Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.7046
Peer-review started: April 10, 2016
First decision: May 12, 2016
Revised: June 10, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: August 21, 2016
Processing time: 127 Days and 23 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a common pancreatic cancer and the fourth leading cause of cancer death in the United States. Treating this life-threatening disease remains challenging due to the lack of effective prognosis, diagnosis and therapy. Apart from pancreatic duct cells, acinar cells may also be the origin of PDAC. During pancreatitis or combined with activating KRasG12D mutation, acinar cells lose their cellular identity and undergo a transdifferentiation process called acinar-to-ductal-metaplasia (ADM), forming duct cells which may then transform into pancreatic intraepithelial neoplasia (PanIN) and eventually PDAC. During ADM, the activation of mitogen-activated protein kinases, Wnt, Notch and phosphatidylinositide 3-kinases/Akt signaling inhibits the transcription of acinar-specific genes, including Mist and amylase, but promotes the expression of ductal genes, such as cytokeratin-19. Inhibition of this transdifferentiation process hinders the development of PanIN and PDAC. In addition, the transdifferentiated cells regain acinar identity, indicating ADM may be a reversible process. This provides a new therapeutic direction in treating PDAC through cancer reprogramming. Many studies have already demonstrated the success of switching PanIN/PDAC back to normal cells through the use of PD325901, the expression of E47, and the knockdown of Dickkopf-3. In this review, we discuss the signaling pathways involved in ADM and the therapeutic potential of targeting reprogramming in order to treat PDAC.
Core tip: Treating pancreatic ductal adenocarcinoma (PDAC) remains challenging due to the lack of effective therapeutics. Apart from pancreatic duct cells, acinar cells may also be the origin of PDAC. During pancreatitis or combined with activating KRasG12D mutation, acinar cells undergo a transdifferentiation process called acinar-to-ductal-metaplasia (ADM), forming duct cells which may then be transformed into PanIN and eventually PDAC. This process involves MAPK, Wnt, Notch and PI3K/Akt signaling. Since ADM may be a reversible process, switching PDAC back to normal cells may also be achieved and developed as a novel therapy.
- Citation: Wong CH, Li YJ, Chen YC. Therapeutic potential of targeting acinar cell reprogramming in pancreatic cancer. World J Gastroenterol 2016; 22(31): 7046-7057
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/7046.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.7046
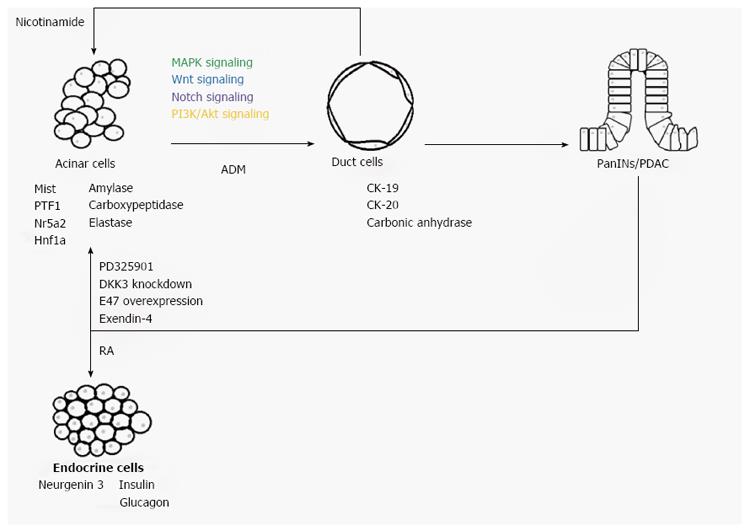
Pancreatic cancer is still the fourth leading cause of cancer death in the United States and Europe, although the incidence rate and death rate in the United States have been stable since the 1970s (but continue to increase in European countries)[1,2]. Most (96%) of the pancreatic cancer is from exocrine pancreas, including pancreatic ductal adenocarcinoma (PDAC)[1]. Due to the lack of reliable diagnostics, over half of patients are usually diagnosed with pancreatic cancer at an advanced stage. This also contributes to the low 5-year survival rate (7%), with median survival around 4 mo[1,3]. Surgery, chemotherapy and radiotherapy are commonly used to treat pancreatic cancer, although they can only extend survival for several months or can be used as an alleviative instead of for curing the patient[3,4]. Also, since many patients already have cancer metastasis when they are diagnosed, only a few patients can receive surgery. The chemotherapy drug gemcitabine is usually used together with surgery or the targeted anti-cancer drug erlotinib. In addition, combining gemcitabine with other chemotherapy drugs, such as nab-paclitaxel or the human monoclonal antibody AGS-1C4D4, can only provide a limited extension of survival by months in exchange for developing adverse effects, including abdominal pain, peripheral neuropathy and myelosuppression[5,6]. Therefore, a novel therapy is urgently needed. Here, we briefly introduce transdifferentiation of acinar cells to ductal cells with tumorigenic potential under the activation of cellular signaling pathways. Also, we discuss the therapeutic potential of reprogramming PDAC back to normal pancreatic cells, such as acinar cells, in order to treat cancer.
Pancreas is composed of the endocrine and exocrine compartments, which are responsible for maintaining body homeostasis and digesting food respectively[7]. The endocrine pancreas is formed by islets cells, which secrete hormones such as insulin and glucagon to regulate the glucose level in the body. The exocrine pancreas consists of acinar cells, which synthesize digestive enzymes such as amylase and duct cells and are responsible for transportation of digestive enzymes to the duodenum for digestion. Studies show that PDAC may originate from pancreatic duct cells through the development of pancreatic intraepithelial neoplasia (PanIN) after the first activating gene mutation in KRas[8]. However, the KRasG12D mutation alone is insufficient to drive the development of PDAC[8]. Further genetic changes, such as in p16, p53 and SMAD4, and overexpression of epidermal growth factor receptor (EGFR), EGF and transforming growth factor alpha (TGFα) are required for development to a higher-grade PanIN and eventually PDAC[9-12].
Apart from pancreatic duct cells, pancreatic acinar cells, which are the source of acinar cell carcinoma, may also contribute to the development of PDAC through acinar-to-ductal metaplasia (ADM)[13]. ADM is a type of transdifferentiation by which a mature and differentiated cell type changes its identity to another differentiated cell type[14]. During ADM, acinar cells leave the quiescent state and transdifferentiate to duct cells, losing their grape-like morphology and changing the transcriptome from acinar-like, such as with expression of Mist, amylase, carboxypeptidase and elastase, to duct-like, such as with expression of cytokeratin-19 (CK-19), cytokeratin-20 (CK-20) and carbonic anhydrase II[13,15]. This transdifferentiation process involves a nestin-positive intermediate[15]. Also, studies using an in vivo model showed that pancreatitis, both acute and chronic, can result in ADM[16,17]. These observations are consistent with the suggestion that chronic pancreatitis may be one of the causes of pancreatic cancer[18].
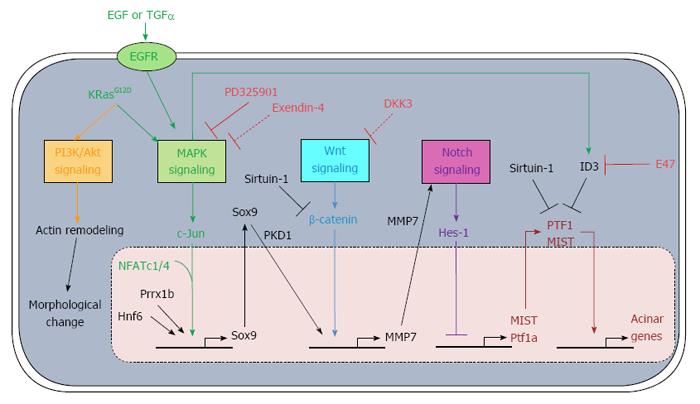
It has been reported that more than 90% of PDAC harbor a KRas activating mutation[10]. In addition, compared to normal acinar cells, acinar cells with KRas mutation fail to regenerate during pancreatitis and undergo ADM-forming PanIN. These findings indicate the importance of an activating mutation of KRas in acinar reprogramming and PDAC development. KRas is involved in many signaling pathways, such as the mitogen-activated protein kinases (MAPK) signaling pathway; therefore, the importance of the MAPK signaling pathway has been studied using three-dimensional culture of mouse acinar cells with activating mutation of KRasG12D after pancreatitis induction[16]. Inhibiting the MAPK signaling pathway after pancreatitis maintains cells in the grape-like acinar morphology and expressing amylase without undergoing ADM. In contrast, ADM is observed for acinar cells without inhibition of the MAPK signaling pathway after pancreatitis.
Upon the activation of EGFR by the EGF family members, such as EGF and TGFα, the growth factor receptor-bound protein 2 (GRB2) binds to the tyrosine kinase on the cytoplasmic side of EGFR through its SH2 domain[19]. This binding recruits the guanine nucleotide exchange factor son of sevenless (SOS) to interact with the SH3 domain of GRB2, resulting in SOS activation. The activated SOS exchanges the GDP in resting Ras with GTP. Therefore, Ras is activated, which in turn activates the protein kinase activity of the serine/ threonine kinase Raf. Raf phosphorylates and activates mitogen-activated protein kinase kinase1/2 (MEK1/2). MEK1/2 phosphorylates and activates MAPK.
The nuclear factor of activated T-cells (NFAT) transcription factor family actively participates in the immune system, which includes T-cells, B-cells and dendritic cells[20]. Apart from the immune system, NFAT is also involved in proliferation, invasion, angiogenesis and drug-resistance in various cancer cells, such as those of prostate, breast and lung[20-24]. In addition, it has been discovered that two family members, NFATc1 and NFATc4 (NFATc1/4), may be the downstream targets of the MAPK signaling pathway and may correlate with ADM and PanIN development[25,26]. NFATc1 is expressed, activated and accumulated in the nucleus of pancreatic cancer cells[27]. In addition, after the activation of MAPK signaling by TGFα, both NFATc1/4 are up-regulated, in contrast to the other NFAT family members such as NFATc2 and NFATc3[25]. Furthermore, acinar to ductal morphological transdifferentiation is observed in NFATc1/4-expressing acinar cells. It has been demonstrated that during ADM, NFATc1 interacts with c-Jun which is activated by the MAPK family member c-Jun N-terminal kinases (JNK) after activation of MAPK signaling, as indicated by co-immunoprecipitation assay[26]. The NFATc1-c-Jun complex is also observed in T-cells, mediating immune response[28,29]. It binds to the promoter of sex determining region Y-box 9 (Sox9), resulting in activation through histone 3 lysine 4 tri-methylation[26]. Hessmann et al[25] also reported similar c-Jun complex formation and Sox9 activation involving NFATc4. Disrupting the NFATc1/4-c-Jun complex results in failure in inducing Sox9. These observations consistently indicate the involvement of Sox9 in ADM.
Although the Sox9 transcription factor is involved in ductal, acinar and endocrine development, it is not expressed in acinar cells[30]. However, it is induced during chronic pancreatitis and ADM[25,26,31]. In addition, it is expressed in ductal cells, PanINs and PDAC, indicating its association in acinar reprogramming, cancer initiation and development[25,26]. On the other hand, acinar cells with Sox9 deletion can still undergo ADM upon pancreatic injury. Kopp et al[31] suggested that another pancreatic development-related transcription factor, hepatocyte nuclear factor 6 (Hnf6), can replace Sox9 with lower ADM-promoting efficiency. Like Sox9, hepatocyte nuclear factor 1 beta (Hnf1β) and Hnf6 are expressed in normal ductal cells but not in acinar cells[32]. However, in contrast to the suggestion by Roy and colleagues[33] that Hnf1β is up-regulated during acinar dedifferentiation, the expression of Hnf1β is not observed during ADM in either human or mouse tissue samples[32].
On the other hand, overexpression of Hnf6 triggers ADM, as indicated by the increase in CK-19 level and decrease in amylase and the acinar transcription factor Mist levels. Although, how Hnf6 is regulated still needs further study. Prévot et al[32] demonstrated that Hnf6 can induce the expression of Sox9, while knockdown of HNF6 alleles cannot fully deplete Sox9. This indicates that Hnf6 is not the sole regulator of Sox9. In addition, the Prrx1b isoform of another transcription factor, Prrx1, is also up-regulated during acinar transdifferentiation and positively regulates the expression of Sox9 through binding to its promoter and induces ADM[34]. Therefore, this may suggest that Sox9 is regulated by at least NFATc1/4-c-Jun, Hnf6 and Prrx1. Although depleting Sox9 can still drive the reprogramming of acinar cells to ductal cells, no progression to PanIN is observed[31]. In addition, Sox9 is expressed in PanIN and PDAC, but not for Hnf6[32]. Although, this may suggest that Sox can be replaced partially by Hnf6 during ADM, but it may still be required for PanIN progression.
Current evidence suggests that there may be a functional correlation between Sox9 and protein kinase D1 (PKD1)[35]. Similar to Sox9, PKD1, which is up-regulated under the activation of MAPK signaling by either EGFR or KRasG12D, promotes acinar cell reprogramming. Also, knockdown of PKD1 or inhibiting PKD1 significantly suppress ADM. PKD1 bridges the MAPK and Notch signaling pathways and promotes PanIN and adenocarcinoma formation[35]. When PKD1 is actively expressed in acinar cells, matrix metalloproteinase 7 (MMP7), a disintegrin and metalloproteinase 7 (Adam7) and Adam10, which are regulators of Notch, are significantly up-regulated, while Notch-suppressor suppressor enhancer lin12/Notch 1-like and casitas B-lineage lymphoma are down-regulated[35,36]. Sawey et al[36] also reported similar findings that MMP7 is involved in ADM via MAPK signaling. In addition, targets of the Notch signaling pathway, such as hairy and enhancer of split-1 (Hes-1), Hairy/enhancer-of-split related with YRPW motif protein-1 (Hey-1) and p21, are significantly up-regulated when PKD1 is activated[32,33]. These results indicate that Notch signaling is involved in ADM.
Wnt signaling is directly and indirectly involved in development and repair of various organs, including pancreas, bone, kidney and heart[37-40]. The Wnt signaling pathway is complex, and the three well-studied pathways are the canonical pathway (or Wnt/β-catenin pathway), the non-canonical planar cell polarity pathway and the non-canonical Wnt/calcium pathway[41]. The canonical pathway is different from the other two in the fact that involvement of β-catenin is required for ADM. When there is no Wnt signaling activation, β-catenin is originally degraded by phosphorylation and then ubiquitination involving the β-catenin destruction complex (formed by protein phosphatase 2A, glycogen synthase kinase3, casein kinase 1α, adenomatosis polyposis coli, and Axin). Wnt signaling can be activated by the binding of Wnt protein to the Frizzled family receptor complex Fz/LRP5/6. This results in membrane translocation and binding of Axin to LRP5/6 through microtubule actin-linking factor 1. In addition, Dsh is activated and binds to the receptor complex, inhibiting glycogen synthase kinase 3. As a result, the β-catenin destruction complex is disrupted and β-catenin accumulates. β-catenin then translocates to the nucleus, activating its target genes.
A growing number of studies suggest the role of the canonical pathway in ADM. However, its role-whether as a promoter or inhibitor-depends on the progression of the reprogramming. Since the canonical pathway is involved in the regeneration process of many organs, it may also actively participate in pancreatic regeneration. During pancreatitis, the canonical pathway is activated within 1 wk, as indicated by the accumulation of β-catenin and Axin[42,43]. Recovered pancreas, with normal grape-like morphology and amylase expression, is observed after 1 wk. In addition, activation of the canonical pathway blocks KRasG12D-driven ADM during pancreatitis, resulting in a normal recovery process. Similarly, overexpressing β-catenin does not result in PDAC but instead in an uncommon intraductal tubular tumor[44,45].
On the other hand, gradual accumulation of β-catenin is observed in acinar cells with KRasG12D after 1 mo of pancreatitis[42]. However, normal acinar morphology and acinar maker expression are not observed. Instead, mice with functional β-catenin and active mutation of KRasG12D express CK-19 and mucin, indicating progression of ADM and PanIN development respectively[44]. Also, inhibition of Frizzled receptor, which is the starting point of the Wnt signaling pathway, by the monoclonal antibody OMP-18R5 hinders progression of ADM. The decrease in Wnt target genes Axin2, lymphoid enhancer-binding factor-1 and MMP7 in mice after treatment with OMP-18R5 confirms the inhibition of Wnt signaling. Therefore, canonical Wnt signaling functions as an inhibitor during the initiation of ADM but promotes the formation of PanIN in the late process.
Sirtuin-1 (Sirt-1) functions as a protein deacetylase, controlling the development of heart, brain and spinal cord[46]. It is also found in both exocrine and endocrine pancreas. In endocrine pancreas, especially in the β-cells, Sirt-1 regulates the secretion of insulin[47]. While in injured exocrine pancreas, it promotes acinar transdifferentiation. During pancreatitis, Sirt-1 is transiently translocated from nucleus to cytoplasm, which is important for ADM[48]. Blocking Sirt-1 translocation significantly interferes with the expression of ductal genes. In addition, inhibiting Sirt-1 expression in nucleus by non-functional gene mutation significantly accelerates and prolongs the progression of acinar reprogramming. These results indicate that Sirt-1 functions as an ADM suppressor in nucleus. On the other hand, similar to suppressing MAPK signaling, the use of nicotinamide, which is an end-product inhibitor of Sirt-1 in protein acetylation, inhibits ADM after cytoplasmic shift of Sirt-1[48,49]. The cytoplasmic Sirt-1 deacetylates β-catenin and acinar-specific transcription factors, thereby inhibiting the acinar regeneration.
Notch signaling is required for regulating the development of various vital organs, including pancreas, bones and blood[50-53]. Notch protein is composed of the Notch extracellular domain (NECD), Notch transmembrane fragment (NTF) and Notch intracellular domain (NICD). Maturation of the Notch receptor involves cleavage of the NECD from the non-NECD and integration of the NECD heterodimer to the cell membrane. During organ development, especially of the pancreas, the Notch receptor is activated by type I transmembrane ligands including jagged-1, resulting in activation of a membrane-bound protease which cleaves the non-NECD into NTF and NICD. The released NICD translocates to the nucleus, resulting in expression of target genes such as Hes-1 and nuclear factor-κB, involving Rbp-J, Mastermind-like, and DEAD-box helicase.
Notch signaling is activated in PDAC and its precursor. Compared to normal pancreatic tissues, the Notch ligand jagged-1, the Notch receptors Notch2 and Notch3, and its target genes Hes-1 and Hey-1 are up-regulated during pancreatitis, ADM and cancer development[13,43,54]. In addition, PDK1, which is up-regulated during ADM, activates the Notch signaling pathway through at least MMP7[35]. Also, MMP7 is the downstream target in Wnt signaling during ADM, since inhibition of Wnt signaling decreases the level of MMP7. MMP7 is one of main metalloproteinases responsible for activating cleavage of the Notch receptor[55]. Therefore, the increase in MMP7 level indicates involvement of Notch signaling during acinar cell reprogramming[36,56].
The downstream effector Hes-1 in the Notch signaling pathway negatively regulates the basic helix-loop-helix (bHLH) transcription factor family, which is involved in pancreatic development. One of the members, neurgenin3, is involved in endocrine differentiation, whereas acinar cell development requires Mist[57]. During development of the exocrine pancreas in the embryo, Notch signaling is activated in the exocrine precursor but it is repressed in normal differentiated acinar cells. Since the downstream target Hes-1 in Notch signaling inhibits the transcription of acinar transcription factors, inactivation of Notch signaling promotes the development of pancreatic acinar cells[58]. During acinar differentiation, Mist activates the genes involved in producing digestive enzymes, such as amylase and carboxypeptidase, in stimulating exocytosis and in maintaining acinar cell homeostasis[57,59]. Inhibition of Mist in pancreatic acinar cells results in ADM, as indicated by the loss of cell organization, the decrease in acinar gene expression and the expression of ductal marker CK-19[60].
Although many studies have focused on the loss of digestive enzymes in acinar cells, little emphasis has been put on the morphological changes during ADM. It has been suggested that activation of Akt signaling is responsible for the morphogenesis of mammary acinar cells during malignant transformation[61]. Similarly, Phosphatidylinositide 3-kinase (PI3K)/Akt signaling is also involved in structural changes, from acinar to ductal, through actin remodeling[62,63]. Under KRas mutation, PI3Kα, but not the other isoforms (β, γ or δ), induces the morphological changes in acinar cells[63]. The activation of PI3Kα phosphorylates phosphatidylinositol 4, 5-bisphosphate (PIP2), forming phosphatidylinositol-3, 4, 5-trisphosphate (PIP3), which activates phosphoinositide-dependent kinase 1 (PDK1) through binding to the pleckstrin homology (PH) domain and eventually activates Akt[64]. In addition, Rho GTPase, especially the RAC subfamily, can be activated by PIP3 via RAC-guanine nucleotide exchange factors (GEFs), such as Vav-1 and Tiam-1, with a PH domain[65]. The downstream effectors RAC and Akt are important for the dynamic changes in actin structure[61,63-66]. Arrangement of actin via polymerization results in morphological changes during ADM[63]. Inhibition or knockdown of PI3K, RAC or PDK1 not only maintains acinar morphology but also inhibits the development of PanIN and PDAC[62-64].
Mutation in p53, which is also a downstream effector in PI3K/Akt signaling, can be found in > 75% of PDAC patients[10]. The importance of p53 inactivation in cancer metastasis has been demonstrated[67,68]. However, whether p53 is involved in the early stage of PDAC development is still poorly understood. It may not participate in ADM but is involved in the development of higher grades of PanINs and PDAC[63].
In addition to Mist, down-regulation of other acinar-specific transcription factors, including Gata6, pancreas transcription factor 1 complex (PTF1), nuclear receptor subfamily 5 group A member 2 (Nr5a2) and Hnf1α are also observed during ADM. During pancreas development, Gata4 and Gata6 are expressed in developing pancreas, as opposed to the other Gata factors, i.e. Gata1-3, 5[69]. In the later stage of development, there is a linage preference for Gata4 and Gata6 expression. Gata4 mRNA and protein can be found only in exocrine pancreas, together with amylase but not glycogen, whereas Gata6 is in the endocrine pancreas[69,70]. However, in the adult pancreas, Gata4 is also expressed in islet cells such as the β-cell[69]. Gata6 expression is also observed in both acinar and islet cells in the adult pancreas[71]. Gata6 regulates the expression of other acinar transcription factors, such as Mist and PTF1, through binding to their promoters[71,72]. The knockdown of Gata6 hinders the expression of Mist, pancreas specific transcription factor1a (Ptf1a) and recombination signal binding protein for immunoglobulin kappa J region1 (Rbpj1), and also their downstream acinar genes, such as amylase, while expression of CK-19 is promoted. In addition, further activation of MAPK signaling upon Gata6 silencing emphasizes the role of Gata6 down-regulation in promoting ADM[72].
PTF1, which is composed of Ptf1α and Rbpj1, is involved in exocrine pancreas development and maintains correct spatial orientation under the regulation of Notch signaling[73-75]. Nr5a2, under the regulation of Gata6 and PTF1, is important in acinar development, maintaining acinar identity and acinar regeneration during pancreatitis[76,77]. During KRasG12D-driven ADM, PTF1 and Nr5a2 are down-regulated and their low expressions are maintained in PanIN and PDAC[76,78]. The loss of these acinar transcription factors sensitizes the cells to KRas mutation, promoting ADM and PDAC development.
Under the stimulation of KRasG12D mutation or combined with pancreatitis, normal acinar cells can be transdifferentiated into ductal cells, followed by the formation of PanIN and PDAC. This process involves the activation of MAPK, Wnt, Notch and PI3K/Akt signaling (Figure 1). Inhibition of these pathways keeps cells in their acinar state. Importantly, treating the transdifferentiated duct cells with nicotinamide reprograms them back to acinar cells, with re-expression of amylase and repression of CK-20, and indicating that ADM may be a reversible process[49]. Therefore, PDAC development from its progenitor acinar cells may also be reversed upon the inhibition of cancer-related signaling (Figure 2).
A growing number of studies have proved the concept of reprogramming PanIN or PDAC to normal pancreatic cell types such as acinar cells and endocrine cells (Table 1). Since the MAPK signaling pathway is up-regulated and is required during ADM and carcinogenesis, attenuation of this pathway may promote cancer transdifferentiation back to quiescent acinar cells. The MEK1/2 inhibitor PD325901 is effective in treating colon cancer, breast cancer and melanoma and has entered phase II clinical trial[79]. In addition, PD325901 can enhance the anti-cancer ability of the PI3K/mTOR inhibitor PF-04691502 in lung cancer and gemcitabine in pancreatic cancer[80]. A recent report has suggested that through inhibiting the MAPK signaling pathway, PD325901 may re-differentiate PanIN back to acinar state in addition to the reduction of the proliferation rate of cancer cells both in vitro and in vivo[16]. However, whether PD325901 can also reprogram cancer cells to acinar cells is still questioned, since more genetic alternations are accumulated in PDAC. Apart from PD325901, exendin-4, for which its synthetic version exenatide is used in treating type II diabetes, may reprogram PDAC[81]. It has been reported that exendin-4 induces cell cycle arrest via p27 and pancreatic cancer reprogramming, as indicated by expression of such digestive enzymes as amylase and chymotrypsinogen B1[82]. However, the detailed mechanism underlying how exendin-4, which is a glucagon-like peptide-1 receptor agonist, initiates cancer reprogramming is not fully understood[83]. In prostate cancer, exendin-4 inhibits cancer proliferation through suppressing MAPK signaling, as indicated by a decrease in phosphorylated ERK1/2 level[84]. On the other hand, ERK1/2 phosphorylation is observed during the process of liver transdifferentiation to pancreas involving exendin-4[85].
| Therapeutic approach | Product | Mechanism | Model studied | Ref. |
| Chemical | PD325901 | Inhibits MEK1/2 in MAPK signaling | Block ADM in in vitro experiment | [16] |
| Reprogram pancreatitis- induced PanIN back to acinar cells in in vivo experiment | ||||
| Exendin-4 | May inhibit ERK1/2 in MAPK signaling | Reprogram PANC-1 cells back to acinar cells and endocrine cells | [82] | |
| RA | Detailed study is needed | Reprogram HPAF cells to endocrine cells | [107] | |
| Genetic | DKK3 knockdown | Detailed study is needed | Reprogram PANC-1 cells back to acinar cells and endocrine cells | [82] |
| E47 overexpression | Functions as a sponge of ID3 to remove the inhibition of acinar transcription factors | Reprogram PDAC cell lines back to acinar cells using both in vitro and in vivo model systems | [100] |
Dickkopf-3 (DKK-3) belongs to the DKK family, which regulates the Wnt signaling pathway. Unlike the other DKK members DKK-1, DKK-2 and DKK-4 which bind to LRP5/6 and Kremem1/2 (Krm1/2) receptors on the cell surface to form a trimer that inhibits Wnt signaling through receptor internalization, DKK-3 interacts with Krm1/2 receptors but not LRP5/6 intracellularly, probably on the membranes of endoplasmic reticulum or Golgi apparatus, before transporting to the cell surface[86,87]. The expression of DKK-3 is regulated by Pax6, which is one of the paired box transcription factors involved in development of the pancreas. DKK-3 can be activated or repressed by Pax6, depending on cancer type; in pancreatic cancer, DKK-3 is positively regulated in pancreatic cells[88]. Pax6 is a downstream target of the MAPK signaling pathway through interaction with Erk1/2 and p38 MAPK[89]. Therefore, DKK-3 links the MAPK and Wnt signaling pathways, controlling development of the pancreas. Although it has been reported that DKK-1 functions as a Wnt signaling inhibitor, the role of DKK-3 may be dependent on cell type; for example, DKK-3 positively regulates Wnt signaling in the embryonic kidney HEK293 cell line but functions as an inhibitor in lung and bone cancer[90-92]. Since it is reported that the Wnt signaling pathway contributes to cancer development, DKK-3 may function as a tumor suppressor through inhibiting the Wnt signaling pathway. Consistently, DKK-3 is down-regulated in various cancer types, such as lung and papillary thyroid cancer, although it promotes cancer invasion in oral squamous cancer[90,93-95]. Also, its expression is significantly decreased or even lost in some pancreatic cancer cell lines and pancreatic cancer tissues, probably via DKK-3 promoter hypermethylation[82,96,97]. However, overexpression of DKK-3 does not inhibit the proliferation of pancreatic cancer cells significantly, although Uchida and colleagues[98] reported inhibition is involved in cancer cell proliferation and induction of apoptosis[82,97]. Interestingly, knockdown of the tumor suppressor DKK-3 also inhibited cell growth, but without apoptosis[82]. In addition, cell cycle arrest, morphological changes to endocrine cell-like status, and expression of the endocrine proteins insulin and glucagon and the acinar proteins amylase, chymotrypsinogen B1 and elastase are observed when DKK-3 is knocked-down. However, these changes in expression of acinar proteins or endocrine proteins have only been studied at the mRNA level but not at the protein level. In addition, DKK-3 level is up-regulated during exendin-4-induced cancer reprogramming. Therefore, whether inhibiting DKK-3 can transdifferentiate pancreatic cancer cells to normal pancreatic endocrine cells or acinar cells is still questioned.
During differentiation of the exocrine pancreas, the bHLH transcription factors Mist and PTF1 are expressed resulting in the development of acinar identity[57]. Since Mist and PTF1 are involved in acinar development, inhibiting Mist and PTF1 at the gene level results in losing acinar identity and promoting ADM[60,78]. In addition, sustained Mist expression hinders the progression of ADM and the development of PanIN[99]. Therefore, targeting acinar transcription factors can be used in cancer reprogramming. One representative example suggested by Kim et al[100] is the use of another bHLH transcription factor, E47. Since the bHLH family usually forms dimers, either homodimers or heterodimers, which then bind to target genes, it has been reported that E47 interacts with Mist or PTF1 to facilitate their entry into the nucleus, followed by activation of acinar genes[101]. This bHLH dimer formation can be disrupted by the inhibitor of DNA (ID) family member ID3, which is mediated by MAPK signaling. This disruption can result from interaction between ID3 and bHLH transcription factors, forming a non-functional ID3/bHLH dimer, or resulting in the decrease in E47 expression[101]. Apart from regulating acinar gene expression, E47 also inhibits the progression from G1 phase to S phase in cell cycle through binding to the p21 promoter[100]. In PDAC, the elevated protein level of ID3 results in decreased E47 activity and cell cycle entry. Because of its role in normal acinar cells and its decreased protein level in PDAC, it has been reported that overexpression of E47 induces growth arrest[100,101]. In addition, Kim et al[100] demonstrated that E47 may reprogram PDAC back to acinar cells, as indicated by the expression of Mist and its target genes such as trypsinogen, connexin32 and ZO.1 which are responsible for protein digestion, gap junctions and tight junctions respectively. The role of overexpressed E47 in suppressing tumor growth and cancer reprogramming has also been confirmed by in vivo study. Therefore, overexpression of bHLH transcription factors that directly target acinar genes may reprogram PDAC to acinar cells.
In addition to pancreatic acinar cells, normal endocrine cells, such as β-cells, may also be another goal in cancer reprogramming. During pancreatic development, retinoic acid (RA) stimulates the development of endocrine progenitor cells and the differentiation into β-cells[102]. Also, RA can be used in treating diabetes through regenerating functional endocrine cells or human embryonic stem cells differentiation to β-cells[103,104]. In addition, RA, which has already been approved for treating acute promyelocytic leukemia, may inhibit the growth or promote differentiation of pancreatic cancer stem cells[105,106]. Since pancreatic duct cells and endocrine cells originate from the same bipotent progenitor, the close developmental relationship favors the transdifferentiation between these two cell types[33]. El-Metwally et al[107] demonstrated the use of RA in reprogramming PDAC back to normal quiescent endocrine cells secreting insulin, glucagon and somatostatin; although, the detailed mechanism needs further study. In addition, induction of insulin production by glucose indicates expression of the insulin receptor gene. This further confirms the formation of functional endocrine cells. Interestingly, apoptosis is observed after cancer transdifferentiation, which contrasts to other reprogramming methods that suggest the formation of quiescent acinar cells without undergoing apoptosis[107]. Increasing attention has been paid to the role played by the mitochondria during cancer reprogramming. Increased mitochondrial activity and mass are observed during cancer transdifferentiation, which is followed by apoptosis[108]. It has been suggested that apoptosis occurring after cancer transdifferentiation to normal endocrine cells may be due to an uncorrectable genetic alternation during cancer development.
It is generally believed that pancreatic duct cells are the origin of PDAC. However, during pancreatitis, acinar cells can undergo ADM, forming duct cells and subsequently PanIN and PDAC under the activation mutation of KRasG12D. Therefore, acinar cells may also be the origin of PDAC through ADM. This acinar transdifferentiation process involves at least MAPK, Wnt, Notch and PI3K/Akt signaling. Inhibition of ADM in transdifferentiated cells can facilitate regaining the acinar identity, indicating ADM may be a reversible process and can be used as a chemopreventive. In addition, PanIN or even PDAC may be reprogrammed back to normal cells. The use of PD325901, exendin-4 and RA, overexpression of E47 and knockdown of DKK-3 have demonstrated the potential of cancer reprogramming. However, more evidence is needed to confirm the success of reprogramming. Future studies are needed to explore other therapeutic targets for regaining normal identity of PDAC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guo JC, Ozaki T S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | American Cancer Society, Cancer Facts and Figures 2015. Atlanta: American Cancer Society 2015; . |
| 2. | Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol. 2015;26:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 3. | Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control. 2006;17:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 5. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4865] [Article Influence: 405.4] [Reference Citation Analysis (0)] |
| 6. | Wolpin BM, O’Reilly EM, Ko YJ, Blaszkowsky LS, Rarick M, Rocha-Lima CM, Ritch P, Chan E, Spratlin J, Macarulla T. Global, multicenter, randomized, phase II trial of gemcitabine and gemcitabine plus AGS-1C4D4 in patients with previously untreated, metastatic pancreatic cancer. Ann Oncol. 2013;24:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569-1580. [PubMed] |
| 8. | Ray KC, Bell KM, Yan J, Gu G, Chung CH, Washington MK, Means AL. Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One. 2011;6:e16786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Bailey JM, Hendley AM, Lafaro KJ, Pruski MA, Jones NC, Alsina J, Younes M, Maitra A, McAllister F, Iacobuzio-Donahue CA. p53 mutations cooperate with oncogenic Kras to promote adenocarcinoma from pancreatic ductal cells. Oncogene. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731-1734. [PubMed] |
| 11. | Friess H, Berberat P, Schilling M, Kunz J, Korc M, Büchler MW. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med (Berl). 1996;74:35-42. [PubMed] |
| 12. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3023] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 13. | Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Schmid RM. Acinar-to-ductal metaplasia in pancreatic cancer development. J Clin Invest. 2002;109:1403-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767-3776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Collins MA, Yan W, Sebolt-Leopold JS, Pasca di Magliano M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology. 2014;146:822-834.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 953] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 18. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1138] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 20. | Müller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 21. | Manda KR, Tripathi P, Hsi AC, Ning J, Ruzinova MB, Liapis H, Bailey M, Zhang H, Maher CA, Humphrey PA. NFATc1 promotes prostate tumorigenesis and overcomes PTEN loss-induced senescence. Oncogene. 2016;35:3282-3292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Mancini M, Toker A, NFAT proteins: emerging roles in cancer progression. Nature Reviews Cancer. 2009;9:810-820. [RCA] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Im JY, Lee KW, Won KJ, Kim BK, Ban HS, Yoon SH, Lee YJ, Kim YJ, Song KB, Won M. DNA damage-induced apoptosis suppressor (DDIAS), a novel target of NFATc1, is associated with cisplatin resistance in lung cancer. Biochim Biophys Acta. 2016;1863:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Yiu GK, Kaunisto A, Chin YR, Toker A. NFAT promotes carcinoma invasive migration through glypican-6. Biochem J. 2011;440:157-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Hessmann E, Zhang JS, Chen NM, Hasselluhn M, Liou GY, Storz P, Ellenrieder V, Billadeau DD, Koenig A. NFATc4 Regulates Sox9 Gene Expression in Acinar Cell Plasticity and Pancreatic Cancer Initiation. Stem Cells Int. 2016;2016:5272498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Chen NM, Singh G, Koenig A, Liou GY, Storz P, Zhang JS, Regul L, Nagarajan S, Kühnemuth B, Johnsen SA. NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas. Gastroenterology. 2015;148:1024-1034.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, Gress TM, Ellenrieder V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714-3724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature. 1998;392:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 393] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992;356:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 400] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 30. | Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA. 2007;104:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 31. | Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JP, Pan FC, Akiyama H, Wright CV, Jensen K. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 545] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 32. | Prévot PP, Simion A, Grimont A, Colletti M, Khalaileh A, Van den Steen G, Sempoux C, Xu X, Roelants V, Hald J. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut. 2012;61:1723-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Roy N, Hebrok M. Regulation of Cellular Identity in Cancer. Dev Cell. 2015;35:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Reichert M, Takano S, von Burstin J, Kim SB, Lee JS, Ihida-Stansbury K, Hahn C, Heeg S, Schneider G, Rhim AD. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev. 2013;27:288-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Liou GY, Döppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, Crawford HC, Fields AP, Murray NR, Wang QJ. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun. 2015;6:6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci USA. 2007;104:19327-19332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Wells JM, Esni F, Boivin GP, Aronow BJ, Stuart W, Combs C, Sklenka A, Leach SD, Lowy AM. Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol. 2007;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Kobayashi Y, Uehara S, Udagawa N, Takahashi N. Regulation of bone metabolism by Wnt signals. J Biochem. 2016;159:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Pahnke A, Conant G, Huyer LD, Zhao Y, Feric N, Radisic M. The role of Wnt regulation in heart development, cardiac repair and disease: A tissue engineering perspective. Biochem Biophys Res Commun. 2016;473:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Baumgart M, Werther M, Bockholt A, Scheurer M, Rüschoff J, Dietmaier W, Ghadimi BM, Heinmöller E. Genomic instability at both the base pair level and the chromosomal level is detectable in earliest PanIN lesions in tissues of chronic pancreatitis. Pancreas. 2010;39:1093-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1164] [Cited by in RCA: 1069] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 42. | Morris JP, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 43. | Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Zhang Y, Morris JP, Yan W, Schofield HK, Gurney A, Simeone DM, Millar SE, Hoey T, Hebrok M, Pasca di Magliano M. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013;73:4909-4922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004;556:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 541] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 48. | Wauters E, Sanchez-Arévalo Lobo VJ, Pinho AV, Mawson A, Herranz D, Wu J, Cowley MJ, Colvin EK, Njicop EN, Sutherland RL. Sirtuin-1 regulates acinar-to-ductal metaplasia and supports cancer cell viability in pancreatic cancer. Cancer Res. 2013;73:2357-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Rooman I, Heremans Y, Heimberg H, Bouwens L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia. 2000;43:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Li XY, Zhai WJ, Teng CB. Notch Signaling in Pancreatic Development. Int J Mol Sci. 2016;17:pii: E48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 442] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 52. | Amsen D, Helbig C, Backer RA. Notch in T Cell Differentiation: All Things Considered. Trends Immunol. 2015;36:802-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 53. | Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 880] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 54. | Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 504] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 55. | Sawey ET, Crawford HC. Metalloproteinases and cell fate: Notch just ADAMs anymore. Cell Cycle. 2008;7:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 59. | Zhu L, Tran T, Rukstalis JM, Sun P, Damsz B, Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol. 2004;24:2673-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 61. | Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003;163:315-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, Hieber M, Arbeiter A, Klein S, Kong B. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 63. | Baer R, Cintas C, Dufresne M, Cassant-Sourdy S, Schönhuber N, Planque L, Lulka H, Couderc B, Bousquet C, Garmy-Susini B. Pancreatic cell plasticity and cancer initiation induced by oncogenic Kras is completely dependent on wild-type PI 3-kinase p110α. Genes Dev. 2014;28:2621-2635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Wu CY, Carpenter ES, Takeuchi KK, Halbrook CJ, Peverley LV, Bien H, Hall JC, DelGiorno KE, Pal D, Song Y. PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology. 2014;147:1405-1416.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 65. | Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1221] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 66. | Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1476] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 67. | Weissmueller S, Manchado E, Saborowski M, Morris JP, Wagenblast E, Davis CA, Moon SH, Pfister NT, Tschaharganeh DF, Kitzing T. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell. 2014;157:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 404] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 68. | Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA. 2010;107:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 500] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 69. | Decker K, Goldman DC, Grasch CL, Sussel L. Gata6 is an important regulator of mouse pancreas development. Dev Biol. 2006;298:415-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Ketola I, Otonkoski T, Pulkkinen MA, Niemi H, Palgi J, Jacobsen CM, Wilson DB, Heikinheimo M. Transcription factor GATA-6 is expressed in the endocrine and GATA-4 in the exocrine pancreas. Mol Cell Endocrinol. 2004;226:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Martinelli P, Cañamero M, del Pozo N, Madriles F, Zapata A, Real FX. Gata6 is required for complete acinar differentiation and maintenance of the exocrine pancreas in adult mice. Gut. 2013;62:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Martinelli P, Madriles F, Cañamero M, Pau EC, Pozo ND, Guerra C, Real FX. The acinar regulator Gata6 suppresses KrasG12V-driven pancreatic tumorigenesis in mice. Gut. 2016;65:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Krapp A, Knöfler M, Ledermann B, Bürki K, Berney C, Zoerkler N, Hagenbüchle O, Wellauer PK. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752-3763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 400] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 74. | Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213-4224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 75. | Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 76. | von Figura G, Morris JP, Wright CV, Hebrok M. Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut. 2014;63:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 77. | Hale MA, Swift GH, Hoang CQ, Deering TG, Masui T, Lee YK, Xue J, MacDonald RJ. The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis. Development. 2014;141:3123-3133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Krah NM, De La O JP, Swift GH, Hoang CQ, Willet SG, Chen Pan F, Cash GM, Bronner MP, Wright CV, MacDonald RJ. The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 79. | Boasberg PD, Redfern CH, Daniels GA, Bodkin D, Garrett CR, Ricart AD. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer Chemother Pharmacol. 2011;68:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 80. | Simmons BH, Lee JH, Lalwani K, Giddabasappa A, Snider BA, Wong A, Lappin PB, Eswaraka J, Kan JL, Christensen JG. Combination of a MEK inhibitor at sub-MTD with a PI3K/mTOR inhibitor significantly suppresses growth of lung adenocarcinoma tumors in Kras(G12D-LSL) mice. Cancer Chemother Pharmacol. 2012;70:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Nielsen LL, Baron AD. Pharmacology of exenatide (synthetic exendin-4) for the treatment of type 2 diabetes. Curr Opin Investig Drugs. 2003;4:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 345] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 82. | Zenzmaier C, Hermann M, Hengster P, Berger P. Dickkopf-3 maintains the PANC-1 human pancreatic tumor cells in a dedifferentiated state. Int J Oncol. 2012;40:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650-19655. [PubMed] |
| 84. | Nomiyama T, Kawanami T, Irie S, Hamaguchi Y, Terawaki Y, Murase K, Tsutsumi Y, Nagaishi R, Tanabe M, Morinaga H. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes. 2014;63:3891-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 85. | Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem. 2009;284:33509-33520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 823] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 87. | Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors. 2010;28:232-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 88. | Forsdahl S, Kiselev Y, Hogseth R, Mjelle JE, Mikkola I. Pax6 regulates the expression of Dkk3 in murine and human cell lines, and altered responses to Wnt signaling are shown in FlpIn-3T3 cells stably expressing either the Pax6 or the Pax6(5a) isoform. PLoS One. 2014;9:e102559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Mikkola I, Bruun JA, Bjorkoy G, Holm T, Johansen T. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:15115-15126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Yue W, Sun Q, Dacic S, Landreneau RJ, Siegfried JM, Yu J, Zhang L. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis. 2008;29:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Nakamura RE, Hunter DD, Yi H, Brunken WJ, Hackam AS. Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol. 2007;8:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 93. | Katase N, Lefeuvre M, Tsujigiwa H, Fujii M, Ito S, Tamamura R, Buery RR, Gunduz M, Nagatsuka H. Knockdown of Dkk-3 decreases cancer cell migration and invasion independently of the Wnt pathways in oral squamous cell carcinomaderived cells. Oncol Rep. 2013;29:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Yin DT, Wu W, Li M, Wang QE, Li H, Wang Y, Tang Y, Xing M. DKK3 is a potential tumor suppressor gene in papillary thyroid carcinoma. Endocr Relat Cancer. 2013;20:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Shien K, Tanaka N, Watanabe M, Soh J, Sakaguchi M, Matsuo K, Yamamoto H, Furukawa M, Asano H, Tsukuda K. Anti-cancer effects of REIC/Dkk-3-encoding adenoviral vector for the treatment of non-small cell lung cancer. PLoS One. 2014;9:e87900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, Shimizu N, Shimizu K. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene. 2002;282:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Guo Q, Qin W. DKK3 blocked translocation of β-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J Cell Mol Med. 2015;19:2832-2841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Uchida D, Shiraha H, Kato H, Nagahara T, Iwamuro M, Kataoka J, Horiguchi S, Watanabe M, Takaki A, Nouso K. Potential of adenovirus-mediated REIC/Dkk-3 gene therapy for use in the treatment of pancreatic cancer. J Gastroenterol Hepatol. 2014;29:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Shi G, DiRenzo D, Qu C, Barney D, Miley D, Konieczny SF. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene. 2013;32:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 100. | Kim S, Lahmy R, Riha C, Yang C, Jakubison BL, van Niekerk J, Staub C, Wu Y, Gates K, Dong DS. The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas. 2015;44:718-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Dufresne M, Clerc P, Dieng M, Edir A, Couvelard A, Delisle MB, Fourmy D, Gigoux V. Id3 modulates cellular localization of bHLH Ptf1-p48 protein. Int J Cancer. 2011;129:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Oström M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, Jeon J, Correa-Medina M, Diez J, Edlund H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3:e2841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | Eltony SA, Elmottaleb NA, Gomaa AM, Anwar MM, El-Metwally TH. Effect of All-Trans Retinoic Acid on the Pancreas of Streptozotocin-Induced Diabetic Rat. Anat Rec (Hoboken). 2016;299:334-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 104. | Bose B, Shenoy SP, Konda S, Wangikar P. Human embryonic stem cell differentiation into insulin secreting β-cells for diabetes. Cell Biol Int. 2012;36:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | El-Metwally TH, Adrian TE. Optimization of treatment conditions for studying the anticancer effects of retinoids using pancreatic adenocarcinoma as a model. Biochem Biophys Res Commun. 1999;257:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Herreros-Villanueva M, Er TK, Bujanda L. Retinoic Acid Reduces Stem Cell-Like Features in Pancreatic Cancer Cells. Pancreas. 2015;44:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | El-Metwally TH, Hussein MR, Abd-El-Ghaffar SKh, Abo-El-Naga MM, Ulrich AB, Pour PM. Retinoic acid can induce markers of endocrine transdifferentiation in pancreatic ductal adenocarcinoma: preliminary observations from an in vitro cell line model. J Clin Pathol. 2006;59:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |