Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.5079
Peer-review started: March 13, 2016
First decision: March 21, 2016
Revised: March 31, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 7, 2016
Processing time: 78 Days and 24 Hours
AIM: To assess the risk of relapse in ulcerative colitis (UC) patients in clinical remission using mucosal status and fecal immunochemical test (FIT) results.
METHODS: The clinical outcomes of 194 UC patients in clinical remission who underwent colonoscopy were based on evaluations of Mayo endoscopic subscores (MESs) and FIT results.
RESULTS: Patients with an MES of 0 (n = 94, 48%) showed a ten-fold lower risk of relapse than those with an MES of 1-3 (n = 100, 52%) (HR = 0.10, 95%CI: 0.05-0.19). A negative FIT result (fecal hemoglobin concentrations ≤ 100 ng/mL) was predictive of patients with an MES of 0, with a sensitivity of 0.94 and a specific of 0.76. Moreover, patients with a negative FIT score had a six-fold lower risk of clinical relapse than those with a positive score (HR = 0.17, 95%CI: 0.10-0.28). Inclusion of the distinguishing parameter, sustaining clinical remission > 12 mo, resulted in an even stronger correlation between negative FIT results and an MES of 0 with respect to the risk of clinical relapse (HR = 0.11, 95%CI: 0.04-0.23).
CONCLUSION: Negative FIT results one year or more after remission induction correlate with complete mucosal healing (MES 0) and better prognosis. Performing FIT one year after remission induction may be useful for evaluating relapse risk.
Core tip: Mucosal healing has been recognized as the treatment goal of. In this study, the relapse rate differed greatly between patients with a Mayo endoscopic subscore (MES) of 0 and an MES of 1 such that mucosal healing should be defined as an MES of 0. We previously reported that a negative fecal immunochemical test (FIT) correlates positively with mucosal healing. This paper indicated that patients with a negative FIT demonstrated a lower risk of clinical relapse than those with a positive FIT and that the risk of relapse in patients in prolonged remission and with a negative FIT was equivalent to that of patients with an MES of 0.
- Citation: Nakarai A, Kato J, Hiraoka S, Takashima S, Takei D, Inokuchi T, Sugihara Y, Takahara M, Harada K, Okada H. Ulcerative colitis patients in clinical remission demonstrate correlations between fecal immunochemical test results, mucosal healing, and risk of relapse. World J Gastroenterol 2016; 22(21): 5079-5087
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/5079.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.5079
Ulcerative colitis (UC) is an idiopathic chronic inflammatory disorder that, when untreated, results in symptoms of diarrhea and bloody stool. Current studies evaluating UC treatment using colonoscopy cite a need to achieve not only clinical responses but also mucosal healing, which is associated with sustained clinical remission and reduced rates of hospitalization and surgical resection[1]. An additional study indicated that early mucosal healing after the administration of infliximab for UC correlates with improved clinical outcomes, including the avoidance of colectomy[2]. Another report showed that a lack of mucosal healing after initial corticosteroid therapy is associated with late negative outcomes[3].
Nevertheless, standardized criteria for evaluating disease severity and the degree of mucosal healing are not presently available[4]. Some reports define mucosal healing as an MES of 0 or 1[2,5], whereas other reports consider only an MES of 0 to be healing[6]. Such inconsistencies complicate interpretations of the significance of mucosal healing in the treatment of UC such that differences in long-term prognosis (evaluated by relapse of clinical symptoms and/or colectomy) between clinically asymptomatic patients with an MES of 0 (complete mucosal healing) and those with an MES of 1 (partial mucosal healing) are rarely reported.
Using colonoscopy to evaluate mucosal status in UC patients is expensive and invasive. Previous work reported by our group demonstrates that a quantitative fecal occult blood test (FIT) effectively reflects the mucosal status of patients with UC and that a negative FIT correlates strongly with mucosal healing[7]. Although we found a significantly higher positive correlation between negative FIT results and an MES of 0 (> 90%) compared with an MES of 0 or 1 (< 60%), the likelihood of relapse in patients in remission with a negative FIT has not been formally evaluated.
In this study, we retrospectively reevaluated and subdivided the colonoscopic findings of UC patients in clinical remission into subcategories of MES 0 or MES 1. Patient prognoses (relapse of clinical symptoms and colectomy rate) were evaluated to determine whether the optimal goal of UC treatment should be either an MES of 0 or 1 or only an MES of 0. Correlations between FIT results and MES in these patients were also evaluated to determine whether FIT scores can function as a surrogate marker for meeting the treatment goals of UC patients in clinical remission.
Between January 2006 and January 2014, ambulatory UC patients who were making periodic visits to Okayama University Hospital were requested to prepare and bring fecal samples to scheduled colonoscopy appointments for an evaluation of disease activity and surveillance. Fecal samples (prior to colonoscopy bowel preparation) were tested for fecal occult blood with an FIT, and the results were evaluated with regard to colonoscopic findings. All of the patients had an established diagnosis of UC according to endoscopic and histologic assessments and had received medical therapy.
Clinical disease activity was scored using the Mayo score, which is based on the following four criteria: stool frequency, rectal bleeding, endoscopic findings, and physician global assessment (0, normal; 1, mild disease; 2, moderate disease; 3, severe disease)[8]. Clinical remission was defined as a partial Mayo score (Mayo score without endoscopic findings) ≤ 2 points, with no individual subscore exceeding 1 point[5]. Clinical relapse was defined by an increase or modification of concomitant medications due to a worsening of symptoms. Patients in clinical remission at the time of colonoscopy were considered eligible for this retrospective cohort study. The study protocol was approved by the Institutional Review Board of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences.
The details of the method used for the FIT were described previously[9-11]. Briefly, patients prepared fecal samples before bowel preparation for colonoscopy using an OC-Hemodia sampling probe (Eiken Chemical, Tokyo, Japan) provided by the manufacturer of the kit. An 8 cm × 2 cm test tube-shaped container holds the sampling probe. The patient inserts the probe into several different areas of stool and then firmly places it back into the tube for sealing. The probe tip with the fecal sample is suspended in a standard volume of hemoglobin-stabilizing buffer. Submitted stool samples were immediately processed and examined using OC-SENSOR neo (Eiken Chemical), which can accurately measure fecal hemoglobin concentrations of 50 ng/mL to 1000 ng/mL. Fecal specimens with a hemoglobin concentration over 1000 ng/mL were measured following dilution. Because FIT results are inaccurate at hemoglobin concentrations below 50 ng/mL, specimens with a hemoglobin concentration in this range were categorized as one (0-50 ng/mL).
On the day of the colonoscopy, patients received a polyethylene glycol-based or magnesium citrate-based electrolyte solution for bowel preparation and ingested it according to the manufacturer’s instructions. After colonic lavage, the patients underwent colonoscopy. Patients were excluded if the colonoscopic examination was incomplete due to problems with the bowel preparation or if the colonoscope could not be inserted into the cecum. At colonoscopy, the colonoscopists were not blinded to the clinical data. However, at data collection for analysis, colonoscopic images were re-evaluated by experienced colonoscopists who did not know the clinical data.
The mucosal status of UC was assessed using the MES classification system. Evaluation was performed at each portion of the colorectum (cecum; ascending, transverse, descending and sigmoid colon; and rectum), and the maximum score in the colorectum of each patient was used for analysis. An MES of “0” throughout the colorectum was defined as complete mucosal healing, whereas a maximum MES of “1” in the colorectum was defined as partial mucosal healing.
Statistical analyses were performed using JMP version 9 (SAS Institute, Cary, NC, United States). A Kaplan-Meier curve estimating the duration of sustained clinical remission was generated for each predefined patient group, and comparisons between groups were performed using a 2-sided log-rank test. The Cox proportional hazards regression model was used to calculate hazard ratios (HRs) between groups, quantifying the likelihood of clinical relapse using a 95%CI. Comparative analyses, such as a χ2 test and the Mann-Whitney test, were used for cross-sectional analysis of categorical data. Spearman rank correlation was performed to measure the association between fecal hemoglobin concentrations and MES, and trends between these values were evaluated using the Cochran-Armitage trend test. A receiver operating characteristic (ROC) curve was generated to estimate appropriate cutoff values for the FIT. The area under the curve (AUC), and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), for detecting mucosal healing based on the FIT results was calculated. All P-values were two-sided and considered significant when less than 0.05.
A total of 248 UC patients who underwent colonoscopy between January 2006 and January 2014 also underwent a corresponding FIT. If a patient underwent two or more colonoscopies during remission, then only the data from the first colonoscopy were included. Among these patients, 194 (78%) demonstrated clinical remission at the time of colonoscopy and were enrolled in the study.
Table 1 summarizes the clinical characteristics of the 194 patients (99 male and 95 female; median age at UC onset 32 years). At the time of colonoscopy, the median duration of sustaining clinical remission was 11 mo. Colonoscopic findings revealed that the maximum MES was 0 in 94 (49%) cases, 1 in 57 (29%) cases, 2 in 39 (20%) cases, and 3 in 7 (2%) cases. On the basis of our definitions, 94 and 57 patients had complete and partial mucosal healing, respectively. Among the 194 patients, 111 (57%) cases showed fecal hemoglobin concentrations of 100 ng/mL or lower and were defined as FIT-negative.
| Total | 194 |
| Gender, n (%) | |
| Male | 99 (51) |
| Female | 95 (49) |
| Extent of disease, n (%) | |
| Pancolitis | 125 (64) |
| Left-side colitis | 48 (25) |
| Proctitis | 21 (11) |
| Median (IQR) age at onset | 32 (22-43) |
| Median (IQR) duration of disease, months | 107 (51-194) |
| Median (IQR) age of undergoing colonoscopy | 44 (33-56) |
| Median (IQR) duration of sustaining clinical remission at the time of colonoscopy, months | 11 (6-23) |
| Concomitant medications, n (%) | |
| Aminosalicylate | 178 (92) |
| Corticosteroids | 27 (14) |
| Mercaptopurine/Azathioprine | 79 (41) |
| Tacrolimus | 10 (5) |
| Biologics | 9 (5) |
| Colonoscopy findings, n (%) (maximum index in the colorectum) | |
| MES 0 | 94 (49) |
| MES 1 | 57 (29) |
| MES 2 | 39 (20) |
| MES 3 | 4 (2) |
| Fecal Hb concentrations (ng/mL), n (%) | |
| 0-100 | 111 (57) |
| 101-1000 | 56 (29) |
| 1001-10000 | 20 (10) |
| 10001- | 7 (4) |
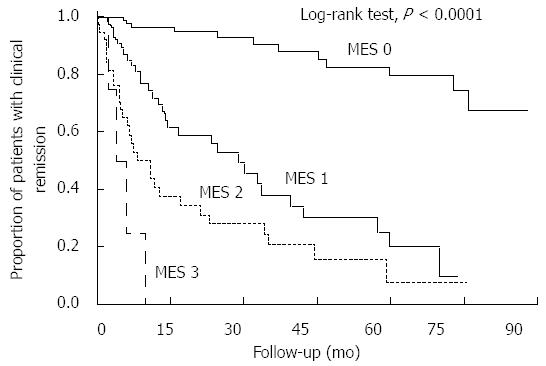
Kaplan-Meier curves comparing the maintenance of clinical remission among patients with an MES of 0-3 are shown in Figure 1. There was a statistically significant difference in remission maintenance rates between each MES group (P < 0.0001, log-rank test). The Cox proportional hazards model suggested that patients with an MES of 1 were more than seven times more likely to relapse than patients with an MES of 0 (HR of MES 1 vs MES 0, 7.40; 95%CI: 3.78-15.06). Conversely, MES 0 patients were approximately ten times less likely to relapse than MES 1-3 patients (HR = 0.10; 95%CI: 0.05-0.19). Furthermore, the risk of colectomy or the occurrence of dysplasia/cancer did not vary significantly between patients in different MES groups (data not shown). Overall, these results suggest that the treatment goal for minimizing relapse in UC patients in clinical remission should be to achieve a score of MES 0, rather than MES 1.
We have shown that achievement of complete mucosal healing (MES 0) is optimal for UC patients with regard to the maintenance of clinical remission. We also compared other clinical characteristics of the 94 patients in clinical remission with complete mucosal healing (MES 0) with those of 100 patients who showed only partial healing (MES 1) or more inflammation (MES 2, 3) (Table 2). The former subgroup had maintained clinical remission for a significantly longer time at the time of colonoscopy (17 mo vs 9 mo, P < 0.0001) and was administered mercaptopurine/azathioprine more frequently than the latter (45 patients vs 34 patients, P = 0.049). The FIT results demonstrated that fecal hemoglobin concentrations were significantly lower (50 ng/mL vs 315 ng/mL, P < 0.0001) in patients with complete mucosal healing than in patients with partial healing or more inflammation.
| Characteristics | MES 0 | MES 1-3 | P value |
| (n = 94) | (n = 100) | ||
| Gender, n (%) | |||
| Male | 54 (57) | 45 (45) | 0.083 |
| Female | 40 (43) | 55 (55) | |
| Median age, yr (IQR) | 46 (32-60) | 41 (33-51) | 0.051 |
| Median duration of disease, mo (IQR) | 99 (53-198) | 112 (44-191) | 0.950 |
| Median age at onset, yr (IQR) | 34 (22-47) | 31 (22-40) | 0.130 |
| Median duration of sustaining clinical remission at the time of colonoscopy, mo (IQR) | 17 (9-33) | 9 (4-13) | < 0.0001 |
| Extent of disease, n (%) | |||
| Pancolitis | 59 (63) | 66 (66) | 0.340 |
| Left-side colitis | 27 (29) | 21 (21) | |
| Proctitis | 8 (8) | 13 (13) | |
| Concomitant medications, n (%) | |||
| Aminosalicylate | 86 (91) | 92 (92) | 0.900 |
| Corticosteroids | 11 (12) | 16 (16) | 0.390 |
| Mercaptopurine/Azathioprine | 45 (48) | 34 (34) | 0.049 |
| Tacrolimus | 4 (4) | 6 (6) | 0.580 |
| Biologics | 2 (2) | 7 (7) | 0.110 |
| Fecal Hb concentrations (ng/mL) | 50 (4-50) | 315 (108-1277) | < 0.0001 |
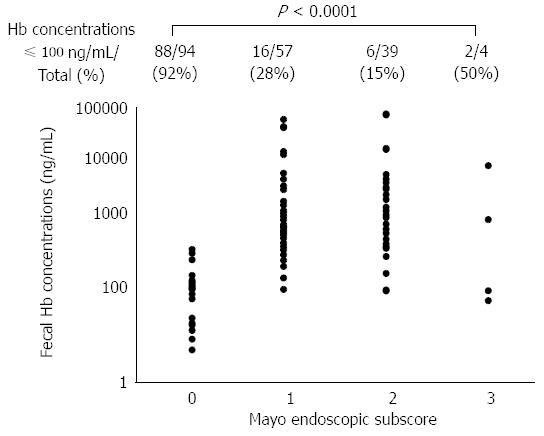
Our data demonstrated that MES 0 patients in clinical remission presented significantly lower fecal hemoglobin concentrations than MES 1-3 patients. The correlation between FIT results and colonoscopic findings among these patient subgroups is illustrated in Figure 2. The Spearman rank correlation coefficient quantifying the relationship between FIT values and MES subgroups was 0.6530 (P < 0.0001). The proportion of cases with fecal hemoglobin concentrations ≤ 100 ng/mL was greatest in the MES 0 patients (88/94, 92%), and decreased gradually as the MES increased (MES 1: 16/57, 28%; MES 2: 6/39, 15%). The trend of the decrease in the relationship to the MES was statistically significant (P < 0.0001, Cochran-Armitage trend test).
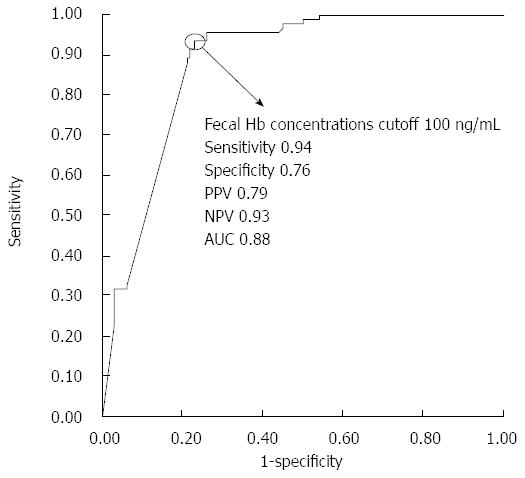
Figure 3 shows the ROC curve for fecal hemoglobin concentrations in relation to complete mucosal healing. A cutoff value of 100 ng/mL was shown to effectively differentiate between patients with and without complete mucosal healing at a sensitivity of 0.94 and a specificity of 0.76. The PPV of applying 100 ng/mL as a cut-off for determining complete mucosal healing was 0.79, whereas the NPV was 0.93. The corresponding AUC was 0.88.
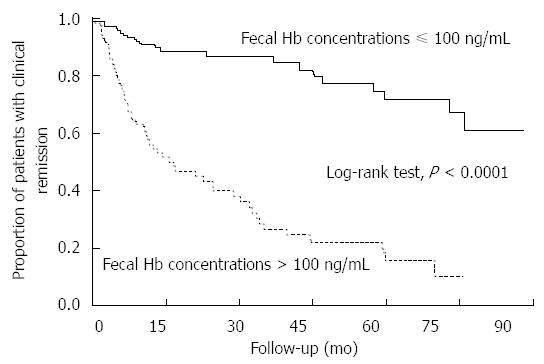
In addition to the results relating FIT to mucosal healing, Kaplan-Meier curves showed that the cumulative remission maintenance rate was also significantly different between patients with fecal hemoglobin concentrations ≤ 100 ng/mL and those with fecal hemoglobin concentrations > 100 ng/mL (P < 0.0001, log-rank test; Figure 4). The Cox proportional hazards model indicated that patients with a negative FIT value had a six-fold lower risk of clinical relapse than those with a positive FIT (HR = 0.17; 95%CI: 0.10-0.28).
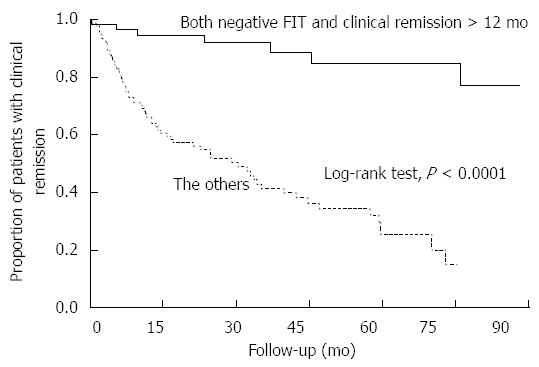
In UC patients in clinical remission, negative FIT values correlate closely with an MES of 0, and patients in either clinical category demonstrate a better prognosis. However, as a marker for a reduced risk of UC relapse, a rating of MES 0 is slightly more accurate in predicting risk than a negative FIT value (HR = 0.10 vs 0.17). In a comparison between Kaplan-Meier curves of MES 0 (Figure 1) and negative FIT (Figure 4), the relapse rate within one year after colonoscopy/FIT was slightly higher in patients with a negative FIT than in those with an MES of 0 (1 year relapse rate 9% vs 3%, and 5 year relapse rate 22% vs 17%, respectively). In addition, the duration of sustaining clinical remission at colonoscopy/FIT was significantly longer in patients with an MES of 0 than in those with an MES of 1-3 among patients with a negative FIT (16 mo vs 8 mo, P = 0.001). These findings suggest that patients who enter clinical remission are more likely to demonstrate a negative FIT first (cessation of colorectal bleeding), followed by complete mucosal healing.
Because the MES 0 patients sustained clinical remission for a longer time than the MES 1-3 patients, we performed multivariate analysis and found that clinical remission > 12 mo was a significant factor for predicting an MES of 0 among our 194 subjects (OR = 8.47; 95%CI: 3.33-24.03). Thus, we used Kaplan-Meier curves to illustrate the relationship between the patients who fulfilled both classifiers - negative FIT and clinical remission > 12 mo - and all others (Figure 5). The Cox proportional hazards model indicated that patients with both a negative FIT and clinical remission > 12 mo have a nine-fold lower risk of relapse than all other patients (HR = 0.11, 95%CI: 0.04-0.23). The one-year and five-year relapse rates for this group (FIT negative, remission > 12 mo) were similar to those of patients with MES 0 (4% and 15%, respectively). Taken together, these findings suggest that a negative FIT in patients who have sustained clinical remission for at least one year is a good indicator of complete mucosal healing and a predictor of low relapse risk.
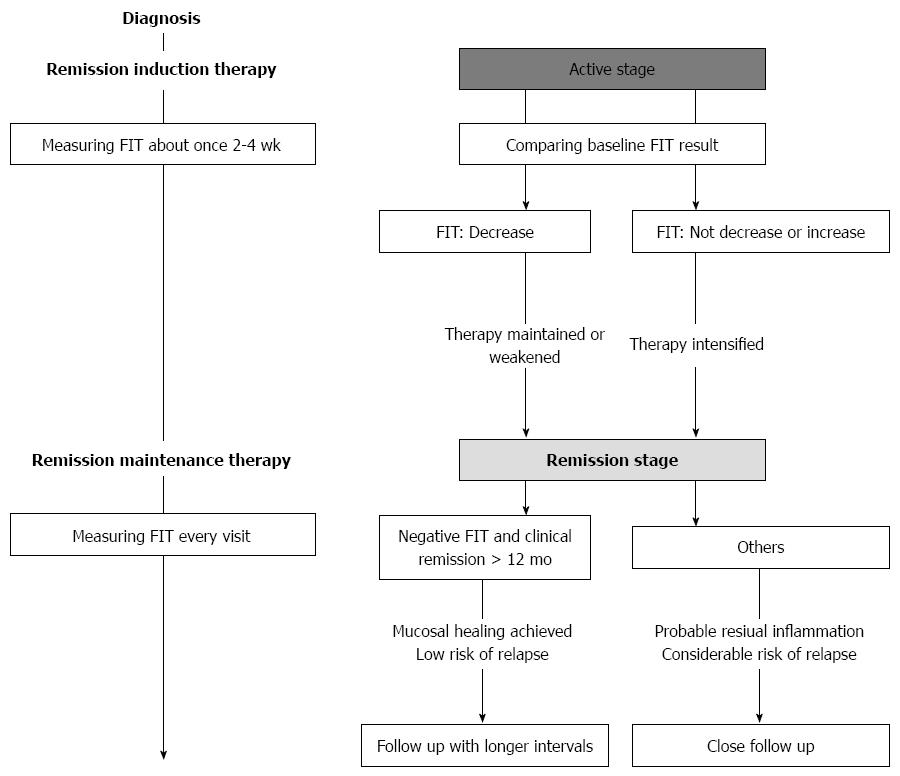
Figure 6 indicated the proposed workflow of the follow-up of UC patients using FIT. During remission induction therapy, we recommend to measure FIT about once 2-4 wk, comparing those results to the baseline FIT result. When FIT results decrease, we make therapy maintained or weakened. On the other hand, when FIT results do not decrease or increase, therapy should be considered to intensify. After remission induction, we recommend to measure FIT every visit. Since patients with both negative FIT and clinical remission > 12 mo are highly probable to have achieved mucosal healing with low risk of relapse, these patients could be followed with longer intervals. Otherwise, patients are considered to have residual inflammation with considerable risk of relapse, they need to be followed up closely.
In this study, our goal was to distinguish which MES scores represent optimal mucosal healing - an MES of 0 alone or an MES of 0 or 1. In addition, because we previously reported that FIT can function as a predictor of mucosal status in UC patients, we further discriminated the predictability of the FIT as a measure of mucosal status and of the prognosis of patients with clinical remission. In retrospective analyses of the differences in long-term clinical outcomes between patients with an MES of 0 and those with an MES 1, we found that negative FIT results were significantly more likely in patients with complete mucosal healing (MES 0) than in patients with partial mucosal healing (MES 1). Our findings showed that patients with an MES of 0 alone were much less likely to relapse and that negative FIT results showed a stronger positive correlation with an MES of 0 alone than with an MES of 0 or 1, as well as with a reduced risk of relapse. Moreover, analyses including the parameter “sustaining clinical remission > 12 mo” revealed a more robust correlation between negative FIT results and complete mucosal healing with regard to the minimum risk of relapse.
Standardized criteria for evaluating the severity of ulcerative colitis and for defining mucosal healing in patients with UC have yet to be established[4]. Many prior clinical studies have defined mucosal healing as maintaining an MES of 0 or 1[2,5], and there are few long-term studies distinguishing the ability of an MES of 0 or an MES of 1 to contribute to the maintenance of clinical remission in UC patients. Detailed analysis of findings from the Active Ulcerative Colitis Trials (ACT-1, 2)[2] showed that the clinical remission rate at 54 wk from the time of colonoscopy (performed after induction of remission at week 8) was 73% in MES 0 patients and 47% in MES 1 patients; more than half of the MES 1 patients relapsed. In addition, other groups including our group, reported that patients in clinical remission with an MES of 0 were less likely to relapse than those with an MES of 1 or more, using a retrospective cohort[12,13]. In contrast, in a study evaluating the effectiveness of mesalazine for maintaining UC remission, Meucci et al[6] reported no significant difference between the rates of relapse at one year in MES 0 vs MES 1 patients.
Negative FIT results among the subjects in clinical remission also correlated closely with an MES of 0, and the patients in clinical remission who showed a negative FIT result were less likely to relapse than those with a positive FIT. These results suggest that a negative FIT result may function as a surrogate marker for complete mucosal healing and should be the treatment goal for UC patients in remission.
Nevertheless, our data do not show a complete overlap between the clinical behavior of patients with negative FIT results and that of MES 0 patients as the former are more likely to relapse within one year after colonoscopy/FIT than the latter. In addition, MES 0 patients with a negative FIT at colonoscopy/FIT maintained clinical remission significantly longer than MES 1-3 patients. These results suggest that UC patients who enter clinical remission achieve a negative FIT first, followed by an MES of 0 (complete healing). The relapse rate among patients with a negative FIT who sustained clinical remission for one year or more was the same as the rate among MES 0 patients.
On the basis of our findings, we recommend that UC patients undergo an FIT one year after induction of clinical remission. If the FIT result is negative, then colonoscopy can be safely skipped, and the optimal treatment goal of complete mucosal healing should be considered met. If the FIT result is positive, then physicians should consider performing a colonoscopy or intensifying treatment. Thus, the FIT (an easy, non-invasive and low-cost test) may function advantageously as a substitute for endoscopy to measure mucosal status and risk of relapse in UC patients one year after remission.
There is accumulating evidence that fecal calprotectin, a major protein found in the cytosol of inflammatory cells, is an effective pioneer and is useful for assessing intestinal inflammation[14-16]. Several studies have reported that fecal calprotectin values can predict relapse in UC patients in clinical remission[17-21]. Although these reports indicated that patients with higher fecal calprotectin levels were more likely to relapse within several to 12 mo, no correlation between fecal calprotectin and mucosal status was identified because endoscopic examinations were not included in the studies.
Other reports, which indicate that fecal calprotectin levels can predict endoscopic mucosal healing[22-24], did not investigate risk of relapse. Thus, clear evidence defining the relationship between fecal markers, mucosal status and risk of relapse is lacking. In contrast, our analyses of FIT results as a marker for complete mucosal healing include all 3 variables: Negative FIT results one year or more after UC remission correlated with complete mucosal healing and also with a minimum risk of relapse. Because fecal calprotectin has recently been reported to also correlate with the presence of histological inflammation[25], testing the correlation between negative FIT results and histological remission is one of our future aims.
Reports comparing fecal hemoglobin and calprotectin levels directly as predictors of mucosal status are scarce. Mooiweer et al[26] demonstrated that both markers are similarly effective in identifying inflammatory bowel disease (IBD) patients with active endoscopic inflammation. However, to the best of our knowledge, no reports have compared the predictability of mucosal healing and/or risk of relapse between the two fecal markers. To further understand the roles of these markers in the clinical management of IBD, we aim to conduct such comparative studies in the future.
The FIT has particular advantages over fecal calprotectin testing: Fetal calprotectin is measured using an enzyme-linked immunosorbent assay (ELISA), which is time-consuming and requires specialized techniques, whereas the FIT can be easily measured automatically in a few minutes. In addition, there is significant inter- and intra-assay variability in measures of fecal calprotectin levels using different ELISA diagnostic kits [such as PhiCal Calprotectin ELISA (R-Biopharm, Darmstadt, Germany), Calprest (Eurospital, Trieste, Italy), and Calprotectin ELISA (Bühlmann, Basel, Switzerland)]. The lack of an established assay kit and an optimal cutoff value for detecting mucosal healing/inflammation in UC patients is another major limitation of fecal calprotectin[14-16]. In this regard, the FIT used in this study is the most widely used system worldwide (OC-Sensor neo), and it maintains a consistent standard cutoff (100 ng/mL) for CRC screening that can also be applied as a robust evaluator of mucosal healing in UC patients.
A retrospective design and single-hospital dataset analyses are limitations of this study. However, we argue that the observational nature of the study, which did not require interventions in clinical practice, should limit bias in the results. Despite its limitations, our study revealed that the clinical prognosis of UC patients in remission differs between patients with complete endoscopic remission (MES 0) and those without (MES 1-3). We also demonstrate that in patients who are one year or more removed from UC remission induction, there is a strong positive correlation between negative FIT results, an MES of 0 and better prognosis. We suggest performing the noninvasive FIT in UC patients in prolonged remission (in place of endoscopy) to simplify the assessment of healing and meeting of treatment goals. These findings may greatly improve clinical practice in the evaluation of UC patients, particularly those in clinical remission.
Mucosal healing is a treatment goal for better prognosis in ulcerative colitis (UC). The authors previously reported that the quantitative fecal immunochemical test (FIT) effectively reflects the mucosal status of UC and that a negative FIT strongly correlates with mucosal healing.
Currently, the definition of mucosal healing is not yet standardized. Some reports have defined mucosal healing with a Mayo endoscopic subscore (MES) of 0 or 1, whereas others define healing as only an MES of 0. The differences in the clinical outcomes between MES 0 and MES 1 patients have not yet been systematically evaluated. In addition, it has not yet been established whether FIT results can function as a surrogate marker for prognosis in UC patients in clinical remission.
Patients with an MES of 0 were much less likely to relapse than those with an MES of 1. Thus, an MES of 0 should be a treatment goal and an optimal definition of mucosal healing. In addition, this is the first study to demonstrate that FIT results can predict the prognosis of UC. In patients in clinical remission, those with a negative FIT result were less likely to relapse than patients with a positive FIT. Moreover, negative FIT results one year or more after remission induction correlated with an MES of 0 and better prognosis.
The endoscopic features of the appropriate treatment goal of UC were indicated. The predictability of the FIT as a measure of mucosal status and the prognosis of patients with clinical remission were shown. Patients with a negative FIT demonstrated a lower risk of clinical relapse than those with a positive FIT. Additional results revealed that the risk of relapse in patients in prolonged remission and with a negative FIT were similar to those with an MES of 0. Taken together, these findings would be useful in an economical follow-up of UC patients.
The manuscript by Asuka Nakarai and colleagues describe a retrospective cohort study on ulcerative colitis. This manuscript is prepared with care and detail. Significance of the study is reflected by analysing human data over time. Ethical data and information are provided. Authors declared no conflict of interest.
P- Reviewer: Braet F, Hokama A S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 728] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 3. | Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, Manes G, Marmo R, Massari A, Molteni P, Maconi G. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483-489.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 664] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 5. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-65.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 943] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 6. | Meucci G, Fasoli R, Saibeni S, Valpiani D, Gullotta R, Colombo E, D’Incà R, Terpin M, Lombardi G. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012;18:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Nakarai A, Kato J, Hiraoka S, Kuriyama M, Akita M, Hirakawa T, Okada H, Yamamoto K. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013;108:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2251] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 9. | Vilkin A, Rozen P, Levi Z, Waked A, Maoz E, Birkenfeld S, Niv Y. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol. 2005;100:2519-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, Birkenfeld S, Leshno M, Niv Y. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Kuriyama M, Kato J, Takemoto K, Hiraoka S, Okada H, Yamamoto K. Prediction of flare-ups of ulcerative colitis using quantitative immunochemical fecal occult blood test. World J Gastroenterol. 2010;16:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical Study of the Relation between Mucosal Healing and Long-Term Outcomes in Ulcerative Colitis. Gastroenterol Res Pract. 2013;2013:192794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Nakarai A, Kato J, Hiraoka S, Inokuchi T, Takei D, Moritou Y, Akita M, Takahashi S, Hori K, Harada K. Prognosis of ulcerative colitis differs between patients with complete and partial mucosal healing, which can be predicted from the platelet count. World J Gastroenterol. 2014;20:18367-18374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, Schmidt C, Trummler M, Pittet V, Vavricka SR. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 624] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 16. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 17. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 543] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 18. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 19. | Gisbert JP, Bermejo F, Pérez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, López P, López-Palacios N, Calvo M. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (3)] |
| 20. | D'Incà R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, Oliva L, Sturniolo GC. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, D’haens GR, Franchimont D, Baert FJ, Torp RA. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | D'Incà R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Lobatón T, Rodríguez-Moranta F, Lopez A, Sánchez E, Rodríguez-Alonso L, Guardiola J. A new rapid quantitative test for fecal calprotectin predicts endoscopic activity in ulcerative colitis. Inflamm Bowel Dis. 2013;19:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 24. | Nancey S, Boschetti G, Moussata D, Cotte E, Peyras J, Cuerq C, Haybrard J, Charlois AL, Mialon A, Chauvenet M. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Guardiola J, Lobatón T, Rodríguez-Alonso L, Ruiz-Cerulla A, Arajol C, Loayza C, Sanjuan X, Sánchez E, Rodríguez-Moranta F. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Mooiweer E, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis. 2014;20:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |