Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5540
Peer-review started: February 1, 2016
First decision: March 7, 2016
Revised: March 30, 2016
Accepted: April 20, 2016
Article in press: April 20, 2016
Published online: June 28, 2016
Processing time: 141 Days and 23.7 Hours
AIM: To investigate the mechanisms and effects of sphincter of Oddi (SO) motility on cholesterol gallbladder stone formation in guinea pigs.
METHODS: Thirty-four adult male Hartley guinea pigs were divided randomly into two groups, the control group (n = 10) and the cholesterol gallstone group (n = 24), which was sequentially divided into four subgroups with six guinea pigs each according to time of sacrifice. The guinea pigs in the cholesterol gallstone group were fed a cholesterol lithogenic diet and sacrificed after 3, 6, 9, and 12 wk. SO manometry and recording of myoelectric activity were obtained by a multifunctional physiograph at each stage. Cholecystokinin-A receptor (CCKAR) expression levels in SO smooth muscle were detected by quantitative real-time PCR (qRT-PCR) and serum vasoactive intestinal peptide (VIP), gastrin, and cholecystokinin octapeptide (CCK-8) were detected by enzyme-linked immunosorbent assay at each stage in the process of cholesterol gallstone formation.
RESULTS: The gallstone formation rate was 0%, 0%, 16.7%, and 83.3% in the 3, 6, 9, and 12 wk groups, respectively. The frequency of myoelectric activity in the 9 wk group, the amplitude of myoelectric activity in the 9 and 12 wk groups, and the amplitude and the frequency of SO in the 9 wk group were all significantly decreased compared to the control group. The SO basal pressure and common bile duct pressure increased markedly in the 12 wk group, and the CCKAR expression levels increased in the 6 and 12 wk groups compared to the control group. Serum VIP was elevated significantly in the 9 and 12 wk groups and gastrin decreased significantly in the 3 and 9 wk groups. There was no difference in serum CCK-8 between the groups.
CONCLUSION: A cholesterol gallstone-causing diet can induce SO dysfunction. The increasing tension of the SO along with its decreasing activity may play an important role in cholesterol gallstone formation. Expression changes of CCKAR in SO smooth muscle and serum VIP and CCK-8 may be important causes of SO dysfunction.
Core tip: This study investigated the role of sphincter of Oddi (SO) motility in cholesterol gallstone formation in a guinea pig model. The myoelectric activity and manometry of SO were measured at different stages of stone formation. As SO motility is controlled by neurological and hormonal factors, we detected the expression of serum vasoactive intestinal peptide (VIP), gastrin, cholecystokinin octapeptide (CCK-8), and CCK-A receptor (CCKAR) in the SO at different stages of stone formation. We found that a cholesterol gallstone-causing diet can induce SO dysfunction and expression changes of CCKAR in SO smooth muscle and serum VIP and CCK-8 may be important causes of SO dysfunction.
- Citation: Rong ZH, Chen HY, Wang XX, Wang ZY, Xian GZ, Ma BZ, Qin CK, Zhang ZH. Effects of sphincter of Oddi motility on the formation of cholesterol gallstones. World J Gastroenterol 2016; 22(24): 5540-5547
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5540
Gallstone disease is one of the most common digestive disorders requiring hospital admission in Western countries, with a morbidity of 10%-15% in adults[1]. In China, the incidence of cholesterol gallstones has been increasing in the past few decades due to changing lifestyles[2]. Cholesterol gallstone formation is a complicated process and is still not fully understood. Cholesterol supersaturation of bile, biliary stasis, and mucus hypersecretion are universally known to be important factors in the process of cholesterol gallstone formation[3,4]. Among them, biliary stasis is thought to be a key factor because it allows time for cholesterol nucleation and then retention of the precipitated microcrystals[4,5]. The sphincter of Oddi (SO) may play an important role in gallstone formation because it is the only way by which bile is discharged into the duodenum. There are very few reports investigating the relationship between SO motility and cholesterol gallstone formation, and the conclusions are controversial[6-8].
It is well-established that cholecystokinin (CCK) is one of the major gastrointestinal hormones responsible for gallbladder contraction and SO relaxation[9]. The biological actions of CCK in the alimentary tract are mediated by the CCK-A receptor (CCK-AR)[10]. A series of studies focused on the expression level of CCK-AR in the gallbladder in the pathogenesis of cholesterol gallstone disease[5,11]. However, no report on CCK-AR expression in the SO in animals with gallstones is available yet.
The aim of this study was to investigate the role of SO motility in cholesterol gallstone formation in a guinea pig model. The myoelectric activity and manometry of SO were measured at different stages of stone formation in this model. As SO motility is controlled by neurological and hormonal factors, we detected serum vasoactive intestinal peptide (VIP), gastrin, CCK-8, and CCK-AR expression in the SO at different stages of stone formation.
Thirty-four adult male Hartley guinea pigs, weighing between 230 g and 270 g, were purchased from Huishan Jiangnan Laboratory Animal Company (License SCXK SU: 2009-0005). The animals were housed in climate-controlled rooms under an alternating 12-h light and dark cycle and permitted continuous access to food and water. After a 2-wk equilibration, the animals were divided randomly into two groups (control and cholesterol stone groups): the control group (n = 10) was fed a normal diet, and the cholesterol stone group (n = 24) was sequentially divided into four subgroups with six guinea pigs each according to time of sacrifice, fed a cholesterol lithogenic diet[6], and sacrificed after 3, 6, 9, and 12 wk. The lithogenic diet consisted of 1% cholesterol[10] (purchased from Trophic Animal Feed High-Tech Co. Ltd., Nantong, China). Gallstones were defined as macroscopically visible sediment. The calculi were tested by infrared spectrometry to verify the sample as cholesterol gallstones. SO manometry and myoelectric activity of the guinea pigs were determined at 3, 6, 9, and 12 wk.
At the end of the feeding period, the animals were anesthetized by injecting pentobarbital sodium (45 mg/kg) into the peritoneal cavity. The guinea pigs were fixed in the supine position, and the skin of the superior abdomen was prepared and sterilized. A longitudinal incision was made and the papilla determined. Details of the myoelectric system were described previously[12]. In brief, two polar hook metal electrodes were inserted 0.2 mm into the subserosa of the SO by megaloscope (× 10 magnification). The fan-out of the two signals was connected with the two polars of the physiological recorder (BL-420 F; Chengdu Taimeng Software, Chengdu, China), and a piece of metal needle was inserted into the legs of the animals to connect with the earth pole of the recorder. The myoelectric signal was collected by the electrode, imported into the computer, and stored after processing by a physiological recorder and the software system specialized in electromyographic signals. The setup parameters were as follows: scanning speed, 500 ms/div; sensitivity, 200 μV; time parameter, 1 s; and frequency filtering, 10 Hz. Finally, the myoelectric figure was dealt with by digital filtering of 10-30 Hz.
Details of manometry were described previously[12]. In brief, a manometry catheter was modified from a pedo bi-lumen central venous catheter (4F and 30 cm long). The catheter was inserted into the common bile duct (CBD) and SO through the duodenal ampulla. The pressure transducer was used for receiving the dynamic pressure change from the manometric lumen. The frequency of SO phasic contraction, SO amplitude, SO basal pressure, and CBD pressure were measured and recorded. A physiological recorder and relevant manometry program were used to record and analyze the tracings.
Four milliliters of venous blood were obtained from the guinea pigs in the early morning before they were sacrificed and placed in a test tube. The blood was centrifuged at 1500 r/min for 15 min, and serum was isolated, placed in Eppendorf tubes and stored at -70 °C. Serum VIP, gastrin, and CCK-8 were measured by enzyme-linked immunosorbent assay (ELISA). The ELISA testing kit was supplied by USCN Life Science Inc. (Houston, TX, United States).
The SO of each animal was quickly removed and transferred to normal saline after animals were euthanized. The smooth muscle was removed carefully using sharp dissection. Total RNA from SO tissue samples was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). Every 50 mg of SO tissue sample was extracted with 1 mL of TRIzol reagent according to the manufacturer’s protocol. One microgram of total RNA was used for the synthesis of cDNA using a FastQuant RT Kit and target genes were assayed using SYBR Green Real-time PCR Master Mix (via Roche Light Cycler, Roche, Basel, Switzerland) with their respective primers. The PCR conditions were as follows: 95 °C for 30 s; 40 cycles of 95 °C for 5 s, 57 °C for 10 s, and 72 °C for 15 s. Transcription levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as an internal control to calculate fold induction, and the fold changes in transcription levels were calculated using the 2-ΔΔCt method. Primer sequences of CCKAR and GAPDH are shown in Table 1.
| Genes | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| CCKAR | ACGGAGGGTAGTGAACTCCA | TCGCAGGCAGAAGTGATGTT |
| GAPDH | GCACCGTCAAGGCTGAGAAT | CATCACGAACATAGGGGCATC |
Statistical analysis was performed using Student’s t test. Data were analyzed with software SPSS 17.0 (SPSS Inc. Chicago, IL, United States), and P < 0.05 was set as the level of significance. The results are expressed as mean ± SE.
No gallstone was found in guinea pigs in the control group. In contrast, the gallstone formation rate in the cholesterol stone group fed with a cholesterol lithogenic diet was 0%, 0%, 16.7%, and 83.3% in the 3, 6, 9, and 12 wk groups, respectively.
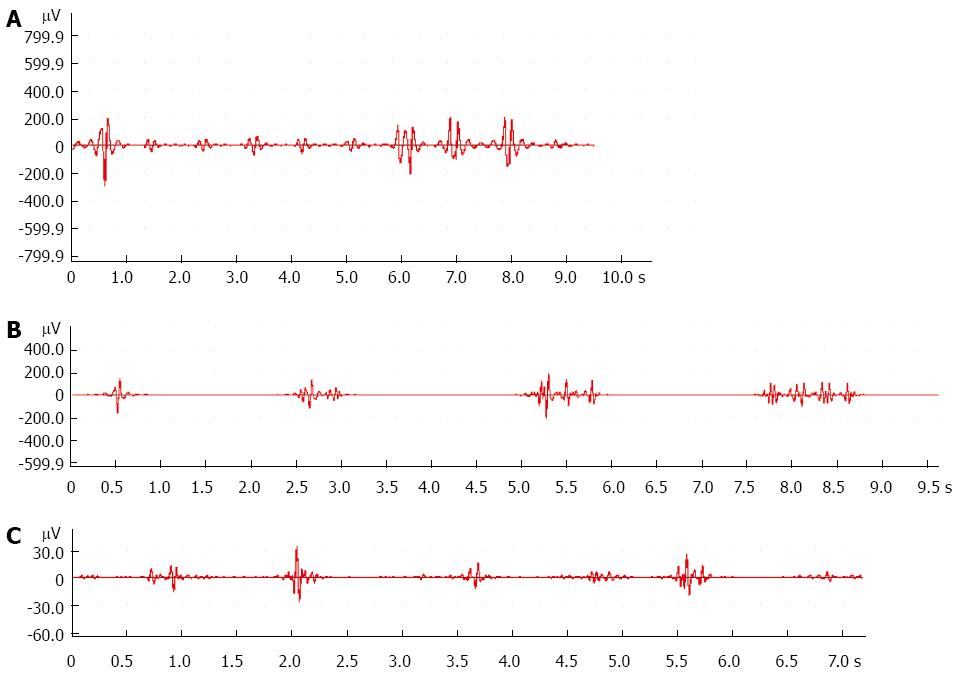
Compared with the control group, the frequency of myoelectric activity decreased markedly in the 9 wk group (Figure 1A and B, Table 2). The amplitude of myoelectric activity decreased significantly in the 9 and 12 wk groups (Figure 1A-C, Table 2).
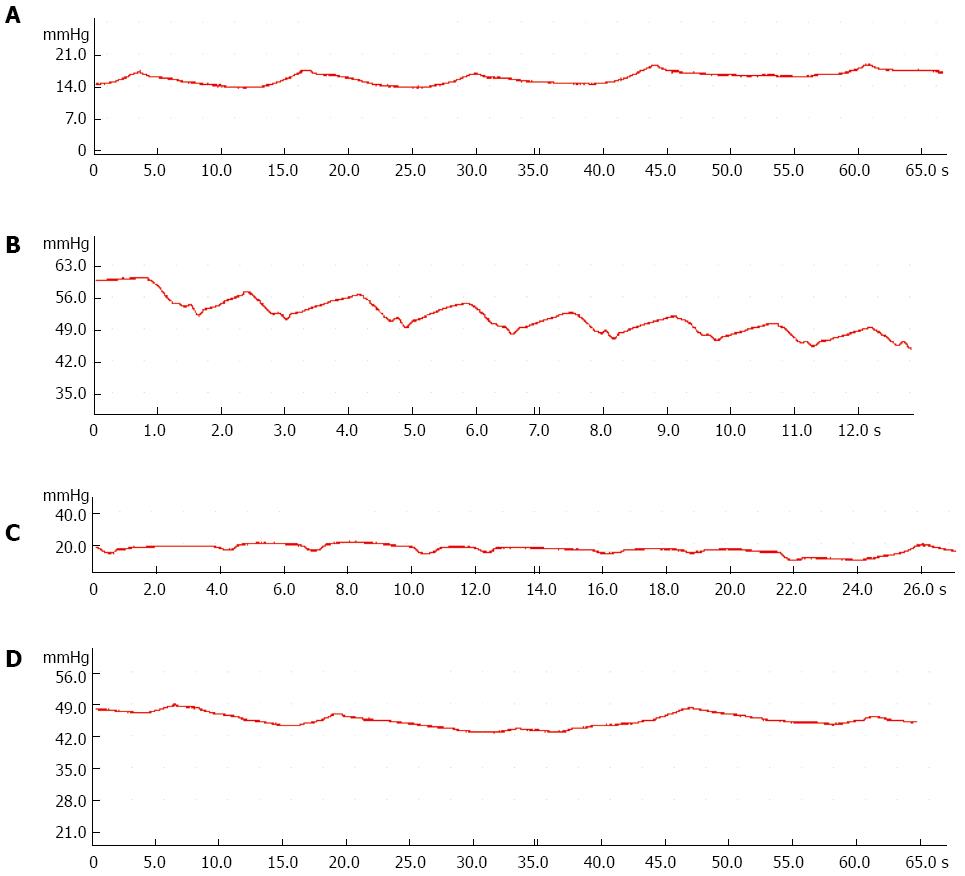
Compared with the control group, the amplitude and frequency of SO decreased significantly in the 9 wk group (Table 3), and SO basal pressure and common bile duct pressure increased significantly in the 12 wk group (Figure 2, Table 3).
| Group | SO basal pressure | CBD pressure | Amplitude of SO | Frequency of SO |
| Control group | 26.59 ± 8.16 | 23.25 ± 8.35 | 8.72 ± 2.05 | 11.27 ± 3.74 |
| 3-wk group | 21.25 ± 1.38 | 18.48 ± 1.94 | 8.33 ± 3.85 | 11.50 ± 1.64 |
| 6-wk group | 27.57 ± 8.67 | 25.82 ± 8.26 | 8.03 ± 3.15 | 9.33 ± 3.27 |
| 9-wk group | 34.11 ± 11.56 | 32.15 ± 11.64 | 5.89 ± 1.41a | 7.67 ± 3.44a |
| 12-wk group | 52.38 ± 12.84c | 50.11 ± 12.59e | 6.82 ± 1.34 | 10.60 ± 3.51 |
Compared with the control group, serum VIP was elevated significantly in the 9 and 12 wk groups (Table 4), and serum gastrin was decreased significantly in the 3 and 9 wk groups (Table 4). There was no difference in serum CCK-8 between the groups (Table 4).
| Group | 3-wk | 6-wk | 9-wk | 12-wk |
| VIP | ||||
| Control | 9.36 ± 2.72 | 9.30 ± 8.91 | 6.91 ± 3.14 | 8.39 ± 0.99 |
| Cholesterol stone | 11.83 ± 3.57 | 7.08 ± 2.31 | 31.20 ± 7.78a | 22.15 ± 2.87c |
| Gastrin | ||||
| Control | 17.83 ± 2.35 | 18.56 ± 5.77 | 17.42 ± 6.39 | 19.41 ± 4.58 |
| Cholesterol stone | 0.08 ± 0.03c | 4.18 ± 2.87 | 2.16 ± 0.44a | 18.92 ± 8.37 |
| CCK-8 | ||||
| Control | 1970.33 ± 439.82 | 2353.32 ± 13.18 | 2100.27 ± 29.70 | 2107.77 ± 171.70 |
| Cholesterol stone | 2214.61 ± 174.96 | 2044.18 ± 147.03 | 2034.75 ± 138.39 | 2042.61 ± 265.01 |
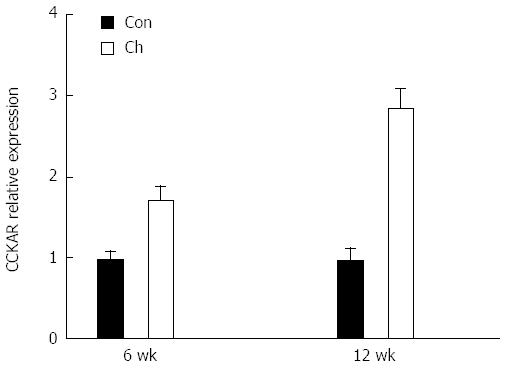
Compared with the control group, expression levels of the CCK-A receptor mRNA were increased significantly in the 6 and 12 wk groups (Figure 3).
Gallstone disease is one of the most common and most expensive digestive disorders requiring admission to the hospital[13-15], with a prevalence of 10%-15% in adults in Europe and the United States. In Western countries, cholesterol gallstones account for 80%-90% of gallstones at cholecystectomy[15], but the mechanism underlying the pathogenesis of cholesterol gallstone disease is not completely understood.
Epidemiological evidence indicates that multiple environmental factors and genetic elements are involved in cholesterol gallstone formation. Among them, biliary stasis is thought to be an important factor. A series of studies in human and animal models have shown that formation of cholesterol gallstones is causatively related to decreased gallbladder contractility[16]. Since SO is the only gate through which bile is discharged into the duodenum, bile filling and excretion of the gallbladder are closely related to the motility state of the SO[17]. However, there is limited research on the role of SO motility in the process of cholesterol gallstone formation. SO manometry (SOM) is the only method that can assess directly the motor function of the SO and is considered the gold standard for assessing SO dysfunction (SOD)[18]. However, manometry changes of the SO in cholelithiasis are controversial. Research by Pang et al[19] indicated that the base pressure of the SO was significantly increased in rabbits fed a cholesterol lithogenic diet. On the other hand, a study in prairie dogs showed that SO resistance remained normal throughout the period of gallstone formation. These completely different results may be species dependent. Meanwhile, the mechanical activity recorded may not represent the features of SO activity, especially in the relaxed SO. Another measurement reflecting SO function is recording the myoelectric activity of the SO[20].
In this study, we investigated whether SOD was present and what role it played in the process of cholesterol gallstone formation in guinea pigs. SOM and myoelectric activity of SO were investigated simultaneously at different stages of stone formation. We found that gallstones did not occur until 9 wk, with an incidence rate of 16.7%, increasing to 83.3% after 12 wk. SO myoelectric activity analysis indicated that the frequency and amplitude of myoelectric activity decreased significantly in the 9 wk group compared to control. Subsequent SO manometry analyses showed the same result: both SO amplitude and SO frequency decreased significantly in the 9 wk group, and the most important indications, the SO basal pressure and common bile duct pressure, increased significantly in the 12 wk group. All these findings are consistent with weakening of the myoelectric activity of SO and gradual increasing of SO tension in the process of gallstone formation. Disturbance of SO motor function impedes the flow of bile into the duodenum, and biliary stasis occurs.
The mechanism of cholesterol gallstone-causing diet-induced SOD has not been fully elucidated. Pang et al[19] considered that the potential mechanisms of hypercholesterolemia (HC)-induced SOD were the intracellular calcium overload and calcium oscillation abnormality. Szilvassy et al[21] suggested that the impairment of the SO relaxation function was related to the alteration of the nitric oxide signal caused by hypercholesterolemia and hypertriglyceridemia. SO motility was controlled by numerous neurotransmitters and gastrointestinal hormones[22]. The most important hormone in the regulation of SO function is CCK[23]. In normal conditions, CCK can contract the gallbladder smooth muscle and reduce tone in the SO to regulate bile flow from the liver through the bile duct into the duodenum. During fasting, hepatic bile enters the gallbladder for storage. Eating initiates gallbladder emptying by neural and hormonal (predominantly CCK) influences. We found serum CCK-8 level was not increased in the 9 and 12 wk groups, meaning that the gallbladder evacuation resistance increased during eating in these groups. Decreases in serum CCK-8 level can cause gallbladder bile stasis, increase the volume of the gallbladder, and induce SOD by increasing the tension of SO.
CCK modulates SO motility mainly via excitatory receptors on the smooth muscle and inhibitory receptors in the neural endings[24]. A study in cats[24] showed that the SO relaxation by CCK was abolished by complete denervation induced by tetrodotoxin. Beagle dogs after cholecystectomy[25] showed an increased CBD pressure, and SO relaxation response to CCK was weakened. These findings were thought to be due to destruction of neural pathways with the operation. In our study, we examined the expression of CCK-AR in the SO using quantitative real-time PCR for the first time. We found that the expression levels of CCK-AR mRNA increased in SO in the 6 and 12 wk groups. The increase in CCK-AR may result in changes in tension of SO, and, therefore, the excreting resistance of the bile may be enhanced significantly.
VIP, an alkaline intestinal peptide composed of 28 amino acids, can relax gallbladder smooth muscle and decrease gallbladder pressure. In the present study, serum VIP level in the 9 and 12 wk groups was higher than that of the control group. We restated the role of VIP in the formation of cholesterol gallstones that had been stressed in our previous study[12]. We found that the frequency of myoelectric activity decreased markedly in the 9 wk group, the amplitude of myoelectric activity decreased in the 9 and 12 wk groups, and the SO basal pressure and common bile duct pressure increased significantly in the 12 wk group. Elevation of VIP may play an important role in the mechanism of SOD.
Gastrin can increase the SO basal pressure amplitude[12]. Elevation of serum gastrin may be related to SOD with characteristics of high tension in patients with post-cholecystectomy pain[26]. However, we found that serum gastrin was decreased significantly in the 3 and 9 wk groups. This effect may have resulted from changes in diet and may not necessarily be related to the formation of gallstones.
In conclusion, we have indicated that a cholesterol gallstone-causing diet may induce SOD, the increasing tension of SO along with its decreasing activity may play an important role in cholesterol gallstone formation, and expression changes in CCK-AR in SO smooth muscle and serum CCK-8 and VIP may be important causes of SOD. The exact mechanism of cholesterol gallstone-causing diet-induced SOD needs further study. Control of a cholesterol diet and regulation of SO motility are important in the prevention of cholesterol gallstone formation.
The sphincter of Oddi (SO) may play an important role in gallstone formation because it is the only way by which bile is discharged into the duodenum. However, there is limited research on how the motor function of the SO works and what mechanism by which stones are formed in the process of cholesterol gallstone formation.
SO manometry is the only method to directly assess the motor function of the SO and is considered the gold standard for assessing SO dysfunction (SOD). However, manometry changes of SO in cholelithiasis are quite controversial. Another measurement reflecting SO function is recording the myoelectric activity of the SO.
This study showed that a cholesterol gallstone-causing diet can induce SOD. The increasing tension of the SO along with its decreasing activity may play an important role in cholesterol gallstone formation. Expression changes of cholecystokinin-A receptor in SO smooth muscle, serum vasoactive intestinal peptide, and cholecystokinin-octapeptide may be important causes of SOD.
The study results suggest that a cholesterol gallstone-causing diet can induce SOD, and disturbance of SO motility may play a role in gallstone formation. Control of a cholesterol diet and regulation of SO motility are important in the prevention of cholesterol gallstone formation.
This is an interesting paper. The authors describe the results of a basic study in a guinea pig model to investigate the role of SO motility in cholesterol gallbladder stone formation. They concluded that a cholesterol gallstone causing diet may induce increasing tension and decreasing activity of SO, and these effects could play an important role in cholesterol gallstone formation. This work represents a well-conducted basic study that contributes useful information about the role of SO motility in the process of cholesterol gallstone formation and confirms previous limited data that indicated a significant increase in the base pressure of the SO in rabbits with a cholesterol lithogenic diet.
P- Reviewer: Antonini F, Beltran MA, Tomkin GH S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Di Ciaula A, Wang DQ, Wang HH, Bonfrate L, Portincasa P. Targets for current pharmacologic therapy in cholesterol gallstone disease. Gastroenterol Clin North Am. 2010;39:245-64, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Hou L, Shu XO, Gao YT, Ji BT, Weiss JM, Yang G, Li HL, Blair A, Zheng W, Chow WH. Anthropometric measurements, physical activity, and the risk of symptomatic gallstone disease in Chinese women. Ann Epidemiol. 2009;19:344-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Kong J, Liu BB, Wu SD, Wang Y, Jiang QQ, Guo EL. Enhancement of interaction of BSEP and HAX-1 on the canalicular membrane of hepatocytes in a mouse model of cholesterol cholelithiasis. Int J Clin Exp Pathol. 2014;7:1644-1650. [PubMed] |
| 4. | Matyja A, Gil K, Pasternak A, Sztefko K, Gajda M, Tomaszewski KA, Matyja M, Walocha JA, Kulig J, Thor P. Telocytes: new insight into the pathogenesis of gallstone disease. J Cell Mol Med. 2013;17:734-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Wang HH, Portincasa P, Liu M, Tso P, Samuelson LC, Wang DQ. Effect of gallbladder hypomotility on cholesterol crystallization and growth in CCK-deficient mice. Biochim Biophys Acta. 2010;1801:138-146. [PubMed] |
| 6. | Wei JG, Wang YC, Du F, Yu HJ. Dynamic and ultrastructural study of sphincter of Oddi in early-stage cholelithiasis in rabbits with hypercholesterolemia. World J Gastroenterol. 2000;6:102-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wang XJ, Wei JG, Wang CM, Wang YC, Wu QZ, Xu JK, Yang XX. Effect of cholesterol liposomes on calcium mobilization in muscle cells from the rabbit sphincter of Oddi. World J Gastroenterol. 2002;8:144-149. [PubMed] |
| 8. | De Masi E, Corazziari E, Habib FI, Fontana B, Gatti V, Fegiz GF, Torsoli A. Manometric study of the sphincter of Oddi in patients with and without common bile duct stones. Gut. 1984;25:275-278. [PubMed] |
| 9. | Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Varga G, Bálint A, Burghardt B, D’Amato M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br J Pharmacol. 2004;141:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Xu GQ, Xu CF, Chen HT, Liu S, Teng XD, Xu GY, Yu CH. Association of caveolin-3 and cholecystokinin A receptor with cholesterol gallstone disease in mice. World J Gastroenterol. 2014;20:9513-9518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Zhang ZH, Qin CK, Wu SD, Xu J, Cui XP, Wang ZY, Xian GZ. Roles of sphincter of Oddi motility and serum vasoactive intestinal peptide, gastrin and cholecystokinin octapeptide. World J Gastroenterol. 2014;20:4730-4736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Portincasa P, Ciaula AD, Bonfrate L, Wang DQ. Therapy of gallstone disease: What it was, what it is, what it will be. World J Gastrointest Pharmacol Ther. 2012;3:7-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 14. | Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140:508-516. [PubMed] |
| 15. | Zhang D, Xiang J, Wang L, Xu Z, Sun L, Zhou F, Zha X, Cai D. Comparative proteomic analysis of gallbladder bile proteins related to cholesterol gallstones. PLoS One. 2013;8:e54489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Maurer KJ, Carey MC, Fox JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. 2009;136:425-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Thune A, Saccone GT, Scicchitano JP, Toouli J. Distension of the gall bladder inhibits sphincter of Oddi motility in humans. Gut. 1991;32:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Rice JP, Spier BJ, Gopal DV, Soni A, Reichelderfer M, Pfau PR. Outcomes of sphincter of oddi manometry when performed in low volumes. Diagn Ther Endosc. 2011;2011:435806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Du P, Cui GB, Wang YR, Zhang XY, Ma KJ, Wei JG. Down regulated expression of the beta1 subunit of the big-conductance Ca2+ sensitive K+ channel in sphincter of Oddi cells from rabbits fed with a high cholesterol diet. Acta Biochim Biophys Sin (Shanghai). 2006;38:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Li ST, Chen XW, Zhao HM, Li N, Yan J, Hu ZA. Effects of orexins on myoelectric activity of sphincter of Oddi in fasted rabbits. Acta Pharmacol Sin. 2006;27:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Szilvássy Z, Nagy I, Madácsy L, Hajnal F, Velösy B, Takács T, Lonovics J. Beneficial effect of lovastatin on sphincter of Oddi dyskinesia in hypercholesterolemia and hypertriglyceridemia. Am J Gastroenterol. 1997;92:900-902. [PubMed] |
| 22. | Sonoda Y, Takahata S, Jabar F, Schloithe AC, Grivell MA, Woods CM, Simula ME, Toouli J, Saccone GT. Electrical activation of common bile duct nerves modulates sphincter of Oddi motility in the Australian possum. HPB (Oxford). 2005;7:303-312. [PubMed] |
| 23. | Pálvölgyi A, Sári R, Németh J, Szabolcs A, Nagy I, Hegyi P, Lonovics J, Szilvássy Z. Interplay between nitric oxide and VIP in CCK-8-induced phasic contractile activity in the rabbit sphincter of Oddi. World J Gastroenterol. 2005;11:3264-3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Behar J, Biancani P. Effect of cholecystokinin and the octapeptide of cholecystokinin on the feline sphincter of Oddi and gallbladder. Mechanisms of action. J Clin Invest. 1980;66:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Fan MM, Li F, Zhang XW. Changes of the sphincter of Oddi motility in dog after cholecystectomy. J Dig Dis. 2012;13:40-46. [PubMed] |
| 26. | Chen XX, Mo JZ, Liu WZ. [A study on motility of sphincter of Oddi in postcholecystectomy syndrome]. Zhonghua Nei Ke Zazhi. 1991;30:337-339, 381. [PubMed] |