Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5616
Peer-review started: March 4, 2016
First decision: April 1, 2016
Revised: April 10, 2016
Accepted: April 20, 2016
Article in press: April 20, 2016
Published online: June 28, 2016
Processing time: 109 Days and 19.4 Hours
Although gastroduodenal ulcers are common in solid organ transplant patients, there are few reports on multiple giant ulcers in the distal ileum and ileocecal valve caused by immunosuppressants Herein, we report on a liver transplant recipient and a renal transplant recipient with multiple large ulcers in the distal ileum and ileocecal valve who rapidly achieved ulcer healing upon withdrawal of sirolimus or tacrolimus and administration of thalidomide. In case 1, a 56-year-old man with primary hepatocellular carcinoma had received a liver transplantation. Tacrolimus combined with sirolimus and prednisolone was used as the anti-rejection regimen. Colonoscopy was performed because of severe abdominal pain and diarrhea at post-operative month 10. Multiple giant ulcers were found at the ileocecal valve and distal ileum. The ulcers healed rapidly with withdrawal of sirolimus and treatment with thalidomide. There was no recurrence during 2 years of follow-up. In case 2, a 34-year-old man with end-stage kidney disease received kidney transplantation and was put on tacrolimus combined with mycophenolate mofetil and prednisolone as the anti-rejection regimen. Twelve weeks after the operation, the patient presented with hematochezia and severe anemia. Colonoscopy revealed multiple large ulcers in the ileocecal valve and distal ileum, with massive accumulation of fresh blood. The bleeding ceased after treatment with intravenous somatostatin and oral thalidomide. Tacrolimus was withdrawn at the same time. Colonoscopy at week 4 of follow-up revealed remarkable healing of the ulcers, and there was no recurrence of bleeding during 1 year of follow-up. No lymphoma, tuberculosis, or infection of cytomegalovirus, Epstein-Barr virus, or fungus was found in either patient. In post-transplantation cases with ulcers in the distal ileum and ileocecal valve, sirolimus or tacrolimus should be considered a possible risk factor, and withdrawing them or switching to another immunosuppressant might be effective to treat these ulcers.
Core tip: There are few reports available on ileal ulcers caused by immunosuppressants. Herein, we report a liver transplant recipient and a renal transplant recipient who had multiple large ulcers in the distal ileum and ileocecal valve. Ulcers rapidly healed after withdrawal of sirolimus or tacrolimus and administration of thalidomide. No lymphoma, tuberculosis, or infection of cytomegalovirus, Epstein-Barr virus, or fungus was found in either patient. There was no recurrence of ulcers or organ rejection. In some post-transplantation cases with ileal ulcers, sirolimus or tacrolimus should be considered as a risk factor because of their inhibitory effects on wound healing. Withdrawing them or switching to other immunosuppressants might be effective.
- Citation: Guo YW, Gu HY, Abassa KK, Lin XY, Wei XQ. Successful treatment of ileal ulcers caused by immunosuppressants in two organ transplant recipients. World J Gastroenterol 2016; 22(24): 5616-5622
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5616.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5616
Solid organ transplant recipients are susceptible to a variety of gastrointestinal (GI) complications, one of which is ulcer disease[1-5]. Most of these ulcers are located at the anastomotic stoma or gastroduodenum; ulcers at the intestine or colon are rare. In addition to Helicobacter pylori infection and ischemia, infection with cytomegalovirus (CMV), Epstein-Barr virus (EBV), mycobacteria, and fungus can contribute to ulcer disease in transplant recipients[1-5]. A few reports suggest that the use of immunosuppressants, especially high-dose immunosuppression after transplantation, might correlate with impairment of the gastrointestinal tract[6,7]. Herein, we report a liver transplant recipient and a renal transplant recipient with multiple large ulcers in the distal ileum and ileocecal valve who rapidly achieved ulcers healing upon withdrawal of sirolimus or tacrolimus and administration of thalidomide.
A 56-year-old man with primary hepatocellular carcinoma received orthotopic liver transplantation in our hospital. Prior to the transplantation, he was diagnosed with chronic hepatitis B virus (HBV), Child-C liver function, and elevated blood glucose. No history of renal or cardiac disease or mycobacterium tuberculosis (TB) infection was noted. He denied smoking and drinking. Tacrolimus combined with sirolimus and prednisolone was used as the anti-rejection regimen. The dose of tacrolimus and sirolimus was adjusted according the drug serum concentration. The patient recovered and liver function improved to normal level. Entecavir was prescribed to prevent HBV reinfection.
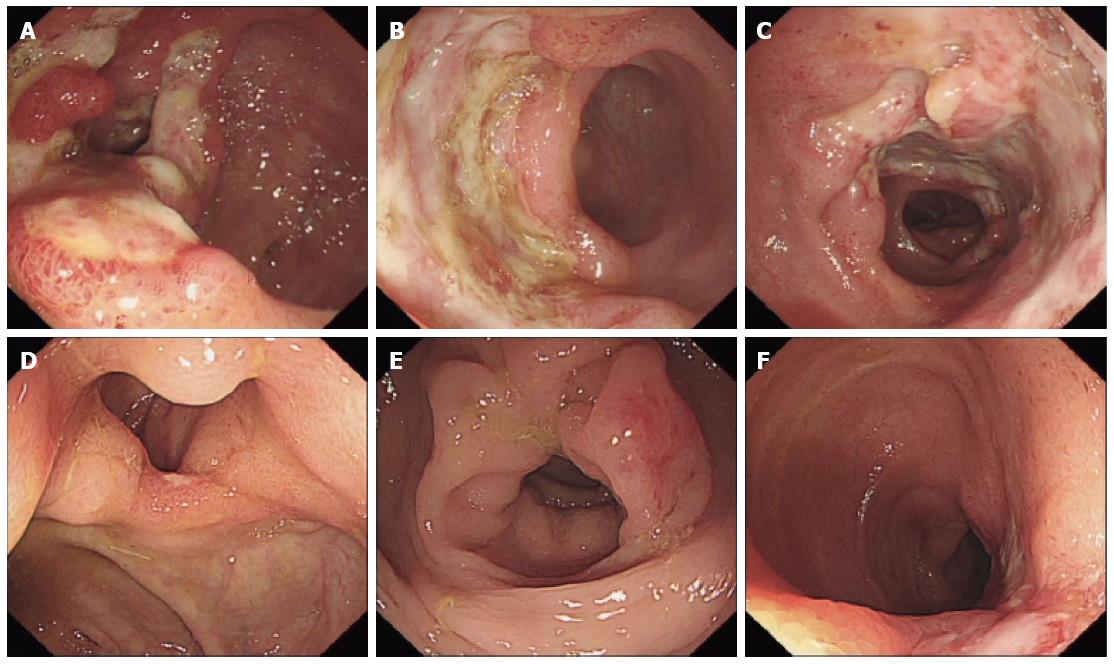
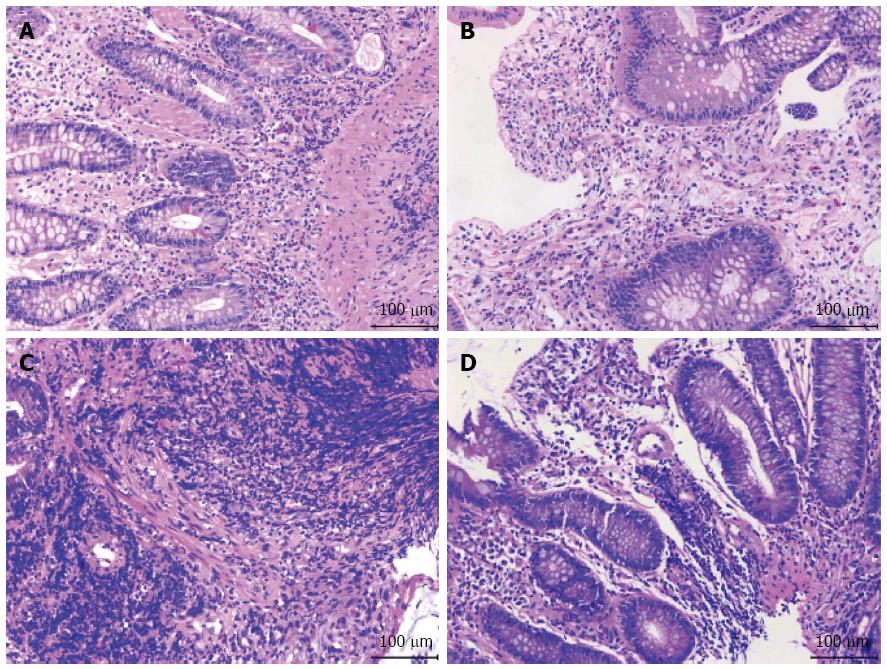
After administration of the immunosuppressants, the patient began to develop mild peri-umbilical pain and diarrhea, which were tolerable at the time. Probiotics and antispasmodic treatment seemed not so effective. At 10 mo post-operation, he was admitted to our hospital because of severe diarrhea and abdominal pain. There were 7-10 bowel movements per day, with mucous blood stool. On physical examination, blood pressure, heart rate, and temperature were normal. There was slight tenderness on the peri-umbilicus area without rebound tenderness. Complete blood count revealed a leukocyte count of 11.50 × 109/L, erythrocyte count of 4.50 × 1012/L, hemoglobin level of 121 g/L, platelet count of 295 × 109/L, neutrophils of 76.4%, and lymphocytes of 16.3%. Leukocytes and erythrocytes were found in the stool. The ratio of coccus to bacillus in stool was 1:9. Serum glutamic-oxaloacetic transaminase, glutamic-pyruvic transaminase, total bilirubin, BUN, creatine, and electrolytes were within normal range. Serum 1-3-β-D dextran was < 10 pg/mL. Blood and stool cultures for fungus and bacteria showed no growth. Chest computed tomography (CT) scan revealed no lesions and serum T-SPOT.TB was negative. Immunoglobulin (Ig)M and IgG of the EB virus were negative. Abdominal enhanced CT scan showed thickening of the distal ileum and ileocecal region, suggesting inflammatory lesions. The spleen was slightly enlarged, muddy stones were found in the common bile duct, and no enlarged lymphonodus was noted. Chronic erosive gastritis with negative Helicobacter pylori infection was confirmed through esophagogastroduodenoscopy. Colonoscopy revealed multiple giant and deep ulcers in the ileocecal valve and distal ileum, with polypoid hyperplasia. The length of the largest ulcer was up to 5.0 cm (Figure 1A-C). Histopathology of biopsy specimens revealed benign ulcers and chronic inflammation with non-caseous granulomas (Figure 2A and B), without signs of fungus and parasites infection. The immunohistochemical study was negative for CMV infection. EBV encoded early small RNA (EBER) was negative by in situ hybridization.
Considering sirolimus to have more gastrointestinal complications than tacrolimus in clinical application, sirolimus was withdrawn first. The patient was also put on oral thalidomide at a dose of 100 mg/d for 2 wk and intravenous antibiotics for one week. Diarrhea and abdominal pain were gradually relieved and subsided. Colonoscopy at week 6 of follow-up revealed remarkable healing of the ulcers in the ileocecal valve and distal ileum, and only two healing 2 stage ulcers were found (Figure 1D-F). No organ rejection was noted after withdrawing sirolimus. No recurrence of diarrhea and abdominal pain was noted during the 2 years of follow-up.
A 34-year-old man with end-stage kidney disease was admitted to the department of renal transplantation for living-donor kidney transplantation. Except for kidney disease, he had no history of primary liver, heart, or head disease. He denied smoking and drinking. Tacrolimus combined with mycophenolate mofetil and prednisolone was applied as the anti-rejection regimen. The dose of tacrolimus was adjusted according to the drug serum concentration. At the same time, oral ganciclovir, voriconazole, and esomeprazole were used to prevent CMV and fungus infection and esophagogastroduodenal complications. The patient recovered smoothly. Serum creatinine level decreased to 152.0 μmol/L, and urine output was normal.
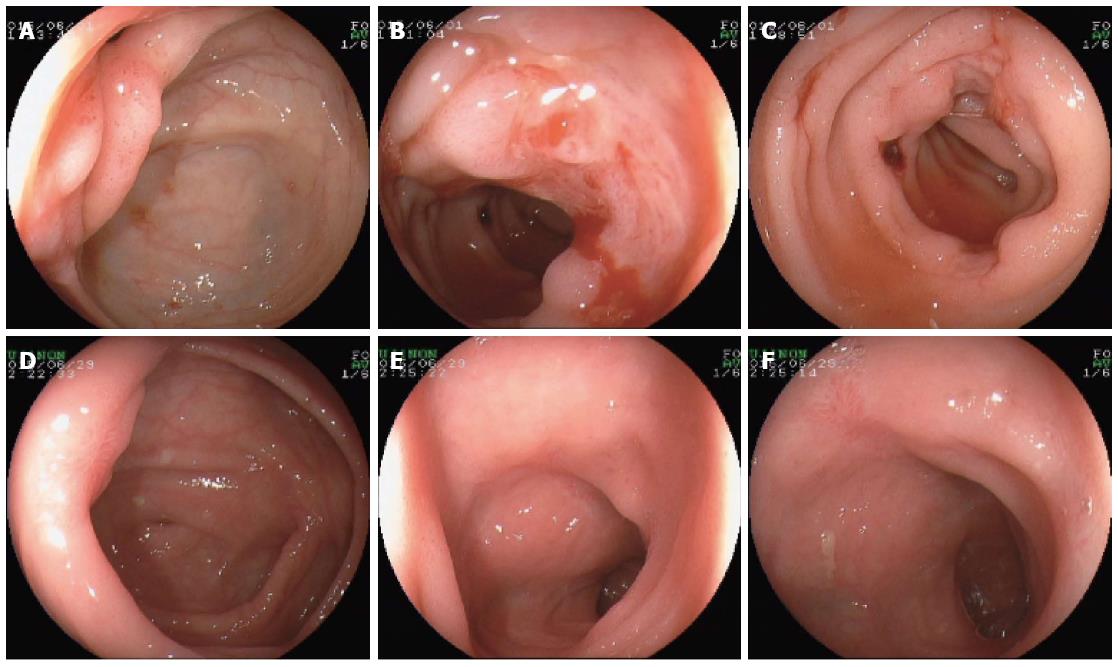
At postoperative week 12, the patient was admitted to our department because of repeated hematochezia for 2 wk, which was accompanied by dizziness and weakness. No abdominal pain, nausea, or vomiting was observed. On physical examination, blood pressure, heart rate, and temperature were 95/55 mmHg, 102 beats/min, 37.5 °C, respectively. There was mild tenderness at the peri-umbilicus area and the lower right abdomen, without rebound tenderness. Complete blood count revealed a leukocyte count of 7.45 × 109/L, erythrocyte count of 2.05 × 1012/L, hemoglobin level of 57 g/L, and platelet count of 241 × 109/L. Mild elevated serum creatinine level of 168.0 μmol/L was noted, with a normal BUN level of 6.8 mmol/L. Prothrombin time, apartprothrombin time, and thrombin time were within the normal range. Laboratory indices about hepatic, cardiac, and respiratory function were all normal. Blood and stool cultures for fungus and bacteria showed no growth. Chest CT scan revealed some pulmonary lesions of previous tuberculosis, and serum T-SPOT.TB was negative. There were no abnormal findings in the abdominal doppler ultrasound. Esophagogastroduodenoscopy was performed first, and only mild gastritis was observed, with negative rapid urease test for Helicobacter pylori infection. Colonoscopy revealed multiple ulcers in the ileocecal valve and distal ileum, with massive accumulation of fresh blood. These ulcers were oval and deep, covered with white fur or a blood scab, and the biggest had a diameter of 2.0 cm (Figure 3A-C). Histopathology revealed chronic inflammation with a large number of lymphocytes infiltration (Figure 2C and D), without signs of fungus or parasite infection. Immunohistochemistry stain for CMV was negative. EBER was also negative by in situ hybridization.
The bleeding lessened and eventually ceased after treatment with intravenous somatostatin (1.2 mg/d) and oral thalidomide (100 mg/d) for 5 d. At the same time, because the multiple intestinal ulcers may be due to immunosuppressors, tacrolimus was withdrawn, and cyclosporine combined with mycophenolate mofetil and prednisolone were administered. Additionally, the patient was put on oral thalidomide at a dose of 100 mg/d for 4 wk. Colonoscopy at week 4 of follow-up revealed remarkable healing of the ulcers with scar tissue in the ileocecal valve and distal ileum (Figure 3D-F). There was no recurrent bleeding during the 1 year of follow-up. In addition, no organ rejection was found after withdrawing tacrolimus.
It is well known that solid organ transplantation patients are particularly at risk for GI complications. Severe GI complications such as GI bleeding and GI perforation may negatively influence long-term outcome and become deadly. It had been reported that GI bleeding occurred in 2.3%-6.4% patients after liver transplantation[8,9] and GI perforation in 2.9% patients after renal transplantation[7]. Ulcer diseases are an important cause of GI bleeding, and perforation can manifest with symptoms of abdominal pain or diarrhea. Most of these ulcers are located at the anastomotic stoma or gastroduodenum, whereas ulcers at the intestine or colon are rare. In the two cases described here, deep and large ulcers were located in the ileocecal valve and distal ileum with no lesions in the gastroduodenum. In case 1, there was a longer period of diarrhea and abdominal pain, while case 2 mainly presented with acute and massive GI bleeding.
Differentiation of ulcer diseases in post-transplantation patients is always difficult. Common and uncommon pathogenesis such as Helicobacter pylori infection, ischemia, infection of CMV, EBV, mycobacteria, and fungus, and post-transplant lymphoproliferative disorders (PTLDs) should be considered. In the present two cases, ulcers were in the ileocecal valve and distal ileum, and Helicobacter pylori infection was ruled out as the pathogen. Clinical manifestation and endoscopic characteristics of ulcers were not consistent with a pathogenesis of ischemia. Because of negative the findings on chest CT scan, serum T-SPOT.TB, and histopathology of ulcers, mycobacteria and fungus infection were also excluded. CMV infection is common in patients with solid organ transplantation, and attention should be paid in cases of gastrointestinal ulcers. In a study of renal transplant patients susceptible to a variety of GI complications, such as infections, ulcer disease, and malignancies, CMV infection occurred in 11% of all patients[1]. Although oral ganciclovir preventive strategy was used in case 2, this treatment might be inefficient in some patients, and atypical symptoms might be present[10,11]. Decreased leukocyte count and interstitial pneumonitis are always found in CMV infection, but in both of our cases, no positive signs about CMV infection were noted in leukocyte count or chest CT scan. More importantly, immunohistochemistry stain for CMV of ulcer biopsy tissue was negative, and both patients recovered without further anti-CMV therapy. Therefore, CMV infection was not considered the cause of ulcers in these two cases.
PTLD was most difficult to be excluded in both patients. This was especially true in case 1, where the ulcers in the ileocecal valve and distal ileum appeared deeper and larger, with surrounding proliferative tissue. PTLD is a severe complication after organ transplantation with a cumulative incidence of 1.1% at 18 mo and 4.7% at 15 years, and it is always associated with EBV infection[12]. Lymph nodes, GI tract, and graft liver are the most common sites of involvement[13]. The involvement of the GI tract could result in deadly perforation and hemorrhage. In both patients, no persistent fever, palpable superficial lymph nodes, enlarged liver, or lymph nodes were found on chest and abdominal CT scan or ultrasound; EBER was negative by in situ hybridization. Pathology was also not consistent with the characteristics of PTLD. Furthermore, PTLD usually deteriorates quickly and is difficult to treat. However, the present two patients were stable within 1-2 wk, and the ulcers healed rapidly in 4-6 wk. Taken together, the evidence did not support the diagnosis of PTLD.
Finally, we considered that ulcer development was related to the use of immunosuppressants. Current studies have shown that some immunosuppressants, such as mammalian target of rapamycin inhibitors, had inhibitory effects on wound healing. Sirolimus is the most common drug that can lead to impairment of wound healing, and the most common wound complication is skin or dermal eruption[14-17]. Therefore, the immunosuppressant use was considered to be a possible cause of GI epithelium impairment. Fortunately, both patients recovered quickly after withdrawing sirolimus and tacrolimus, supporting our speculation. In Smith et al[6], three liver transplant patients taking sirolimus suffered from gastrointestinal hemorrhage due to complications of gastroduodenal ulcers. The ulcers in two patients healed only after discontinuation of sirolimus, and the third patient died of massive gastrointestinal bleeding[6].
Thalidomide has anti-angiogenic properties and seems effective in some cases of GI bleeding, especially angiodysplasia-related bleeding[18-20]. It is also used in some inflammatory and ulcerative diseases like inflammatory bowel disease and some skin and oral ulcers, because of its anti-inflammatory and immunomodulatory effects[20-22]. In our clinical practice, thalidomide is effective in some unexplained and refractory multiple ulcers of intestine and related GI bleeding. It also seemed to work in our present two patients. Thalidomide was administrated at a dose of 100 mg/d for 2 wk and 4 wk respectively, and no severe side effects were found.
In summary, some types of immunosuppressants, such as sirolimus and tacrolimus, can lead to impairment of the GI track and sometimes to the development of severe ulcers. Withdrawing them or switching to other immunosuppressants might be effective to treat these ulcers.
A 56-year-old man presented with severe diarrhea and abdominal pain after orthotopic liver transplantation, and a 34-year-old man presented with hematochezia and severe anemia after living-donor kidney transplantation.
Multiple giant ulcers in the distal ileum and ileocecal valve were caused by immunosuppressants.
Common and uncommon pathogenesis of gastrointestinal (GI) ulcers in solid organ transplant recipient, such as Helicobacter pylori infection, ischemia, infection of cytomegalovirus (CMV), Epstein-Barr virus (EBV), mycobacteria and fungus, and post-transplant lymphoproliferative disorders (PTLDs), should be considered.
Blood and stool cultures for fungus and bacteria showed no growth, and serum T-SPOT.TB was negative.
Computed tomography (CT )scan revealed no current tuberculosis, and there were no abnormal findings on abdominal enhanced CT or doppler ultrasound.
Colonoscopy revealed multiple giant and deep ulcers in the ileocecal valve and distal ileum.
Histopathology revealed chronic inflammation without signs of fungus or parasite infection, negative immunohistochemistry stain for CMV, and negative stain for EBV encoded early small RNA by in situ hybridization.
Sirolimus or tacrolimus was withdrawn, and thalidomide was administrated.
Most GI ulcers are located at the anastomotic stoma or gastroduodenum in post-transplant recipients, and ulcers at the intestine or colon are rare. Besides ischemia and infection of CMV, EBV, mycobacteria, and fungus, use of immunosuppressants might contribute to the impairment of gastrointestinal tract.
PTLDs are a severe complication of solid organ and hematopoietic stem cell transplantation, including lymphoproliferative entities varying from reactive hyperplasia to malignant lymphoma. EBV is the main pathogen of PTLD.
In some post-transplantation cases with ileal ulcers, sirolimus or tacrolimus should be considered as a risk factor, and withdrawing them or switching to other immunosuppressants might be effective.
This article is very interesting for those in the field of liver and kidney transplantation. Since ulcers in the distal ileum in solid organ transplant recipients are very rare, experience about the management of these patients is very useful.
P- Reviewer: Markic D S- Editor: Yu J L- Editor: Filipodia E- Editor: Ma S
| 1. | Ishaque M, Rashid R, Mubarak M. Gastrointestinal complications in renal transplant recipients detected by endoscopic biopsies in a developing country. Indian J Gastroenterol. 2015;34:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Lahon B, Mordant P, Thabut G, Georger JF, Dauriat G, Mal H, Lesèche G, Castier Y. Early severe digestive complications after lung transplantation. Eur J Cardiothorac Surg. 2011;40:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Telkes G, Peter A, Tulassay Z, Asderakis A. High frequency of ulcers, not associated with Helicobacter pylori, in the stomach in the first year after kidney transplantation. Nephrol Dial Transplant. 2011;26:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Grass F, Schäfer M, Cristaudi A, Berutto C, Aubert JD, Gonzalez M, Demartines N, Ris HB, Soccal PM, Krueger T. Incidence and Risk Factors of Abdominal Complications After Lung Transplantation. World J Surg. 2015;39:2274-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Gad EH, Alsebaey A, Lotfy M, Eltabbakh M, Sherif AA. Complications and mortality after adult to adult living donor liver transplantation: A retrospective cohort study. Ann Med Surg (Lond). 2015;4:162-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Smith AD, Bai D, Marroquin CE, Tuttle-Newhall JE, Desai DM, Collins BH, Muir A, Kuo PC, McHutchison J, Rockey DC. Gastrointestinal hemorrhage due to complicated gastroduodenal ulcer disease in liver transplant patients taking sirolimus. Clin Transplant. 2005;19:250-254. [PubMed] |
| 7. | Catena F, Ansaloni L, Gazzotti F, Bertelli R, Severi S, Coccolini F, Fuga G, Nardo B, D’Alessandro L, Faenza A. Gastrointestinal perforations following kidney transplantation. Transplant Proc. 2008;40:1895-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kimura K, Ikegami T, Bekki Y, Ninomiya M, Yamashita Y, Yoshizumi T, Yoshiya S, Soejima Y, Harada N, Shirabe K. Clinical significance of gastrointestinal bleeding after living donor liver transplantation. Transpl Int. 2014;27:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ma Y, He XS, Zhu XF, Wang GD, Wang DP, Hu AB, Ju WQ, Wu LW, Tai Q. [Etiology and management of postoperative gastrointestinal bleeding after orthotopic liver transplantation]. Zhonghua Wei Chang Wai Ke Zazhi. 2010;13:26-28. [PubMed] |
| 10. | Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, Zandotti C, Berland Y, Vacher-Coponat H. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transpl Infect Dis. 2010;12:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Pérez-Valentín MA, Cofán F, Solé M, Llach J, Esforzado N, Campistol JM, Oppenheimer F. [Atypical cytomegalovirus in renal transplantation: a new form of presentation]. Nefrologia. 2002;22:381-385. [PubMed] |
| 12. | Cornejo A, Bohnenblust M, Harris C, Abrahamian GA. Intestinal perforation associated with rituximab therapy for post-transplant lymphoproliferative disorder after liver transplantation. Cancer Chemother Pharmacol. 2009;64:857-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Lo RC, Chan SC, Chan KL, Chiang AK, Lo CM, Ng IO. Post-transplant lymphoproliferative disorders in liver transplant recipients: a clinicopathological study. J Clin Pathol. 2013;66:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94:547-561. [PubMed] |
| 15. | Tiong HY, Flechner SM, Zhou L, Wee A, Mastroianni B, Savas K, Goldfarb D, Derweesh I, Modlin C. A systematic approach to minimizing wound problems for de novo sirolimus-treated kidney transplant recipients. Transplantation. 2009;87:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int. 2011;24:1216-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 17. | Knight RJ, Villa M, Laskey R, Benavides C, Schoenberg L, Welsh M, Kerman RH, Podder H, Van Buren CT, Katz SM. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant. 2007;21:460-465. [PubMed] |
| 18. | Boey JP, Hahn U, Sagheer S, McRae SJ. Thalidomide in angiodysplasia-related bleeding. Intern Med J. 2015;45:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Moser S, Tischer A, Karpi A, Schleicher M, Stavjanik S, Gschwantler M. Evidence that thalidomide is effective in recurrent bleeding from watermelon stomach associated with liver cirrhosis. Endoscopy. 2014;46 Suppl 1 UCTN:E384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Amirshahrokhi K, Khalili AR. The effect of thalidomide on ethanol-induced gastric mucosal damage in mice: involvement of inflammatory cytokines and nitric oxide. Chem Biol Interact. 2015;225:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Diamanti A, Capriati T, Papadatou B, Knafelz D, Bracci F, Corsetti T, Elia D, Torre G. The clinical implications of thalidomide in inflammatory bowel diseases. Expert Rev Clin Immunol. 2015;11:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Barrons RW. Treatment strategies for recurrent oral aphthous ulcers. Am J Health Syst Pharm. 2001;58:41-50; quiz 51-53. [PubMed] |