Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5495
Peer-review started: March 28, 2016
First decision: April 14, 2016
Revised: April 28, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: June 28, 2016
Endoscopic bariatric therapy may be a useful alternative to pharmacological treatment for obesity, and it provides greater efficacy with lower risks than do conventional surgical procedures. Among the various endoscopic treatments for obesity, the intragastric balloon is associated with significant efficacy in body weight reduction and relief of comorbid disease symptoms. Anatomically, this treatment is based on gastric space-occupying effects that increase the feeling of satiety and may also affect gut neuroendocrine signaling. The simplicity of the intragastric balloon procedure may account for its widespread role in obesity treatment and its applicability to various degrees of obesity. However, advances in device properties and procedural techniques are still needed in order to improve its safety and cost-effectiveness. Furthermore, verification of the physiological outcomes of intragastric balloon treatment and the clinical predictive factors for treatment responses should be considered. In this article, we discuss the types, efficacy, safety, and future directions of intragastric balloon treatment.
Core tip: Obesity is a complex metabolic illness that is associated with several comorbid diseases. There has been a constant demand for safe and more effective weight reduction interventions to fill the gap in the treatment of obesity. The intragastric balloon is a fascinating intermediate alternative solution between medical obesity treatment and bariatric surgical procedures for obese patients that may provide better efficacy and have a more favorable risk profile.
- Citation: Kim SH, Chun HJ, Choi HS, Kim ES, Keum B, Jeen YT. Current status of intragastric balloon for obesity treatment. World J Gastroenterol 2016; 22(24): 5495-5504
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5495.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5495
Obesity is a complex metabolic illness that results from excess accumulation of body fat and may lead to negative health consequences. Obesity increases the prevalence of various diseases, including diabetes mellitus, hypertension, coronary heart disease, sleep apnea, stroke, gastroesophageal reflux disease, gall bladder disease, certain types of malignancy, and non-alcoholic fatty liver disease[1]. Moreover, it is also a major avoidable health detriment. Current therapeutic approaches to obesity are lifestyle changes, pharmacologic treatment, and bariatric surgery. Although intensive lifestyle modification was reportedly associated with only limited weight reduction[2-4], when it is combined with weight-loss drugs approved for long-term use, an additional weight reduction of 3%-9% can occur within 1 year[5]. Such drugs are said to improve several cardiometabolic risk factors, but they are also related to harmful adverse effects[5]. Although new obesity medications have recently been approved and introduced[6-8], they are associated with issues of safety and high costs. Weight-loss surgery provides the most sustained and effective therapeutic choice for obesity. Accessible methods include the adjustable gastric band, Roux-en-Y gastric bypass, or sleeve gastrectomy[9,10]. Regardless of its proven effectiveness, only 1% of obese patients eligible for the surgical procedure choose to undergo it[11]. The major issues with surgery are difficult accessibility, high costs, patient non-preference, and significant morbidity and mortality. Although its associated mortality has decreased considerably, the complication rate in the early and late stages of the bariatric procedure persist at 17% (95%CI: 11%-23%)[10].
Therefore, minimally invasive and effective methods are needed for the treatment of obesity. As such, endoscopic bariatric treatment was recently introduced. It includes intragastric balloons, gastroplasty techniques, aspiration therapy, and gastrointestinal bypass sleeves. Among them, the intragastric balloon has been the most frequently used in practice and the most studied for obesity treatment.
In 1985, the Garren-Edwards gastric bubble (GEGB) was the first intragastric balloon approved for obesity treatment and was introduced in the United States market. It was made of polyurethane, had a cylindrical design, and was filled with 200-220 mL of air[12]. However, several adverse events were associated with its use, including small bowel obstruction associated with spontaneous deflation and gastric mucosal injury. Although the GEGB is no longer used, considerable advancements to its design have led to the development of a more effective and safer intragastric balloon. It is now being used in numerous countries. Additionally, the United States Food and Drug Administration (FDA) recently approved two new intragastric balloons.
The increased prevalence of obesity has motivated experts in bariatric medicine to advance minimally invasive endoscopic treatment for obesity management as well as innovative techniques that address important features of treatments, such as their efficiency and safety. A new meta-analysis showed that endoscopic obesity treatment could be effective and of substantial value if combined with a multidisciplinary and comprehensive treatment plan[13].
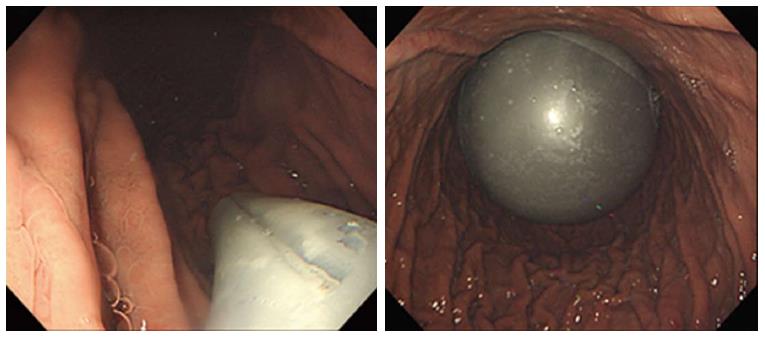
The intragastric balloon technique has become an effective method of achieving significant weight reduction in obese people (Figure 1). One or more intragastric balloons can be placed in the stomach using endoscopic procedures under mild sedation in an outpatient setting. Intragastric balloons allow patients to sense fullness and ultimately reduce their food intake. It is hypothesized that the intragastric balloon facilitates satiety peripherally by being an obstacle to food consumption, decreasing intragastric volume, and delaying gastric emptying[14]. Additionally, signals transmitted centrally through the vagal nerves by activated gastric stretching receptors could affect satiety[14]. The intragastric balloon permits an early feeling of satiety, which is thought to be a consequence of gastric distention. The mechanical intragastric distention to a meaningful volume during mealtime significantly decreases the amount of food eaten[15,16].
The intragastric balloon may also act via its relationship with various neurohumoral factors. It affects hunger control and gastric emptying by altering gut hormones and peptides such as ghrelin, leptin, cholecystokinin, and pancreatic polypeptide[17,18] and may also be related to physiological adaptation to weight loss.
The intragastric balloon may play diverse roles in obesity treatment as a preemptive therapy, a metabolic therapy, or a primary therapy.
The most frequently used balloon is the Orbera Intragastric Balloon (Apollo Endosurgery, Austin, TX, United States), which was known previously as the BioEnterics Intragastric Balloon (BIB). It is an elastic silicone balloon containing saline (450-700 mL) (Table 1). The positioning assembly, which comprises a balloon-filling tube and a catheter with the deflated balloon, is blindly advanced to the gastro-esophageal junction. An endoscopic device is inserted to ensure the precise deployment of the intragastric balloon, which is then filled with methylene-mixed saline under direct observation via the catheter. If an unexpected balloon rupture occurs, the methylene blue turns the urine green. The Orbera balloon is usually implanted for 6 mo, removed endoscopically by needle aspiration of the intragastric fluid, and retrieved with a snare or grasper. The FDA approved the use of the Orbera balloon on August 6, 2015. It is expected that the Orbera balloon could provide a valuable and less invasive therapeutic approach to bariatric treatment.
| Balloon | Material | Volume | Weight loss1 | Note |
| Fluid-supplied | ||||
| Orbera (Apollo Endosurgery) | Silicone | 400-700 mL (saline) | 16.9 ± 0.9 kg (at 6 mo)[58] | Most widely used and studied intragastric balloon |
| Volume adjustment at the time of placement | ||||
| Spatz Adjustable Balloon system (Spatz FGIA) | Silicone | 400-600 mL (saline) | 24 kg (at 12 mo)[64] | Totally adjustable balloon |
| Approved for 12 mo of implantation | ||||
| ReShape Duo® Integrated DualBalloon System (ReShape medical) | Silicone | 900 mL (450 mL × 2) (saline) | 25.1 ± 1.6 %EWL (at 6 mo)[62] | Two independent balloons connected to silicone shaft |
| The Elipse™ (Allurion Technologies) | N/A | 450-550 mL (filling fluid) | 2.4 kg (at 6 wk)[19] | Naturally swallowed, self-emptying, and naturally excreted |
| Air-supplied | ||||
| Obalon® Gastric Balloon (Obalon Therapeutics) | N/A | 250 mL (air, nitrogen) | 5 kg (at 12 wk)[66] | Can be swallowed |
| A second or a third balloon can be swallowed depending on patient’s progress | ||||
| Heliosphere BAG® (Helioscopie) | Polyurethane and silicone | 950 mL (air) | 16 ± 7 kg (at 6 mo)[23] | Less than 30 g |
The ReShape Duo® (ReShape Medical, San Clemente, CA, United States) aims to improve the space-occupying effects of intragastric balloons. This non-invasive device is delivered via the mouth through a 30-min endoscopic procedure. It is inflated with 900 mL of saline, which is equally distributed to each of two balloons. The balloon is inflated by a controller with methylene blue-mixed saline. This dual balloon system may reduce deflation-associated complications. If one of the balloons ruptures, the other balloon could sustain the location of the device in the stomach. It is recommended that the balloon be retrieved 6 mo after placement. The ReShape Duo was also approved by the FDA, on July 29, 2015.
The Spatz Adjustable Balloon System (Spatz Medical, Great Neck, NY, United States) is a silicone balloon that is inflated with saline. It includes a filling catheter, which is extractable endoscopically, that permits an intragastric volume adjustment of 400-800 mL. The volume of the intragastric balloon can be modified to improve the patient’s tolerance and increase weight reduction. The Spatz Adjustable Balloon System is allowed to be implanted in the stomach for 12 mo in locations outside the United States.
The Elipse™ (Allurion Technologies, Wellesley, MA, United States) is a swallowable, self-draining, and naturally expelled intragastric balloon device for weight reduction. It is covered with a vegetarian shell and fixed to a flexible, slim tube. A resorbable substance inside the balloon degrades, allowing the balloon to empty naturally after a certain period. The deflated intragastric balloon is devised to be excreted via the digestive tract[19]. It is inflated with 550 mL of fluid and can be placed in the stomach for 4 mo. Endoscopic procedures are not needed for Elipse™ placement or removal.
The Obalon Gastric Balloon® (Obalon Therapeutics Inc, Carlsbad, CA, United States) is a balloon filled with 250 mL of air. It has a self-sealing valve linked to a catheter and is set inside a gelatin capsule. The balloon is packaged in the capsule and swallowed, and the thin catheter is extended alongside the esophagus into the stomach. Fluoroscopy is used to define the location of the intragastric balloon, and the gelatin capsule disintegrates and releases the balloon. The balloon is then inflated using a gas-contained canister. The catheter is separated from the balloon and removed after balloon inflation.
The Heliosphere Bag® (Helioscopie, Vienne, France) comprises a double-bagged polymer covered with an external silicone pouch. It is slowly filled with 960 mL of air for a final inflation volume of 700 mL[20,21]. The weight of the Heliosphere Bag is less than 30 g[22,23].
The Silimed Gastric Balloon is a spherical silicone balloon set inside a thin silicone sheath[24]. It is attached to the endoscope by a snare, advanced by an endoscope, and placed in the gastric fundus. It is filled with 650 mL of saline solution mixed with contrast dye and 10 mL of methylene blue.
The adjustable totally implantable intragastric prosthesis (ATIIP) is a polyurethane intragastric balloon that is rugby-shaped, 12 cm long, and has a volume of 300 mL when inflated with air[25]. This balloon is placed with an endoscopic percutaneous gastrostomy technique followed by the deployment of a subcutaneous totally implantable system through a surgical procedure. This method may reduce the likelihood of device dislocation and allows for balloon volume adjustment. The proximal balloon position in the gastric fundus-corpus lesion could affect gastric accommodation, neurohormonal process, and electrical action, thus modifying various control processes related to satiety[14].
The intragastric balloon may offer a minimally invasive and valuable method for managing obesity and related conditions. It is used to achieve weight loss in obese people, generally those with a body mass index (BMI) > 35 kg/m2, or 30 kg/m2 with certain comorbidities. Intragastric balloon treatment may play a different role in bariatric treatment based on the grade of obesity.
Early intervention and preemptive therapy for weight reduction can be performed in obese patients (BMI ≥ 30 kg/m2) at risk for disease development, at high risk for all-cause mortality, and with a high cardiovascular risk profile[26].
The objective of preemptive treatment is to achieve modest weight reduction, and, therefore, the overall risk/benefit ratio could validate the standards for procedures with this indication. Depending on the circumstances, indications for placement of intragastric balloons could also be extended to manage overweight people with a BMI < 30 kg/m2 who desire weight reduction but who cannot achieve body weight loss with a controlled dietary program or with pharmacotherapy[27].
Body weight loss achieved with intragastric balloon placement is associated with improvements in obesity-related metabolic illness. The intragastric balloon for metabolic therapy may be performed in patients with mild obesity (BMI ≥ 30 kg/m2), where recovery from metabolic disease is the primary concern[28]. Co-existing illnesses, such as hyperlipidemia, type II diabetes mellitus, and hypertension, could be particularly improved or resolved with even a modest reduction in body weight[29,30]. Treatment for metabolic issues should be relatively low-risk and have superior stability.
The goal of intragastric balloon treatment is to achieve weight reduction in severely obese people, generally those with a BMI > 35 kg/m2 with or without comorbidities, and who could not achieve long-term weight loss with a weight-control regimen. In addition, intragastric balloon therapy could be performed in patients with a BMI ≥ 40 kg/m2, primarily as a preparation for bariatric treatment or in patients with increased surgical risks. Obese patients who reject bariatric surgical procedures or who do not have an approach for surgery can also opt for it. Intragastric balloon therapy used as a primary therapy could induce weight reduction and improve obesity-related comorbidities with a level of safety and efficiency comparable to that of bariatric surgery[28]. However, lower efficiency is also acceptable because of the lower risk profile of intragastric balloon therapy.
Exclusion criteria include any situation that could increase the risks related to intragastric balloon insertion, such as a large hiatal hernia (> 5 cm), active ulcer in the stomach or duodenum, previous surgical resection of the stomach, inflammatory bowel disease, gastrointestinal neoplasm, oropharyngeal abnormalities, active gastrointestinal bleeding, coagulative disorder, variceal disease, alcoholic disease or drug abuse, psychiatric disease, pregnancy, use of anti-coagulants or anti-inflammatory drugs, and cardiovascular, pulmonary or cerebrovascular diseases[31].
The purpose of intragastric balloon treatment is to stimulate weight loss and assist with recovery from associated comorbidities with adequate safety. Gastric capacity restriction is an essential factor in surgical bariatric management.
Surgical gastric restriction could induce early satiety and potentiate gastric mechanical and chemical stimulation through a relationship with various gastric or exogenic factors. It affects hunger control and gastric emptying through alterations in gut hormones and peptides[32-34]. The intragastric balloon attempts to mimic surgical weight loss procedures by restricting the effective gastric volume.
Results from previous studies indicated that the mean weight loss associated with intragastric balloon therapy ranged between 10.5 and 13.7 kg after 3 mo and between 12 and 26.3 kg after a 6-mo placement of the Orbera intragastric balloon[22,23,35-49]. After balloon removal at 6 mo, the achieved weight reduction endured to some extent. The excess weight loss (EWL) at the 12-mo implantation mark (6 mo after removal) was 14% to 50.9%[37,40,42,44,50-54]. The EWL at 12 mo after balloon extraction ranged from 14.2% to 27.2%[51,55-57]. The Orbera intragastric balloon was most effective during the first 3 mo of therapy. During that time, average weight loss of obese patients was 12.9 kg, or 80% of the total achieved weight reduction[58]. Additionally, the initial body weight loss (BWL) following intragastric balloon placement was associated with significant long-term weight maintenance. The percentage of BWL 1 mo after intragastric balloon placement was significantly associated with weight loss after 6, 12, and 18 mo (Pearson correlation coefficient = 0.77, 0.65, and 0.62, respectively, P < 0.001 for all)[59].
A study reported that insertion of a second balloon is not difficult and achieved good results. The mean %EWL was 31.5 ± 23.2 after the second balloon removal[43]. Some studies showed long-term results after intragastric balloon placement. A multi-center European study presented results for weight loss 3 years after balloon removal, which accounted for 29.1% of mean EWL[54]. A study that included 195 patients who were followed up for 5 years after intragastric balloon insertion demonstrated a %EWL of 12.97 ± 8.54[44].
Additional limited studies have utilized other intragastric balloons. A Spanish study with 60 patients showed a total body weight reduction of 16.6 ± 9.33 kg 6 mo after implantation of the ReShape Duo double-balloon system[60]. A prospective study with a double-balloon system showed a mean EWL of 18.3% in the control group compared to 31.8% in the treatment group 6 mo post-balloon implantation[61]. Furthermore, 64% of the reduced body weight was maintained at 12 mo post-implantation (6 mo after removal). In the REDUCE Pivotal Trial, a prospective randomized trial with 326 patients, patients assigned to the double-balloon system plus exercise and diet showed significantly superior EWL compared to the sham endoscopy plus exercise and diet alone group at 6 mo post-implantation (25.1% vs 11.3%, P < 0.05) in an intent-to-treat analysis[62].
A pilot trial showed 15.6 kg of mean weight loss at 24 wk and 24.4 kg at 52 wk after Spatz adjustable balloon placement[63]. A small observational investigation in 73 obese patients showed 45.7% of EWL 12 mo after deployment of the Spatz adjustable balloon[64].
Two preliminary studies with the Silimed Gastric Balloon reported results after the completion of 6 mo of treatment. The mean weight loss was 11.3 kg[24] and 8.1 kg[65]. A total of 57 morbidly obese patients underwent ATIIP placement. Mean EWL was 28.7% at 6 mo (38 patients) and 39.2% at 12 mo (20 patients)[25]. The Obalon (orally ingestable intragastric balloon) showed median weight losses after 4 weeks, 8 weeks, and 12 wk as 2.2 kg, 4.0 kg, and 5 kg, respectively[66]. A prospective study with 50 obese patients compared the effects of intragastric balloon therapy or pharmacotherapy on weight reduction. At 6 mo, patients in the intragastric balloon group had lost more weight than had patients in the pharmacotherapy group (percent of initial weight lost, %IWL = 14.5 ± 1.2; percent of excess BMI lost, %EBL = 37.7 ± 3.2 vs %IWL = 9.1 ± 1.5, %EBL = 25.3 ± 4.1, respectively, P < 0.005)[67].
Although the intragastric balloon has been shown to be effective in causing a meaningful weight loss, several studies have reported that the results were short-lasting, with most patients regaining weight following intragastric balloon removal[31,36,51,55,68-72].
Obesity in patients is related to several comorbidities that are significant targets for obesity treatment. A study with 143 obese patients described the effects of the Orbera intragastric balloon at the 12-mo follow-up[51]. The incidence of metabolic syndrome declined from 34.8% (before balloon insertion) to 14.5%, 13%, and 11.6% at the time of removal, at the 6-mo follow-up, and at the 1-year follow-up, respectively. The incidence of type 2 diabetes mellitus decreased from 32.6% to 20.9%, 22.5%, and 21.3%, respectively. Likewise, the occurrence of hyperuricemia, hypertriglyceridemia, and hypercholesterolemia decreased from 26.1%, 37.7%, and 33.4% to 25.4%, 14.5%, and 16.7%, respectively, at the time of removal, 25.9%, 15.2%, and 16.7%, respectively, at the 6-mo follow-up, and 26.4%, 17.4%, and 18.9%, respectively, at the 1-year follow-up. A multi-center study presented data following treatment with the Orbera intragastric balloon in overweight populations[54]. The percentage of patients with co-morbidities at baseline and at the 3-year follow-up was 29% and 16% for hypertension, 15% and 10% for diabetes mellitus, 20% and 18% for dyslipidemia, 32% and 21% for hypercholesterolemia, and 25% and 13% for osteoarthropathy, respectively. A study with 119 obese patients assessed the effects of the Orbera intragastric balloon on obesity-associated diseases and quality of life[42]. Six months after placement of the intragastric balloon, the rate of metabolic syndrome in the patients decreased from 42.9% to 15.1% (P < 0.0005). Cholesterol, triglycerides, fasting glucose, C-reactive protein levels, and blood pressure also improved after balloon treatment (P < 0.005). In patients with diabetes mellitus, the HbA1c level was decreased at 6 mo compared to that at baseline (7.4% to 5.8%; P < 0.0005). In addition, the quality of life of patients increased.
Obesity is one of the risk factors for nonalcoholic fatty liver disease (NAFLD). About 27% of patients with NAFLD can develop fibrosis, and 19% can develop cirrhosis[73]. A randomized controlled study showed that intragastric balloon therapy improved the histology of nonalcoholic steatohepatitis[74]. Six months after implantation, a significant reduction in the median NAFLD activity scores was observed in the intragastric balloon-treated group (2 vs 4, P = 0.030) compared to that in the control group. Additionally, the median steatosis scores displayed a trend toward improvement in the balloon-treated group compared to those in the control group (1 vs 1, P = 0.075).
Body weight is controlled by multifaceted coordination of both central and peripheral factors. It is now obvious that a physiologic change in gut neurohumoral signaling is one important contributor to weight reduction and improvement in related diseases obtained by anatomic surgical manipulation of the gastrointestinal tract[75]. Moreover, intragastric balloon treatment may affect weight changes through an interaction of gastric neurohumoral factors (Table 2). These factors include several gut peptides and hormones, such as ghrelin, leptin, cholecystokinin, peptide YY, pancreatic polypeptide, and glucagon-like peptide-1.
| Ref. | Balloon | Weight loss at 6 mo | Ghrelin (at 0 mo) | Ghrelin (after 3 mo) | Ghrelin (after 6 mo) | Ghrelin (after 12 mo) | Leptin (at 0 mo) | Leptin (after 3 mo) | Leptin (after 6 mo) | Leptin (after 12 mo) |
| Mathus-Vliegen et al[17] | Orbera | 17.4 ± 7.8 kg | 722.3 ± 151.5 pg/mL | 791.5 ± 239.0 pg/mL | 743.7 ± 115.2 pg/mL | N/A | N/A | N/A | N/A | N/A |
| Fuller et al[48] | N/A | 14.2% | 414.1 pmol/L | 448 pmol/L | 452.4 pmol/L | 379.4 pmol/L | 23.4 ng/mL | 18.5 ng/mL | 11.7 ng/mL | 19.7 ng/mL |
| Bužga et al[85] | MedSil® | 18.4 ± 8.2 kg | 240.5 ± 101.5 μg/L | 378.1 ± 155.8 μg/L | 335.8 ± 149.2 μg/L | N/A | 30.4 ± 17.2 μg/L | 18.2 ± 15.8 μg/L | 14.9 ± 15.5 μg/L | N/A |
| 1Nikolic et al[86] | Orbera | N/A | 958.3 pg/mL | 1346.2 pg/mL | 1050.1 pg/mL | 922.6 pg/mL | 25.1 ng/mL | 14.3 ng/mL | 10.5 ng/mL | 17.5 ng/mL |
| Konopko-Zubrzycka et al[39] | Orbera | 17.1 ± 8.0 kg | 621.9 ± 182.4 pg/mL | 903.9 ± 237 pg/mL (1 mo) | N/A | N/A | 61.3 ± 36.7 ng/mL | 39.9 ± 17.5 ng/mL (1 mo) | N/A | N/A |
| Martinez-Brocca et al[79] | Orbera | 12.7 ± 5.6 kg (4 mo) | 934.4 ± 199.2 pg/mL | 947.1 ± 195.1 pg/mL (1 mo) | N/A | N/A | 31.9 ± 16.4 ng/mL | 22.4 ± 15.1 ng/mL (1 mo) | N/A | N/A |
| Mion et al[78] | Orbera | 8.7 kg | 3.2 ± 0.4 ng/mL | N/A | 1.9 ± 0.1 ng/mL | N/A | 27.8 ± 3.7 ng/mL | N/A | 18.7 ± 2.7 ng/mL | N/A |
These factors are interrelated and participate in the peripheral mediation of satiety[76,77]. Ghrelin is a hormone that has been known to influence energy balance. However, several studies presented varying results regarding ghrelin concentrations after balloon treatment. A study with 40 obese patients who underwent balloon placement indicated no effect on ghrelin levels when patients were fasting or meal-suppressed[17]. In another study, 17 patients with non-morbid obesity underwent balloon placement, and fasting plasma ghrelin concentrations significantly decreased (3.2 to 1.9 ng/mL; P = 0.021) as a result[78]. Martinez-Brocca et al[79] reported that fasting and meal-suppressed plasma ghrelin levels did not differ significantly between groups in morbidly obese patients. Konopko-Zubrzycka et al[39] reported that body weight reduction after balloon treatment is related to a transient elevation in plasma ghrelin levels and a decrease in plasma leptin levels.
Another study evaluated fasting and postprandial cholecystokinin and pancreatic polypeptide secretion after 13 wk of balloon treatment in obese patients. Baseline and meal-stimulated cholecystokinin levels were decreased[18].
Maintenance of weight loss is controlled by the collaboration of several processes, including environmental, behavioral, and homeostatic factors[80]. Physiological adaptation to weight reduction and weight regain show interplaying alterations in the stability of the levels of hunger hormones and energy homeostasis, in addition to changes in subjective appetite and nutrient metabolism. Appetite-related gastrointestinal hormones may play a crucial function in body weight regain after weight reduction[81]. Bariatric procedures may prove to be effective methods to aid in altering obese patients’ physiology and may provide a good opportunity for long-term maintenance. Procedures that affect the physiology of weight loss and regain may eventually fortify future approaches for obesity treatment.
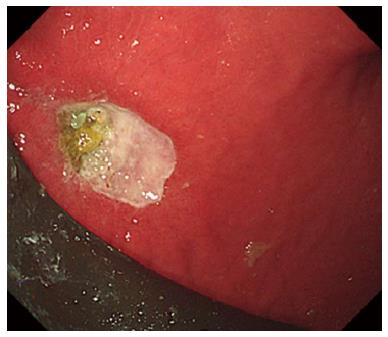
Adverse events rates following Orbera intragastric balloon placement showed pooled incidences of pain (33.7%) and nausea (29%) as common adverse side effects[82]. The incidence of GERD, gastric ulcers (Figure 2), and balloon migration was 18.3%, 2%, and 1.4%, respectively. Serious adverse events with the Orbera balloon are uncommon, with prevalences of small bowel obstruction, perforation, and death as 0.3%, 0.1% and 0.08%, respectively[82]. Similarly, the REDUCE pivotal trial (n = 264), which evaluated the efficacy and safety of the ReShape Duo intragastric balloon, showed that symptoms such as nausea, abdominal pain, and vomiting were common, but decreased rapidly with medical management. Spontaneous intragastric balloon deflation occurred in 6% of patients, but balloon migrations did not occur concurrently. Early balloon removal occurred in 9.1% of study participants for non-ulcer-related intolerance. In 35% of the study participants, a gastric ulcer presented initially, but ulcer incidence and size decreased following subsequent device modification[62].
New emerging technology trends in endoscopic treatment for obesity require an extensive and meticulous research plan to promote finding and recognize their optimal role in managing obese patients and their applications for clinical practice[83]. Intragastric balloon placement can be performed through a simple endoscopic method and is easily reversible. This simplicity offers an expansive role in obesity treatment based on the degree of obesity. It is important to establish an appropriate method for intragastric balloon treatment by classifying the degree of obesity. Although serious complications with intragastric balloon treatment are rare, safety is an issue that cannot be overlooked. Various factors are associated with balloon safety, such as its durability and the simplicity of the procedure. Advances in device properties and procedural techniques could offer more cost-effectiveness with this outpatient procedure[84]. There is also uncertainty about the ideal filling medium and content. A preference for fluid other than air has been mentioned[14], but a debate still exists, with no established guidelines to date. There is a need to establish standards of intragastric balloon treatment practice, which could comprise pre- and post-procedural guidelines and long-term schedule management. In addition, collaboration with dietary counseling and an exercise program could offer quality assurance with intragastric balloon treatment.
Intragastric balloon treatment might produce only short-lasting effects in obesity treatment. Thus, it is important to maintain weight loss following intragastric balloon removal. Long-term management for weight reduction after intragastric balloon removal can also comprise intensive lifestyle modification, alone or with pharmacotherapy, and could be suggested to protect against weight regain.
Material and safety issues regarding the intragastric balloon should be further investigated. More research should be performed to investigate a pathophysiologic pattern of obesity, the uncertain role of gut hormones, potential predictive factors for the efficacy of the intragastric balloon in obesity treatment, and individualized treatment-induced changes.
Effective and safe weight reduction is very important for the treatment of obesity, which is responsible for about 5% of all obesity-related deaths globally. Intragastric balloon treatment is more than just a transient curiosity. It shows promise in improving the quality of life and health status for obese patients. It offers a minimally invasive and effective method for managing obesity and associated conditions. Although there remains the possibility of improvement with research and development, gastroenterologists should maintain attention and interest in determining satisfactory outcomes and verifying standards of intragastric balloon treatment for the obesity epidemic.
P- Reviewer: Badiu C, Dogan UB, Farhat S, Shin JM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 698] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 2. | Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489-2496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 3. | Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1999] [Cited by in F6Publishing: 1922] [Article Influence: 128.1] [Reference Citation Analysis (1)] |
| 4. | Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 455] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 5. | Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 552] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 6. | Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64:1376-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Rankin W, Wittert G. Anti-obesity drugs. Curr Opin Lipidol. 2015;26:536-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Fujioka K. Current and emerging medications for overweight or obesity in people with comorbidities. Diabetes Obes Metab. 2015;17:1021-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Ochner CN, Gibson C, Carnell S, Dambkowski C, Geliebter A. The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity. Physiol Behav. 2010;100:549-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1083] [Article Influence: 108.3] [Reference Citation Analysis (1)] |
| 11. | Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1020] [Cited by in F6Publishing: 957] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 12. | Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016;12:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425-438.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Mathus-Vliegen EM. Endoscopic treatment: the past, the present and the future. Best Pract Res Clin Gastroenterol. 2014;28:685-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 15. | Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48:592-594. [PubMed] [Cited in This Article: ] |
| 16. | Geliebter A, Melton PM, McCray RS, Gage D, Heymsfield SB, Abiri M, Hashim SA. Clinical trial of silicone-rubber gastric balloon to treat obesity. Int J Obes. 1991;15:259-266. [PubMed] [Cited in This Article: ] |
| 17. | Mathus-Vliegen EM, Eichenberger RI. Fasting and meal-suppressed ghrelin levels before and after intragastric balloons and balloon-induced weight loss. Obes Surg. 2014;24:85-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Mathus-Vliegen EM, de Groot GH. Fasting and meal-induced CCK and PP secretion following intragastric balloon treatment for obesity. Obes Surg. 2013;23:622-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Machytka E, Chuttani R, Bojkova M, Kupka T, Buzga M, Stecco K, Levy S, Gaur S. Elipse™, a Procedureless Gastric Balloon for Weight Loss: a Proof-of-Concept Pilot Study. Obes Surg. 2016;26:512-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Mion F, Gincul R, Roman S, Beorchia S, Hedelius F, Claudel N, Bory RM, Malvoisin E, Trepo F, Napoleon B. Tolerance and efficacy of an air-filled balloon in non-morbidly obese patients: results of a prospective multicenter study. Obes Surg. 2007;17:764-769. [PubMed] [Cited in This Article: ] |
| 21. | Forestieri P, De Palma GD, Formato A, Giuliano ME, Monda A, Pilone V, Romano A, Tramontano S. Heliosphere Bag in the treatment of severe obesity: preliminary experience. Obes Surg. 2006;16:635-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | De Castro ML, Morales MJ, Del Campo V, Pineda JR, Pena E, Sierra JM, Arbones MJ, Prada IR. Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study. Obes Surg. 2010;20:1642-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Giardiello C, Borrelli A, Silvestri E, Antognozzi V, Iodice G, Lorenzo M. Air-filled vs water-filled intragastric balloon: a prospective randomized study. Obes Surg. 2012;22:1916-1919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Carvalho GL, Barros CB, Okazaki M, Novaes ML, Albuquerque PC, Almeida NC, Albuquerque PP, Wakiyama C, Vilaça TG, Silva JS. An improved intragastric balloon procedure using a new balloon: preliminary analysis of safety and efficiency. Obes Surg. 2009;19:237-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Gaggiotti G, Tack J, Garrido AB, Palau M, Cappelluti G, Di Matteo F. Adjustable totally implantable intragastric prosthesis (ATIIP)-Endogast for treatment of morbid obesity: one-year follow-up of a multicenter prospective clinical survey. Obes Surg. 2007;17:949-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2562] [Cited by in F6Publishing: 2340] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 27. | Mitura K, Garnysz K. In search of the ideal patient for the intragastric balloon - short- and long-term results in 70 obese patients. Wideochir Inne Tech Maloinwazyjne. 2016;10:541-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ginsberg GG, Chand B, Cote GA, Dallal RM, Edmundowicz SA, Nguyen NT, Pryor A, Thompson CC. A pathway to endoscopic bariatric therapies. Gastrointest Endosc. 2011;74:943-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Poobalan A, Aucott L, Smith WC, Avenell A, Jung R, Broom J, Grant AM. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes--a systematic review. Obes Rev. 2004;5:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007;8:503-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg. 2008;18:1611-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766-R769. [PubMed] [Cited in This Article: ] |
| 33. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [PubMed] [Cited in This Article: ] |
| 34. | Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1709] [Cited by in F6Publishing: 1493] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 35. | Bonazzi P, Petrelli MD, Lorenzini I, Peruzzi E, Nicolai A, Galeazzi R. Gastric emptying and intragastric balloon in obese patients. Eur Rev Med Pharmacol Sci. 2005;9:15-21. [PubMed] [Cited in This Article: ] |
| 36. | Genco A, Balducci S, Bacci V, Materia A, Cipriano M, Baglio G, Ribaudo MC, Maselli R, Lorenzo M, Basso N. Intragastric balloon or diet alone? A retrospective evaluation. Obes Surg. 2008;18:989-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Genco A, Cipriano M, Materia A, Bacci V, Maselli R, Musmeci L, Lorenzo M, Basso N. Laparoscopic sleeve gastrectomy versus intragastric balloon: a case-control study. Surg Endosc. 2009;23:1849-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Göttig S, Daskalakis M, Weiner S, Weiner RA. Analysis of safety and efficacy of intragastric balloon in extremely obese patients. Obes Surg. 2009;19:677-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Konopko-Zubrzycka M, Baniukiewicz A, Wróblewski E, Kowalska I, Zarzycki W, Górska M, Dabrowski A. The effect of intragastric balloon on plasma ghrelin, leptin, and adiponectin levels in patients with morbid obesity. J Clin Endocrinol Metab. 2009;94:1644-1649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Ohta M, Kitano S, Kai S, Shiromizu A, Eguchi H, Endo Y, Masaki T, Kakuma T, Yoshimatsu H. Initial Japanese experience with intragastric balloon placement. Obes Surg. 2009;19:791-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Coskun H, Bostanci O. Assessment of the application of the intragastric balloon together with sibutramine: a prospective clinical study. Obes Surg. 2010;20:1117-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Mui WL, Ng EK, Tsung BY, Lam CH, Yung MY. Impact on obesity-related illnesses and quality of life following intragastric balloon. Obes Surg. 2010;20:1128-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Lopez-Nava G, Rubio MA, Prados S, Pastor G, Cruz MR, Companioni E, Lopez A. BioEnterics® intragastric balloon (BIB®). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes Surg. 2011;21:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg. 2012;22:896-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 45. | Papavramidis TS, Grosomanidis V, Papakostas P, Penna S, Kotzampassi K. Intragastric balloon fundal or antral position affects weight loss and tolerability. Obes Surg. 2012;22:904-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Genco A, Dellepiane D, Baglio G, Cappelletti F, Frangella F, Maselli R, Dante MC, Camoirano R, Lorenzo M, Basso N. Adjustable intragastric balloon vs non-adjustable intragastric balloon: case-control study on complications, tolerance, and efficacy. Obes Surg. 2013;23:953-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Gümürdülü Y, Doğan ÜB, Akın MS, Taşdoğan BE, Yalakı S. Long-term effectiveness of BioEnterics intragastric balloon in obese patients. Turk J Gastroenterol. 2013;24:387-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Fuller NR, Lau NS, Denyer G, Caterson ID. An intragastric balloon produces large weight losses in the absence of a change in ghrelin or peptide YY. Clin Obes. 2013;3:172-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Totté E, Hendrickx L, Pauwels M, Van Hee R. Weight reduction by means of intragastric device: experience with the bioenterics intragastric balloon. Obes Surg. 2001;11:519-523. [PubMed] [Cited in This Article: ] |
| 50. | Sallet JA, Marchesini JB, Paiva DS, Komoto K, Pizani CE, Ribeiro ML, Miguel P, Ferraz AM, Sallet PC. Brazilian multicenter study of the intragastric balloon. Obes Surg. 2004;14:991-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Crea N, Pata G, Della Casa D, Minelli L, Maifredi G, Di Betta E, Mittempergher F. Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg. 2009;19:1084-1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Fuller NR, Pearson S, Lau NS, Wlodarczyk J, Halstead MB, Tee HP, Chettiar R, Kaffes AJ. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity (Silver Spring). 2013;21:1561-1570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Al Kahtani K, Khan MQ, Helmy A, Al Ashgar H, Rezeig M, Al Quaiz M, Kagevi I, Al Sofayan M, Al Fadda M. Bio-enteric intragastric balloon in obese patients: a retrospective analysis of King Faisal Specialist Hospital experience. Obes Surg. 2010;20:1219-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Genco A, López-Nava G, Wahlen C, Maselli R, Cipriano M, Sanchez MM, Jacobs C, Lorenzo M. Multi-centre European experience with intragastric balloon in overweight populations: 13 years of experience. Obes Surg. 2013;23:515-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Herve J, Wahlen CH, Schaeken A, Dallemagne B, Dewandre JM, Markiewicz S, Monami B, Weerts J, Jehaes C. What becomes of patients one year after the intragastric balloon has been removed? Obes Surg. 2005;15:864-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Tai CM, Lin HY, Yen YC, Huang CK, Hsu WL, Huang YW, Chang CY, Wang HP, Mo LR. Effectiveness of intragastric balloon treatment for obese patients: one-year follow-up after balloon removal. Obes Surg. 2013;23:2068-2074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Dastis NS, François E, Deviere J, Hittelet A, Ilah Mehdi A, Barea M, Dumonceau JM. Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy. 2009;41:575-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Gaur S, Levy S, Mathus-Vliegen L, Chuttani R. Balancing risk and reward: a critical review of the intragastric balloon for weight loss. Gastrointest Endosc. 2015;81:1330-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Dogan UB, Gumurdulu Y, Akin MS, Yalaki S. Five percent weight lost in the first month of intragastric balloon treatment may be a predictor for long-term weight maintenance. Obes Surg. 2013;23:892-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Lopez-Nava G, Bautista-Castaño I, Jimenez-Baños A, Fernandez-Corbelle JP. Dual Intragastric Balloon: Single Ambulatory Center Spanish Experience with 60 Patients in Endoscopic Weight Loss Management. Obes Surg. 2015;25:2263-2267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9:290-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 63. | Machytka E, Klvana P, Kornbluth A, Peikin S, Mathus-Vliegen LE, Gostout C, Lopez-Nava G, Shikora S, Brooks J. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic weight loss management. Obes Surg. 2011;21:1499-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg. 2014;24:813-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Carvalho GL, Barros CB, Moraes CE, Okazaki M, Ferreira Mde N, Silva JS, de Albuquerque PP, Coelho Rde M. The use of an improved intragastric balloon technique to reduce weight in pre-obese patients--preliminary results. Obes Surg. 2011;21:924-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Mion F, Ibrahim M, Marjoux S, Ponchon T, Dugardeyn S, Roman S, Deviere J. Swallowable Obalon® gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg. 2013;23:730-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Farina MG, Baratta R, Nigro A, Vinciguerra F, Puglisi C, Schembri R, Virgilio C, Vigneri R, Frittitta L. Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity. Obes Surg. 2012;22:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18:841-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 69. | Forlano R, Ippolito AM, Iacobellis A, Merla A, Valvano MR, Niro G, Annese V, Andriulli A. Effect of the BioEnterics intragastric balloon on weight, insulin resistance, and liver steatosis in obese patients. Gastrointest Endosc. 2010;71:927-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Angrisani L, Lorenzo M, Borrelli V, Giuffré M, Fonderico C, Capece G. Is bariatric surgery necessary after intragastric balloon treatment? Obes Surg. 2006;16:1135-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Mathus-Vliegen EM. Intragastric balloon treatment for obesity: what does it really offer? Dig Dis. 2008;26:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Ganesh R, Rao AD, Baladas HG, Leese T. The Bioenteric Intragastric Balloon (BIB) as a treatment for obesity: poor results in Asian patients. Singapore Med J. 2007;48:227-231. [PubMed] [Cited in This Article: ] |
| 73. | Younossi ZM, Diehl AM, Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology. 2002;35:746-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 74. | Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, Wee A, Lim SG, Ho KY. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76:756-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Ochner CN, Kwok Y, Conceição E, Pantazatos SP, Puma LM, Carnell S, Teixeira J, Hirsch J, Geliebter A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 76. | Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116-2130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 77. | Delzenne N, Blundell J, Brouns F, Cunningham K, De Graaf K, Erkner A, Lluch A, Mars M, Peters HP, Westerterp-Plantenga M. Gastrointestinal targets of appetite regulation in humans. Obes Rev. 2010;11:234-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 78. | Mion F, Napoléon B, Roman S, Malvoisin E, Trepo F, Pujol B, Lefort C, Bory RM. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes Surg. 2005;15:510-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Martinez-Brocca MA, Belda O, Parejo J, Jimenez L, del Valle A, Pereira JL, Garcia-Pesquera F, Astorga R, Leal-Cerro A, Garcia-Luna PP. Intragastric balloon-induced satiety is not mediated by modification in fasting or postprandial plasma ghrelin levels in morbid obesity. Obes Surg. 2007;17:649-657. [PubMed] [Cited in This Article: ] |
| 80. | Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 81. | Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martínez JA, Casanueva FF. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95:5037-5044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Abu Dayyeh BK, Edmundowicz SA, Jonnalagadda S, Kumar N, Larsen M, Sullivan S, Thompson CC, Banerjee S. Endoscopic bariatric therapies. Gastrointest Endosc. 2015;81:1073-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 83. | Kim SH, Chun HJ. Endoscopic Treatment for Obesity: New Emerging Technology Trends. Gut Liver. 2015;9:431-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Dai SC, Paley M, Chandrasekhara V. Intragastric balloons: an introduction and removal technique for the endoscopist. Gastrointest Endosc. 2015;82:1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Bužga M, Evžen M, Pavel K, Tomáš K, Vladislava Z, Pavel Z, Svagera Z. Effects of the intragastric balloon MedSil on weight loss, fat tissue, lipid metabolism, and hormones involved in energy balance. Obes Surg. 2014;24:909-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Nikolic M, Boban M, Ljubicic N, Supanc V, Mirosevic G, Pezo Nikolic B, Krpan R, Posavec L, Zjacic-Rotkvic V, Bekavac-Beslin M. Morbidly obese are ghrelin and leptin hyporesponders with lesser intragastric balloon treatment efficiency: ghrelin and leptin changes in relation to obesity treatment. Obes Surg. 2011;21:1597-1604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |