chitosan-cysteine/TPP NPs compared to trimethyl chitosan-cysteine/TPP NPs | [75] |
| F4/80 Ab Fab' | Poly(lactic acid)-poly(ethylene glycol) block copolymer | Improved DSS-induced colitis in vivo, and higher therapeutic efficacy was obtained using Fab'-bearing NPs compared to non-conjugated NPs | [77] |
| Amphiphilic hyaluronic acid | Decylamine | Budesonide loaded HANPs demonstrated higher anti-inflammatory effect on IL-8 and TNF-α secretion in inflamed cell model compared to the same dose of free drug | [78] |
Lectin is a type of naturally occurring protein that is highly specific for carbohydrate residues. It has been widely used as a ligand facilitating colon-specific drug delivery.
Moulari et al[54] prepared two types of lectin-functionalized NPs that were peanut (PNA)-functionalized NPs and wheat germ (WGA)-functionalized NPs. Ex vivo quantitative adhesion analyses showed that lectin-functionalized NP exhibited a much higher binding and selectivity to inflamed tissue compared to plain NP. In terms of therapeutic efficacy, all glucocorticoid containing formulations revealed an enhanced therapeutic effect with lectin functionalization, especially by the PNA-NP compared to plain NP.
Healthy colon tissue has low expression level of transferrin receptor (TfR), whereas inflamed colon overexpresses TfR. Its elevated expression was detected in both basolateral and apical membranes of enterocytes from the colon biopsies of IBD patients and rats with colitis[55]. In addition, TfR levels are also found to be elevated in activated immune cells (e.g., lymphocytes and macrophages)[56,57]. Thus, it is reasonable to speculate that TfR could be used as a targeting receptor for colitis-targeted drug delivery.
Harel et al[58] investigated the ex vivo adhesion capacity of anti-TfR antibody-functionalized immunoliposome to inflamed mucosal tissue[59]. The results indicated that this liposome exhibited mucopenetration and a 4-fold increase in uptake by colitis tissues from TNBS-induced colitis mice, compared to healthy colon tissues.
CD98 is a heterodimeric neutral amino acid transporter, which consists of a heavy chain (CD98hc or SLC3A2) and one of several versions of the L-type amino acid transporter 1[60]. In colonic tissues from mice with active colitis, the expression level of CD98 is highly up-regulated at the surface of intestinal B cells, CD4+ T cells, and CD8+ T cell[61-63]. Additionally, CD98 is over-expressed in intestinal macrophages[64] and colonic epithelial cells from mice with colitis[61,65].
Recently, Xiao et al[66] fabricated an orally delivered hydrogel that releases CD98 antibody-functionalized NPs with the diameter of around 200 nm. In mice with acute or chronic colitis, orally administrated CD98 antibody-functionalized NPs significantly reduced the CD98 expression level in colitis tissue. Further studies revealed that about 24% of colonic macrophages internalize the targeted NPs within 12 h after oral administration, and the severity of colitis was significantly alleviated compared to the DSS control group.
The mannose receptor is a 175-kDa transmembrane protein, and it is exclusively expressed on the surfaces of macrophages[67]. MRs have high-affinity binding to infectious agents with terminal mannose groups[61]. Moreover, this receptor can also bind to mannose-functionalized NPs, inducing the subsequent rapid internalization[68]. Numerous reports demonstrated that the functionalization of mannose on NP surface provides selective macrophage targeting and promotes cell internalization efficiency[69,70].
Xiao et al[26] synthesized a mannosylated bioreducible cationic polymer (PPM). It could spontaneously complex with siRNA to form NPs assisted by TPP. The resultant TPP-PPM/siTNF NPs exhibited obvious macrophage-targeting property. Further ex vivo experiment indicated that these NPs markedly inhibited TNF-α secretion in DSS-induced colitis tissues. Importantly, flow cytometry results showed that TPP-PPM/siTNF NPs were efficiently taken up by macrophages (29.5%), whereas there was no significant uptake by the epithelial cells.
Another study presented the similar result by Huang et al[71]. They fabricated a drug formulation by cationic konjac glucomannan (cKGM), phytagel and an antisense oligonucleotide (ASO) against TNF-α. The unique swelling properties of cKGM induced the spontaneous release of cKGM/ASO NPs from the phytagel matrix to colon lumen. Subsequently, mannose groups can be recognized by MRs that are abundant on the surface of macrophages. The treatment of this oral drug formulation significantly decreased TNF-α expression level and alleviated the symptoms of colitis in DSS-treated mice.
It was reported that galactose receptor is highly overexpressed on the surface of activated macrophages[72,73]. Recently, Zhang et al[74] fabricated galactosylated trimethyl chitosan-cysteine (GTC) NPs loaded with siRNAs against mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4), which is a key enzyme of TNF-α production. In vitro results showed that the cellular uptake efficiency of GTC/TPP NPs was significantly higher than that of trimethyl chitosan-cysteine/TPP NPs, owing to galactose receptor-mediated endocytosis. Further in vivo experiments demonstrated that oral administration of siMAP4K4-loaded GTC/TPP NPs obviously improved DSS-induced colitis, as characterized by body weight, histology, and myeloperoxidase activity.
F4/80 is one of the vital macrophage-specific markers, and its antibody has been widely applied to separate macrophages[75,76]. Laroui et al[77] synthesized poly(lactic acid)-poly(ethylene glycol) block copolymer (PLA-PEG), and then conjugated with a macrophage-specific ligand (F4/80 Ab Fab’) via maleimide/thiol group-mediated covalent bonding. They further used this polymer to encapsulate TNF-α siRNA (siTNF) to obtain F4/80 Fab’-functionalized siTNF-loaded NPs. Oral administration of siTNF-loaded NPs markedly improved DSS-induced colitis, and exhibited much higher therapeutic efficacy for F4/80 Fab’-functionalized NPs, in comparison to non-functionalized NPs. Further flow cytometry experiments indicated that functionalization of F4/80 Fab’ significantly improved their macrophage targeting ability and the endocytosis of these NPs.
Hyaluronic acid (HA) is a natural anionic polysaccharide, composed of alternating D-glucuronic acid and N-acetyl-D-glucosamine units. It has a high affinity for the CD44 receptor, which is overexpressed on the surface of epithelial cells and activated inflammatory cells in colitis tissue[78]. Recently, Vafaei et al[78] synthesized HA-decylamine (DA) by chemical conjugation of DA to the backbone of HA. This amphiphilic HA-DA polymer could then form self-assembled HANPs. FITC-labeled HANPs revealed greater cellular uptake in inflamed Caco-2 BBE cell compared to untreated Caco-2 BBE cell and CD44-negative cell line (NIH3T3). Cytotoxicity test reveals that budesonide (BDS)-loaded HANPs displayed almost no toxicity, indicating HANPs were biocompatible nano-carriers. Importantly, BDS-loaded HANPs exhibited much higher anti-inflammatory effect in inflamed cell model compared to the same dose of free drug.
Overall, these studies demonstrate that active targeting is a very promising approach to enhance the accumulation and uptake of drugs by inflamed tissues.
Nanotherapeutic has been widely used as a novel approach for IBD treatment, and is much more effective than traditional drug formulation. Surface functionalization of these nanotherapeutics with targeting moieties (e.g., antibody, monosaccharide, or polysaccharide) can further promote drug accumulation in the IBD therapy-related cells through receptor-mediated endocytosis and decrease the potential adverse effects. As summarized in this review, we provide some novel nano-formulations specific to the organs, tissues, cells, or organelles. We must clearly understand the barriers impeding the specific delivery of drugs to inflamed colonic tissues or IBD therapy-related key cells. Specifically, there is a need for further investigation of the pathophysiology of IBD (especially in the molecular level) and the development of intelligent NPs.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gaertner W, Perse M, Rigoli L, Zouiten-Mekki L S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Vong LB, Yoshitomi T, Matsui H, Nagasaki Y. Development of an oral nanotherapeutics using redox nanoparticles for treatment of colitis-associated colon cancer. Biomaterials. 2015;55:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1043] [Article Influence: 94.8] [Reference Citation Analysis (18)] |
| 3. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 746] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 4. | Doshi N, Mitragotri S. Designer biomaterials for nanomedicine. Adv Funct Mater. 2009;19:3843-3854. [DOI] [Full Text] |
| 5. | Xiao B, Han MK, Viennois E, Wang L, Zhang M, Si X, Merlin D. Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale. 2015;7:17745-17755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Lautenschläger C, Schmidt C, Lange K, Stallmach A. [Drug delivery strategies for targeted treatment of inflammatory bowel disease]. Z Gastroenterol. 2015;53:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Isaacs KL, Lewis JD, Sandborn WJ, Sands BE, Targan SR. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis. 2005;11 Suppl 1:S3-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Wachsmann P, Lamprecht A. Polymeric nanoparticles for the selective therapy of inflammatory bowel disease. Methods Enzymol. 2012;508:377-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Meissner Y, Lamprecht A. Alternative drug delivery approaches for the therapy of inflammatory bowel disease. J Pharm Sci. 2008;97:2878-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Xiao B, Merlin D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv. 2012;9:1393-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J Control Release. 2012;162:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 12. | Maroni A, Zema L, Del Curto MD, Foppoli A, Gazzaniga A. Oral colon delivery of insulin with the aid of functional adjuvants. Adv Drug Deliv Rev. 2012;64:540-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Loretz B, Föger F, Werle M, Bernkop-Schnürch A. Oral gene delivery: Strategies to improve stability of pDNA towards intestinal digestion. J Drug Target. 2006;14:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 1050] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 15. | Wang YY, Lai SK, So C, Schneider C, Cone R, Hanes J. Mucoadhesive nanoparticles may disrupt the protective human mucus barrier by altering its microstructure. PLoS One. 2011;6:e21547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064-15069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1533] [Article Influence: 90.2] [Reference Citation Analysis (1)] |
| 17. | Maisel K, Ensign L, Reddy M, Cone R, Hanes J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release. 2015;197:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Ijssennagger N, van der Meer R, van Mil SW. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol Med. 2016;22:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 19. | Ingersoll SA, Ayyadurai S, Charania MA, Laroui H, Yan Y, Merlin D. The role and pathophysiological relevance of membrane transporter PepT1 in intestinal inflammation and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2012;302:G484-G492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Lamprecht A. IBD: selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol Hepatol. 2010;7:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Collnot EM, Ali H, Lehr CM. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 2012;161:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J Pharmacol Exp Ther. 2005;315:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 976] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 24. | Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi JF, Blot G, Leuba F, Escola JM, Demaurex N, Marsh M. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Forbes DC, Peppas NA. Polycationic nanoparticles for siRNA delivery: comparing ARGET ATRP and UV-initiated formulations. ACS Nano. 2014;8:2908-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Xiao B, Laroui H, Ayyadurai S, Viennois E, Charania MA, Zhang Y, Merlin D. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials. 2013;34:7471-7482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Dean DA. Nuclear transport: an emerging opportunity for drug targeting. Adv Drug Deliv Rev. 2003;55:699-702. [PubMed] |
| 28. | Escriou V, Carrière M, Scherman D, Wils P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv Drug Deliv Rev. 2003;55:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Wang HY, Chen JX, Sun YX, Deng JZ, Li C, Zhang XZ, Zhuo RX. Construction of cell penetrating peptide vectors with N-terminal stearylated nuclear localization signal for targeted delivery of DNA into the cell nuclei. J Control Release. 2011;155:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Xiao B, Zhang M, Viennois E, Zhang Y, Wei N, Baker MT, Jung Y, Merlin D. Inhibition of MDR1 gene expression and enhancing cellular uptake for effective colon cancer treatment using dual-surface-functionalized nanoparticles. Biomaterials. 2015;48:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 31. | Xiao B, Si X, Han MK, Viennois E, Zhang M, Merlin D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J Mater Chem B Mater Biol Med. 2015;3:7724-7733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Mahajan N, Sakarkar D, Manmode A, Pathak V, Ingole R, Dewade D. Biodegradable nanoparticles for targeted delivery in treatment of ulcerative colitis. Adv Sci Lett. 2011;4:349-356. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Rosenholm J, Sahlgren C, Linden M. Cancer-cell targeting and cell-specific delivery by mesoporous silica nanoparticles. J Mater Chem. 2010;20:2707-2713. [RCA] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Moulari B, Pertuit D, Pellequer Y, Lamprecht A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials. 2008;29:4554-4560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Xiao B, Si X, Zhang M, Merlin D. Oral administration of pH-sensitive curcumin-loaded microparticles for ulcerative colitis therapy. Colloids Surf B Biointerfaces. 2015;135:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Ali H, Weigmann B, Neurath MF, Collnot EM, Windbergs M, Lehr CM. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release. 2014;183:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Beloqui A, Coco R, Memvanga PB, Ucakar B, des Rieux A, Préat V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int J Pharm. 2014;473:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | Bhavsar MD, Tiwari SB, Amiji MM. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Control Release. 2006;110:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Kriegel C, Attarwala H, Amiji M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Adv Drug Deliv Rev. 2013;65:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS). J Control Release. 2007;119:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Bhavsar MD, Amiji MM. Development of novel biodegradable polymeric nanoparticles-in-microsphere formulation for local plasmid DNA delivery in the gastrointestinal tract. AAPS PharmSciTech. 2008;9:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Bhavsar MD, Amiji MM. Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Gene Ther. 2008;15:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Kriegel C, Amiji M. Oral TNF-α gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J Control Release. 2011;150:77-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Cario E. Nanotechnology-based drug delivery in mucosal immune diseases: hype or hope? Mucosal Immunol. 2012;5:2-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138:843-853.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 46. | Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48:743-751. [PubMed] |
| 47. | Eberlein M, Scheibner KA, Black KE, Collins SL, Chan-Li Y, Powell JD, Horton MR. Anti-oxidant inhibition of hyaluronan fragment-induced inflammatory gene expression. J Inflamm (Lond). 2008;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9:923-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 49. | Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 399] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 50. | Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 220] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Araki Y, Sugihara H, Hattori T. The free radical scavengers edaravone and tempol suppress experimental dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143:1027-1036.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Laroui H, Geem D, Xiao B, Viennois E, Rakhya P, Denning T, Merlin D. Targeting intestinal inflammation with CD98 siRNA/PEI-loaded nanoparticles. Mol Ther. 2014;22:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Moulari B, Béduneau A, Pellequer Y, Lamprecht A. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J Control Release. 2014;188:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 55. | Tirosh B, Khatib N, Barenholz Y, Nissan A, Rubinstein A. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol Pharm. 2009;6:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Pallone F, Fais S, Squarcia O, Biancone L, Pozzilli P, Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn’s disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Tacchini L, Gammella E, De Ponti C, Recalcati S, Cairo G. Role of HIF-1 and NF-kappaB transcription factors in the modulation of transferrin receptor by inflammatory and anti-inflammatory signals. J Biol Chem. 2008;283:20674-20686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Harel E, Rubinstein A, Nissan A, Khazanov E, Nadler Milbauer M, Barenholz Y, Tirosh B. Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa. PLoS One. 2011;6:e24202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 353] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 60. | Verrey F, Jack DL, Paulsen IT, Saier MH, Pfeiffer R. New glycoprotein-associated amino acid transporters. J Membr Biol. 1999;172:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Kucharzik T, Lugering A, Yan Y, Driss A, Charrier L, Sitaraman S, Merlin D. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation. Lab Invest. 2005;85:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Nguyen HT, Dalmasso G, Yan Y, Laroui H, Dahan S, Mayer L, Sitaraman SV, Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem. 2010;285:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020-1030. [PubMed] |
| 64. | MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 65. | Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, Zhang Z, Baker MT, Zhang B, Gewirtz AT. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146:1289-1300.e1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 67. | Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci USA. 1986;83:2501-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Sato Y, Beutler E. Binding, internalization, and degradation of mannose-terminated glucocerebrosidase by macrophages. J Clin Invest. 1993;91:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Jiang HL, Kang ML, Quan JS, Kang SG, Akaike T, Yoo HS, Cho CS. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials. 2008;29:1931-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Jain A, Agarwal A, Majumder S, Lariya N, Khaya A, Agrawal H, Majumdar S, Agrawal GP. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Control Release. 2010;148:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Huang Z, Gan J, Jia L, Guo G, Wang C, Zang Y, Ding Z, Chen J, Zhang J, Dong L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | van Vliet SJ, Saeland E, van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 2008;29:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Coombs PJ, Taylor ME, Drickamer K. Two categories of mammalian galactose-binding receptors distinguished by glycan array profiling. Glycobiology. 2006;16:1C-7C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Zhang J, Tang C, Yin C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials. 2013;34:3667-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 75. | van den Berg TK, Kraal G. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 2005;26:506-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1230] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 77. | Laroui H, Viennois E, Xiao B, Canup BS, Geem D, Denning TL, Merlin D. Fab’-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J Control Release. 2014;186:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 78. | Vafaei SY, Esmaeili M, Amini M, Atyabi F, Ostad SN, Dinarvand R. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr Polym. 2016;144:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |