Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5532
Peer-review started: February 20, 2016
First decision: March 21, 2016
Revised: April 11, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 28, 2016
Processing time: 122 Days and 21 Hours
AIM: To explore the regulatory mechanism of the target gene of microRNA-21 (miR-21), phosphatase gene (PTEN), and its downstream proteins, protein kinase B (AKT) and phosphatidylinositol 3-kinase (PI3K), in colorectal cancer (CRC) cells.
METHODS: Quantitative real-time PCR (qRT-PCR) and Western blot were used to detect the expression levels of miR-21 and PTEN in HCT116, HT29, Colo32 and SW480 CRC cell lines. Also, the expression levels of PTEN mRNA and its downstream proteins AKT and PI3K in HCT116 cells after downregulating miR-21 were investigated.
RESULTS: Comparing the miR-21 expression in CRC cells, the expression levels of miR-21 were highest in HCT116 cells, and the expression levels of miR-21 were lowest in SW480 cells. In comparing miR-21 and PTEN expression in CRC cells, we found that the protein expression levels of miR-21 and PTEN were inversely correlated (P < 0.05); when miR-21 expression was reduced, mRNA expression levels of PTEN did not significantly change (P > 0.05), but the expression levels of its protein significantly increased (P < 0.05). In comparing the levels of PTEN protein and downstream AKT and PI3K in HCT116 cells after downregulation of miR-21 expression, the levels of AKT and PI3K protein expression significantly decreased (P < 0.05).
CONCLUSION: PTEN is one of the direct target genes of miR-21. Thus, phosphatase gene and its downstream AKT and PI3K expression levels can be regulated by regulating the expression levels of miR-21, which in turn regulates the development of CRC.
Core tip: RT-PCR and Western blot were applied to detect the expression level of microRNA-21 (miR-21) and Phosphatase gene (PTEN), including its downstream proteins protein kinase B (AKT) and phosphatidylinositol 3-kinase (PI3K) in colorectal cancer (CRC) cell lines, and to explore the regulatory mechanism of the expression of miR-21 in inhibiting CRC, respectively. Their associations were investigated to clarify whether one of the direct target genes of miR-21 is PTEN. The expression levels of miR-21, PTEN and its downstream proteins AKT and PI3K are regulated and controlled to manage the occurrence and progression of CRC.
- Citation: Sheng WZ, Chen YS, Tu CT, He J, Zhang B, Gao WD. MicroRNA-21 promotes phosphatase gene and protein kinase B/phosphatidylinositol 3-kinase expression in colorectal cancer. World J Gastroenterol 2016; 22(24): 5532-5539
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5532
MicroRNA (miRNA) is a kind of non-coding macromolecule RNA that includes nearly 22 nucleotides, and it is able to conjugate the 3’UTR of mRNA to facilitate target mRNA degradation and inhibition of the translation process by virtue of lowering gene expression[1-4]. The research of Calin et al indicated that approximately 50% of miRNA was located in tumor-related genomic regions. This work also revealed aberrant expression levels in a number of tumors, which was probably on account of the oncogenic and tumor suppressing gene functions of miRNA molecules. In addition, miRNA is involved in the occurrence and progression of human tumors[5-8]. Phosphatase gene (PTEN) is a phosphatase and tensin homologue gene derived from chromosome ten, which is associated with phosphohydrolase; its inactivation induces tumor occurrence in the human body[9-11]. In recent years, the protein kinase B/phosphatase gene/phosphatidylinositol 3-kinase (AKT/PTEN/PI3K) signaling pathway has raised increasing concern, and a number of studies found that the atypical AKT/PTEN/PI3K signaling pathway was intimately linked with numerous tumor occurrences and progression, immunity, drug resistance, metastasis, angiogenesis, etc.[12-16]. In addition, the AKT/PTEN/PI3K signaling pathway has been reported in pulmonary, nasopharyngeal, gastric and renal tumors, as well as in neuroglioma[17-21]. However, literature with regard to colorectal cancer (CRC) is rare. Moreover, there were reports that revealed that microRNA-21 (miR-21) could regulate the AKT/PTEN/PI3K signaling pathway to promote tumor occurrence and progression, and even tumor invasion[22-25]. Hence, quantitative real-time PCR (qRT-PCR) and Western blot were applied to detect the expression level of miR-21 and PTEN, including its downstream proteins AKT and PI3K, in CRC cell lines. Furthermore, the mechanism of miR-21 expression in inhibiting CRC and their correlations were also explored. This study will provide a theoretical and experimental basis for the early diagnosis and therapy of CRC.
Primary reagents and equipment: PTEN antibody, PI3K mouse anti-human monoclonal antibody, immunohistochemistry (IHC) kit and AKT rabbit anti-human polyclonal antibody were purchased from Beijing Zhongshan Golden Bridge Biotechnology Company. Quantitative RT-PCR kit and miR-21 primer were purchased from Takara. Fluorescent quantitative PCR detection system was purchased from ABI (United States). The PCR instrument was purchased from Bio-Rad (United States). The inverted microscope was purchased from Olympus (Japan). The refrigerated centrifuge was purchased from Thermo Scientific (United States). HCT116, HT29, Colo32 and SW480 CRC cell strains were all purchased from the Cell Bank of the Chinese Academy of Sciences.
Preparation of primary reagents: Requisite reagents: phosphate buffer solution (PBS), bovine serum albumin (BSA) solution, Tris-buffered saline and Tween-20 (TBST) buffer, BSA blocking buffer, 0.25% trypsin solution, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis buffer, transmembrane buffer, SDS-PAGE separation and stacking gel.
CRC cell culture and transfection: A Dulbecco’s modified Eagle’s medium (DMEM) high glucose medium with 10% calf serum was used to culture HCT116, Ht29, Colo32 and SW480 CRC cell strains in 5% CO2 at 37 °C. Cells in the logarithmic phase were used in experiments. All cells were sorted into three groups: miR-21 inhibition group (IG), negative control (NC) and blank control (BC). HCT116 cells were inoculated in 500 μL of medium (no antibiotics) to 30%-60% degree to spare. Then, 20 pmol/L of miR-21 inhibitor was diluted with 50 mL of DMEM medium (no serum) and incubated for five minutes at room temperature after mixing. Next, 1 μL of mixing Lipofectamine 2000 was diluted in 50 μL of DMEM (no antibiotics and serum) and incubated at room temperature for 5 min. The diluted miR-21 inhibitor was mixed with Lipofectamine 2000 and incubated at room temperature for 20 min. Then, a 100-μL transfection buffer was added, mixed and incubated for six hours in an incubator (5% CO2 at 37 °C). Afterward, the medium was changed to normal medium and cells were incubated for another 48-72 hours for detection.
Transwell assay: Pre-cooling non-serum DMEM was used to dilute the Matrigel matrix gel. Then, it was paved on the chamber of the polycarbonate filtering membrane followed by inoculation of diluted HCT116 cells (100 μL). Afterwards, 10% fetal calf serum was added to the lower chamber. After 24 h of culture in 5% CO2 at 37 °C, the chamber was washed twice, stained with 0.1% crystal violet for five minutes, and washed again. Cells were randomly counted in five views at 100 × objective and the average was calculated. This was performed in three replicates for each group.
Real-time quantitative RT-PCR to detect miR-21 and PTEN mRNA levels: Total RNA was extracted using Trizol RNA extraction methods, based on the Molecular Clone Technique Experimental Manual. One mL of Trizol reagent could be added to approximately 100-mg tissues. A mortar was used to grind the tissues to powder, which eventually reached complete decomposition. The lysate was drawn into a 1.5-mL tube and incubated for 10 min on ice. After centrifuging at 12000 rpm for 10 min at 4 °C, the supernatant was drawn into a new 1.5-mL tube. Isopropanol in equal volume was added and mixed, plated on ice for 10 min, and centrifuged at 12000 rpm for another 10 min at 4 °C. The supernatant was discarded, 1 mL of 75% ethanol was added, the precipitate was washed, and centrifuged for another five minutes. Afterwards, the supernatant was discarded, placed into a tube in room temperature for 10 min, waited until the residue entirely volatilized, then 100 μL of diethyl pyrocarbonate H2O was added to dissolve the precipitate, and it was stored in liquid nitrogen for use. Extracted RNA purity and concentration was detected using a spectrophotometer. The normal value of optical density (OD) OD260/OD280 was between 1.8 and 2.1, respectively. Then, cDNA was synthesized by reverse transcription reaction. RT-PCR was used to detect miR-21 reaction conditions: 95 °C for 10 s, one cycle, 95 °C for 5 s, 60 °C for 34 s, 45 cycles; computational formula: relative amount = 2-ΔΔCT, where -ΔΔCT = (CTmiR-21-CTu6) tumor-(CTmiR-21-CTu6) normal tissue. RT-PCR was used to detect PTEN mRNA reaction conditions: 95 °C for 10 s, one cycle, 95 °C for 5 s, 60 °C for 20 s, 45 cycle; and PTEN mRNA expression level was calculated on the basis of ΔCt = Ct(PTEN) - Ct(β-actin), where Folds = 2-ΔΔCT, and the average result was used with three repeats.
Western blot: After 48-72 h of transfection, the medium was discarded and 100 μL of RIPA lysate was added into each well. The lysate was mixed for 5 min, centrifuged at 12000 rpm for 15 min at 4 °C, and the supernatant was stored for use. Samples mixed with 5 × loading were boiled for 10 min at 95 °C and loaded after centrifugation. Eight μL of purified and desalted antibody was loaded, and 2-3 μL of the marker was loaded. The antibody was confirmed according to the marker position. Electrophoresis: stacking gel in 80 V for 20-30 min, and separation gel in 120 V for 40 min. Transmembrane: 300 mA for 90 min. After transmembrane, the membrane was blocked with non-fat milk, washed with PBS for three times, and incubated with the primary antibody (PTEN antibody, PI3K antibody, AKT antibody and β-actin antibody; 1:500 dilution) overnight at 4 °C. On the next day, cells were washed with TBST for seven times and incubated with the secondary antibody (horseradish peroxidase-labeled goat-anti rabbit secondary antibody, 1:2500 dilution) for one hour. Then, cells were washed with TBST for another six times. Chemiluminescence reagent A and B solutions were mixed and added on the membrane based on a 1:1 proportion, and β-actin protein was used as an internal reference.
SPSS 19.0 was used to analyze all data; measurement data were presented as mean ± SD. The t-test was used to compare miR-21 and PTEN expression levels, and the variance analysis method was used to compare the mean value of transfection in the IG, NC and BC groups. P < 0.05 was considered statistically significant.
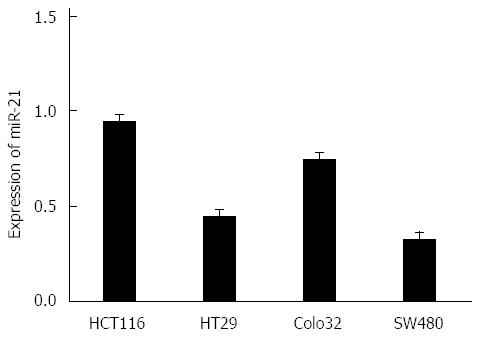
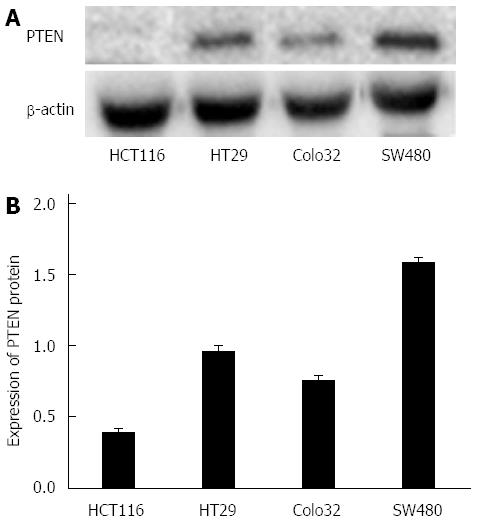
miR-21 had the highest protein expression in HCT116 cells, but had the lowest expression in SW480 cells (Figure 1). In contrast, PTEN protein expression was the lowest in HCT116 cells, but had the highest expression in SW480 cells (Figure 2). The protein expression level between miR-21 and PTEN was inversely related, and the difference was statistically significant (P < 0.01).
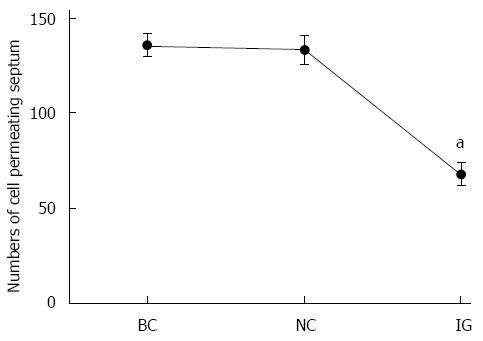
In counting the cells that crossed after transfection in the Transwell assay, we found that cells transfected with miR-21 in IG were reduced by 39.1% compared to BC and 36.9% compared to NC. However, the difference between BC and NC was not statistically significant (Figure 3).
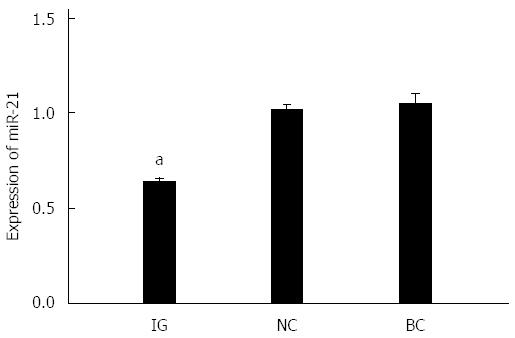
miR-21 expression levels after transfection in IG, NC and BC are shown in Figure 4. It can be observed that miR-21 expression levels in BC and NC were higher than in IG, and the difference was statistically significant (P < 0.05).
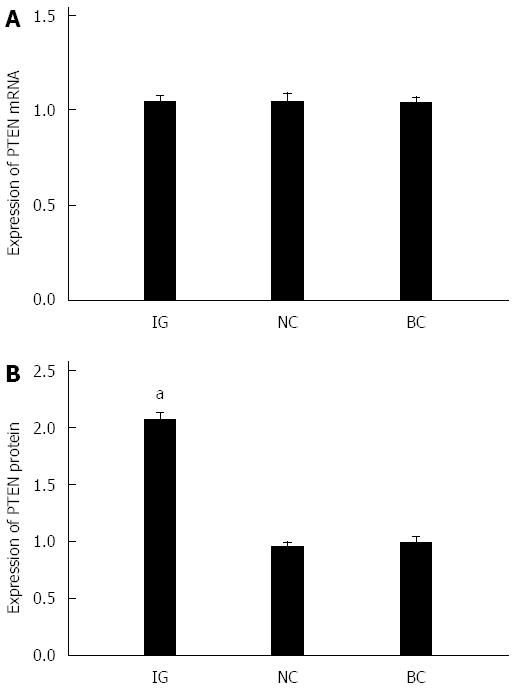
As shown in Figure 5A, after reducing the expression of miR-21, PTEN mRNA expression levels in HCT116 cells revealed no statistical significance after transfection in IG, NC and BC (P > 0.05). As illustrated in Figure 5B, when miR-21 was downregulated, PTEN protein expression levels were markedly elevated, and the difference was statistically significant (P < 0.05). The difference between BC and NC was not statistically significant (P > 0.05)
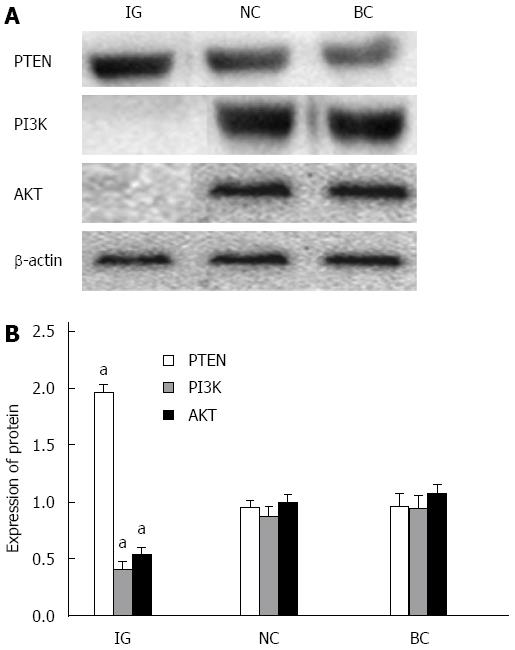
As shown in Figure 6, PTEN protein expression levels in HCT116 cells were elevated in IG, and the difference was statistically significant (P < 0.05). However, AKT and PI3K expression levels in HCT116 cells in IG decreased; and the difference was statistically significant (P < 0.05).
Much research has indicated that CRC occurrence in patients is a complicated process, which consists of multiple stages and factors. One momentous feature is its oncogenic activation and tumor-suppressing gene expression dysregulation or absence[26,27]. In recent years, it has been found miRNA possesses oncogenic and tumor suppressing gene functions[22]. One representative miRNA, miR-21, possesses a crucial oncogenic function and exhibits high expression levels in a number of tumors; it is closely associated with tumor occurrence and chemotherapy sensitivity[28,29]. PTEN is derived from chromosome ten, and acts as a tumor-suppressing gene. PTEN exerts vital effects on cell growth, proliferation, migration, signal transmission, invasion and apoptosis; it has an inhibitory role on tumor cell growth, invasion, proliferation, metastasis and apoptosis[30]. The AKT/PTEN/PI3K signaling pathway plays a significant part in regulating cell growth, metabolism, differentiation and apoptosis; it is closely associated with tumor occurrence and progression[31-36]. PTEN protein phosphatase activity (dephosphorylation) can inhibit AKT function, leading to cell apoptosis. Furthermore, the deletion or mutation of PTEN is able to enhance AKT activity to increase PI3K expression[37]. AKT/PTEN/PI3K signaling pathway activation is capable of inhibiting cell apoptosis to facilitate cell differentiation and proliferation. This also exerts a crucial role in tumor occurrence and progression, and participates in tumor metastasis and invasion[38-44].
miR-21 and PTEN protein expression was detected in HCT116, HT29, Colo32 and SW480 CRC cell lines through RT-PCR and Western blot. Results revealed that miR-21 expression level was highest in HCT116 cells and lowest in SW480 cells. Furthermore, PTEN expression level was lowest in HCT116 cells and highest in SW480 cells. The protein expression level between miR-21 and PTEN was inversely correlated. These results indicate that miR-21 could be involved in the regulation of PTEN protein expression. In addition, due to the high expression level of miR-21 in HCT116 cells, HCT116 cells were selected for this study. Transwell invasion assay revealed that the invasive capacity of tumor cells was obviously reduced after inhibiting CRC cell miR-21 expression, which in turn indicates that miR-21 probably plays a promoting role in cell malignancy transformation.
After downregulating the expression of miR-21, the expression level of miR-21 in HCT116 cells in IG was obviously lower compared to NC and BC, which illustrates the successful inhibition of miR-21. As a known miR-21 target gene, PTEN has been verified to be a target gene of miR-21 by Western blot. When we inhibited the expression of miR-21, PTEN mRNA expression levels did not reveal apparent change, but protein levels were obviously elevated; which indicates that miR-21 could regulate PTEN expression at the post-transcriptional level and that PTEN was a target gene for miR-21. Meng et al[45] recently found that miR-21 targeted tumor-suppressing gene PTEN in liver and bile duct cancer cells to promote tumor growth, invasion and migration, manifesting that PTEN was a target gene of miR-21.
After inhibiting the expression of miR-21, PTEN protein level was significantly elevated, but its downstream AKT and PI3K expression obviously decreased, indicating that miR-21 could regulate and control PTEN and the expression level of downstream AKT and PI3K in terms of its effects on tumor cell invasion and migration. Certain studies have shown that change in miR-21 expression level is able to induce PTEN downstream molecule AKT phosphorylation, metalloproteinase 2 and focal adhesion kinase expression; these are altered to restrain tumor cell invasion and migration[45-50].
In conclusion, our study revealed that PTEN is one of the direct target genes of miR-21. By altering miR-21 expression, downstream AKT and PI3K expression levels could be regulated and controlled, in order to control CRC occurrence and progression. Nevertheless, the mechanisms of miR-21 and PTEN function, as well as their effects in tumors, need to be further investigated. As more in-depth research on miRNA is being undertaken, especially regarding tumor cell regulatory mechanisms, more roles and targets of miR-21 will probably be unraveled.
Colorectal cancer (CRC) is complicated and combines multiple stages and factors. In recent years, we have found that miRNA possesses oncogenic and tumor-suppressing gene functions. One representative miRNA, microRNA-21 (miR-21), possesses a crucial oncogenic function that shows high expression levels in a number of tumors; this is closely associated with tumor occurrence and chemotherapy sensitivity. PTEN acts as a tumor-suppressing gene derived from chromosome ten. PTEN exerts vital effects in cell growth, proliferation, migration, signal transmission, invasion and apoptosis; it has an inhibitory role in tumor cell growth, invasion, proliferation, metastasis and apoptosis. The AKT/PTEN/PI3K signaling pathway plays a significant part in regulating cell growth, metabolism, differentiation, and apoptosis; it is closely associated with tumor occurrence and progression.
In addition, the AKT/PTEN/PI3K signaling pathway has been reported in pulmonary, nasopharyngeal, gastric and renal tumors, as well as in neuroglioma; however, literature with regard to CRC is rare. Moreover, there have been reports that revealed that miR-21 could regulate the AKT/PTEN/PI3K signaling pathway, in order to promote tumor occurrence and progression, and even tumor invasion. Hence, RT-PCR and Western blot were applied to detect the expression levels of miR-21 and PTEN, including its downstream proteins AKT and PI3K in CRC cell lines, as well as the inhibition of miR-21 expression in CRC cell lines. Furthermore, their association was also explored. This study hopes to provide a theoretical and experimental basis for the early diagnosis and therapy of CRC.
RT-PCR and Western blot were applied to detect the expression level of miR-21 and PTEN including its downstream proteins AKT and PI3K, and the inhibition of miR-21 expression in CRC cell lines, respectively. This association was also investigated to demonstrate that PTEN was one of the direct target genes of miR-21. Through regulating and controlling the expression level of miR-21, PTEN and its downstream proteins AKT and PI3K expression level are regulated to control the occurrence and progression of CRC.
This study revealed that PTEN was one of the direct target genes of miR-21, and that its downstream AKT and PI3K expression levels could be regulated and controlled through altering miR-21 expression, in order to control CRC occurrence and progression. Nevertheless, the mechanisms of miR-21 and PTEN function, as well as their effects in tumors, need to be further investigated. As in-depth research on mRNA is currently being implemented, particularly regarding the regulatory mechanism of tumor cells, more roles and targets of miR-21 will probably be unraveled.
This is an interesting manuscript about the miR-21 promotion of PTEN and its downstream proteins AKT and PI3K in CRC. Over all, this study is well designed and the manuscript is well written.
P- Reviewer: Imai K, Mangiola F S- Editor: Gong ZM L- Editor: Logan S E- Editor: Ma S
| 1. | Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8847] [Cited by in RCA: 9285] [Article Influence: 464.3] [Reference Citation Analysis (0)] |
| 2. | Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1665] [Cited by in RCA: 1701] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 3. | Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1316] [Cited by in RCA: 1260] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 4. | Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1298] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 5. | Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol. 2012;138:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1519] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 10. | Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 11. | Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 983] [Cited by in RCA: 964] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 12. | Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 14. | Hafsi S, Pezzino FM, Candido S, Ligresti G, Spandidos DA, Soua Z, McCubrey JA, Travali S, Libra M. Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of drug-resistance (review). Int J Oncol. 2012;40:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Hemmings BA, Restuccia DF. The PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2015;7:pii a026609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, Fu S, Falchook GS, Hong DS, Garrido-Laguna I. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 17. | Shih MC, Chen JY, Wu YC, Jan YH, Yang BM, Lu PJ, Cheng HC, Huang MS, Yang CJ, Hsiao M. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2012;31:2389-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Qu C, Liang Z, Huang J, Zhao R, Su C, Wang S, Wang X, Zhang R, Lee MH, Yang H. MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle. 2012;11:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Wang KF, Yang H, Jiang WQ, Li S, Cai YC. Puquitinib mesylate (XC-302) induces autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells. Int J Mol Med. 2015;36:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Mueller S, Phillips J, Onar-Thomas A, Romero E, Zheng S, Wiencke JK, McBride SM, Cowdrey C, Prados MD, Weiss WA. PTEN promoter methylation and activation of the PI3K/Akt/mTOR pathway in pediatric gliomas and influence on clinical outcome. Neuro Oncol. 2012;14:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Xiong B, Cheng Y, Ma L, Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol. 2013;42:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One. 2012;7:e39520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Wei J, Feng L, Li Z, Xu G, Fan X. MicroRNA-21 activates hepatic stellate cells via PTEN/Akt signaling. Biomed Pharmacother. 2013;67:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Elez E, Argilés G, Tabernero J. First-Line Treatment of Metastatic Colorectal Cancer: Interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015;16:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1691] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 28. | Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin Chem. 2015;61:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 358] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 29. | Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu RX, Liu SH, Yi QT, Li J, Song CH. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36:3035-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Fragoso R, Barata JT. Kinases, tails and more: regulation of PTEN function by phosphorylation. Methods. 2015;77-78:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, Pu H, Li WW, Tang B, Wang Y. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112:2853-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 32. | Yang X, Cheng Y, Li P, Tao J, Deng X, Zhang X, Gu M, Lu Q, Yin C. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015;36:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | He C, Dong X, Zhai B, Jiang X, Dong D, Li B, Jiang H, Xu S, Sun X. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6:28867-28881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 34. | Paul-Samojedny M, Pudełko A, Suchanek-Raif R, Kowalczyk M, Fila-Daniłow A, Borkowska P, Kowalski J. Knockdown of the AKT3 (PKBγ), PI3KCA, and VEGFR2 genes by RNA interference suppresses glioblastoma multiforme T98G cells invasiveness in vitro. Tumour Biol. 2015;36:3263-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Xu J, Cai J, Jin X, Yang J, Shen Q, Ding X, Liang Y. PIG3 plays an oncogenic role in papillary thyroid cancer by activating the PI3K/AKT/PTEN pathway. Oncol Rep. 2015;34:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Malemud CJ. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem. 2015;7:1137-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 37. | McCarroll JA, Gan PP, Erlich RB, Liu M, Dwarte T, Sagnella SS, Akerfeldt MC, Yang L, Parker AL, Chang MH. TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in non-small cell lung cancer. Cancer Res. 2015;75:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Hevner RF. Brain overgrowth in disorders of RTK-PI3K-AKT signaling: a mosaic of malformations. Semin Perinatol. 2015;39:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4837] [Cited by in RCA: 5253] [Article Influence: 262.7] [Reference Citation Analysis (0)] |
| 40. | Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1584] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 41. | Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4896] [Cited by in RCA: 5077] [Article Influence: 195.3] [Reference Citation Analysis (0)] |
| 42. | Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3583] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 43. | Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1451] [Cited by in RCA: 1369] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 44. | Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375-13378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2257] [Cited by in RCA: 2354] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 45. | Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2191] [Cited by in RCA: 2184] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 46. | Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett. 2013;337:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Vinciguerra M, Sgroi A, Veyrat-Durebex C, Rubbia-Brandt L, Buhler LH, Foti M. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog (PTEN) via microRNA-21 up-regulation in hepatocytes. Hepatology. 2009;49:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Ye JJ, Cao J. MicroRNAs in colorectal cancer as markers and targets: Recent advances. World J Gastroenterol. 2014;20:4288-4299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Gayral M, Jo S, Hanoun N, Vignolle-Vidoni A, Lulka H, Delpu Y, Meulle A, Dufresne M, Humeau M, Chalret du Rieu M. MicroRNAs as emerging biomarkers and therapeutic targets for pancreatic cancer. World J Gastroenterol. 2014;20:11199-11209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 713] [Cited by in RCA: 714] [Article Influence: 47.6] [Reference Citation Analysis (0)] |