Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.4041
Peer-review started: December 17, 2015
First decision: January 13, 2016
Revised: January 26, 2016
Accepted: February 20, 2016
Article in press: February 22, 2016
Published online: April 21, 2016
Processing time: 108 Days and 21.3 Hours
AIM: To evaluate the current status of gastric cancer surgery worldwide.
METHODS: An international cross-sectional survey on gastric cancer surgery was performed amongst international upper gastro-intestinal surgeons. All surgical members of the International Gastric Cancer Association were invited by e-mail to participate. An English web-based survey had to be filled in with regard to their surgical preferences. Questions asked included hospital volume, the use of neoadjuvant treatment, preferred surgical approach, extent of the lymphadenectomy and preferred anastomotic technique. The invitations were sent in September 2013 and the survey was closed in January 2014.
RESULTS: The corresponding specific response rate was 227/615 (37%). The majority of respondents: originated from Asia (54%), performed > 21 gastrectomies per year (79%) and used neoadjuvant chemotherapy (73%). An open surgical procedure was performed by the majority of surgeons for distal gastrectomy for advanced cancer (91%) and total gastrectomy for both early and advanced cancer (52% and 94%). A minimally invasive procedure was preferred for distal gastrectomy for early cancer (65%). In Asia surgeons preferred a minimally invasive procedure for total gastrectomy for early cancer also (63%). A D1+ lymphadenectomy was preferred in early gastric cancer (52% for distal, 54% for total gastrectomy) and a D2 lymphadenectomy was preferred in advanced gastric cancer (93% for distal, 92% for total gastrectomy)
CONCLUSION: Surgical preferences for gastric cancer surgery vary between surgeons worldwide. Although the majority of surgeons use neoadjuvant chemotherapy, minimally invasive techniques are still not widely adapted.
Core tip: Since surgical techniques might differ over time and between countries, we aimed to evaluate international preferences in gastric cancer surgery by means of a cross-sectional survey. Surgical preferences for gastric cancer surgery vary between surgeons worldwide. Minimally invasive gastrectomy is still not widely adapted, but most popular in Asia to treat patients with early gastric cancer. Neo-adjuvant chemotherapy is used by the majority of surgeons worldwide. A D1+ lymphadenectomy is preferred for early gastric cancer and a D2 lymphadenectomy is preferred for advanced gastric cancer.
- Citation: Brenkman HJ, Haverkamp L, Ruurda JP, van Hillegersberg R. Worldwide practice in gastric cancer surgery. World J Gastroenterol 2016; 22(15): 4041-4048
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/4041.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.4041
Gastric cancer is the fifth most common type of cancer worldwide[1]. Its treatment consists of (neo-) adjuvant chemotherapy and/or chemoradiation and surgical resection of the tumor and lymph nodes. The worldwide surgical practices may vary between surgeons, countries and continents. Current topics of debate in gastric cancer surgery are: (1) The influence of volume of hospitals and surgeons on the outcome after gastrectomy; (2) The technique of surgery: open or minimally invasive gastrectomy; (3) Reconstruction of the alimentary tract by means of a jejunal pouch. (4) The extent of lymph node dissection and need for omental resection and/or pancreaticosplenectomy; and (5) The type of (neo-)adjuvant treatment in patients with gastric cancer.
In this article the current practice of surgeons worldwide will be evaluated by means of a survey.
An international cross-sectional survey about the surgical treatment of gastric cancer was performed amongst international gastric surgeons. All surgical members of the International Gastric Cancer Association (IGCA) were invited by email to participate after approval of the IGCA was obtained. An English web-based survey had to be filled in according to the surgeons’ preferences. Questions asked included hospital volume, the use of neoadjuvant treatment, preferred surgical approach, extent of the lymphadenectomy and preferred anastomotic technique and are attached to the manuscript (Appendix 1). Definitions of the extent of lymph node dissection and gastric cancer classification were according to the Japanese gastric cancer classification system[2,3]. The invitations were sent in September 2013 and the survey was closed in January 2014. Statistical analysis was performed with the χ2 test using the IBM SPSS Statistics (version 21; IBM Corporation, Armonk, NY, United States). Data were considered significant if P < 0.05.
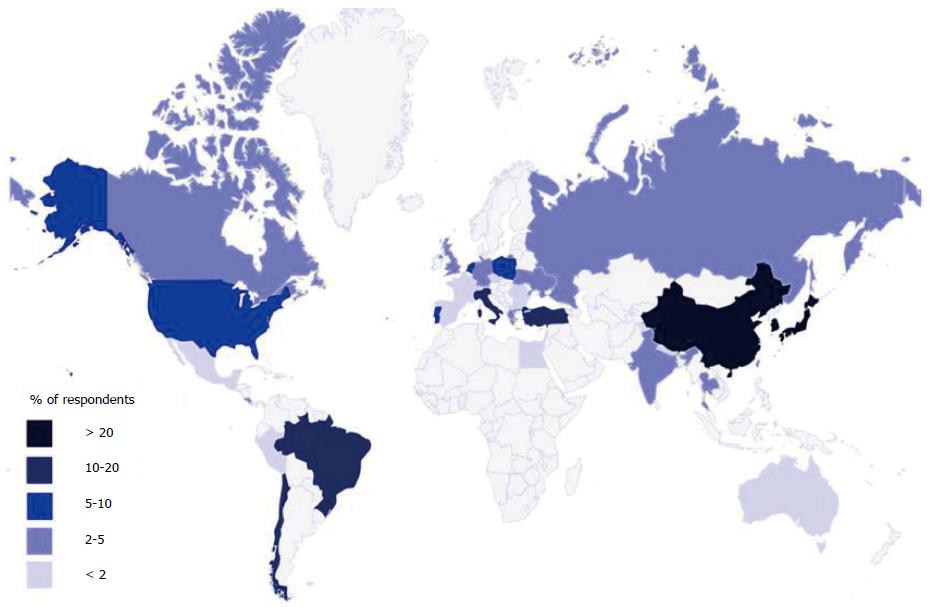
The survey was completed by 248 of 615 (40%) members of the IGCA. The 21 duplicate respondents were consequently excluded. The corresponding specific response rate was therefore 227/615 (37%). The respondents originated from Asia (54%), Europe (27%), South America (12%), North America (6%), Africa (0.4%), and Oceania (0.4%) (Figure 1).
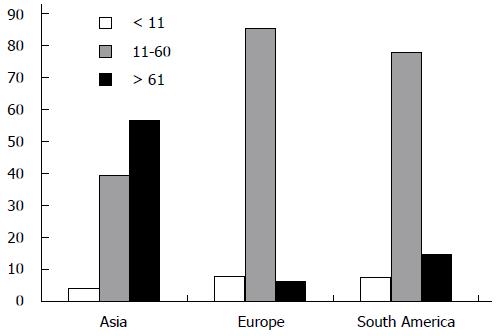
The volume of the participating surgeons was ≤ 10 gastrectomies per year in 16 (7%) respondents and > 21 resections in 180 (79%) respondents. In Asia, the majority of respondents (57%) performed > 61 resections (Figure 2). Medium and high volume surgeons worked in a university hospital (74%) more often than in a regional hospital (5%, P = 0.048).
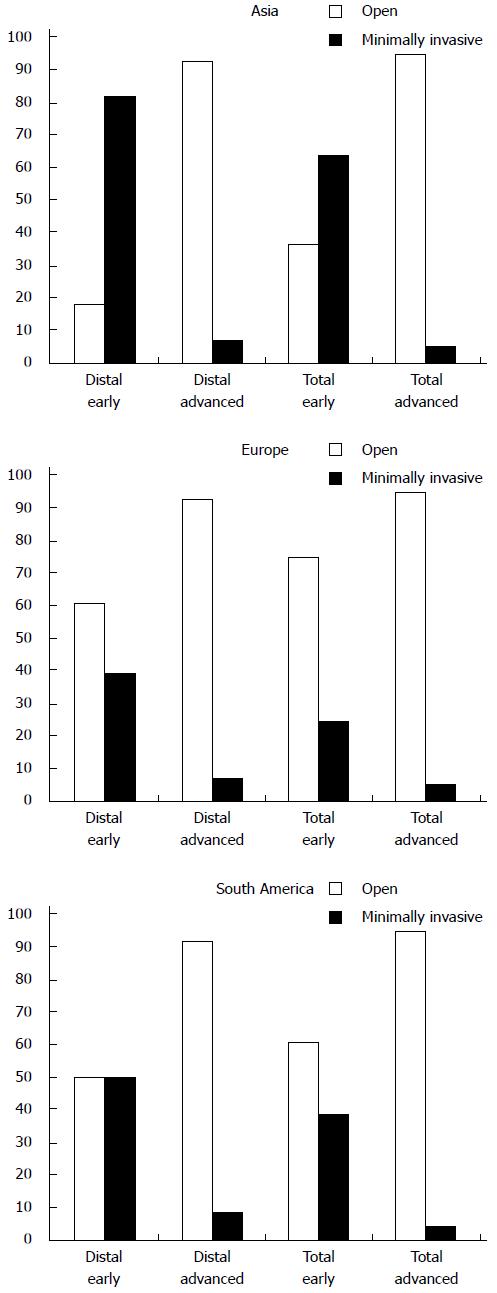
The current survey revealed that minimally invasive distal gastrectomy was preferred by 65% of surgeons in the treatment of early gastric cancer. The Asian respondents performed minimally invasive distal gastrectomy for early gastric cancer in 82% of the cases (Figure 3). In South America minimally invasive and open distal gastrectomy were equally performed. Minimally invasive distal gastrectomy for advanced gastric cancer was performed by only 9% of respondents. These results were comparable in all continents. For total gastrectomy, minimally invasive total techniques were favored by 49% for early gastric cancer and by 6% for advanced gastric cancer. However, in Asia the majority (64%) of respondents performed minimally invasive total gastrectomy for early gastric cancer, whereas other continents preferred the open procedure. For total gastrectomy for advanced cancer, there was no difference between continents.
The preference of 83% of the participating surgeons in the survey was to construct a direct esophagojejunostomy without jejunal pouch reconstruction after total gastrectomy. In merely 17% of surgeons a pouch was the preferred method of reconstruction. This percentage was consistent between all continents. A pouch reconstruction was slightly more popular amongst surgeons from a university hospital than surgeons from a regional hospital (19% vs 11%, P = 0.50). The data from the survey reveal that anastomoses were preferably performed by means of a mechanical stapler by 92% of respondents, compared to 8% of surgeons who favored a hand-sewn anastomosis.
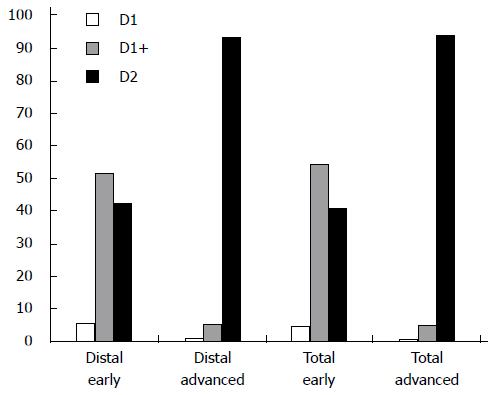
The current survey indicated that surgeons preferred a D1+ resection in 52% of distal gastrectomies and 54% of total gastrectomies for early cancers (Figure 4). In Asia and Northern America a D2 dissection was performed less frequently for early stage tumors compared to Europe and Southern America (Table 1). A D2 resection was favored by 93% of distal gastrectomies and 92% of total gastrectomies for advanced tumors. Resection of the spleen was preferably performed by 20% of all respondents: 33% of Asian respondents, 19% of South American respondents, and 15% of European respondents. The survey reveals that resection of the greater omentum was preferred by 89% of the participating surgeons.
| Asia | Europe | South America | North America | |
| Distal early | 33.9 | 57.1 | 54.2 | 38.5 |
| Total early | 27.7 | 66.7 | 50.0 | 30.8 |
| Distal advanced | 98.2 | 85.7 | 91.7 | 92.3 |
| Total advanced | 95.5 | 93.0 | 95.8 | 92.3 |
The results of our survey on the use of neoadjuvant treatment for gastric cancer indicated that chemotherapy was preferred by 73% and chemoradiation was favored by 12% of respondents. Only 16% favored treatment without neoadjuvant treatment. These results did not differ significantly over continents (Table 2).
| Asia | Europe | South America | North America | |
| Chemotherapy | 69.6 | 76.8 | 68.2 | 84.6 |
| Radiotherapy | 0.0 | 1.8 | 0.0 | 0.0 |
| Chemoradiation | 12.5 | 12.5 | 9.1 | 7.7 |
| None | 17.9 | 8.9 | 22.7 | 7.7 |
In this study the current worldwide trends in gastric surgery for cancer were evaluated by means of a survey amongst surgeons. It was found that the majority of surgeons have a high annual volume of gastrectomies. Open gastrectomy was still the preferred procedure in most procedures (68%) combined with neoadjuvant chemotherapy in the majority of cases (73%). Differences in surgical approach and lymphadenectomy were found across continents.
In our survey, 79% of respondents performed > 20 gastrectomies per year which is considered a high volume in literature. With regard to annual volume of resection, both the volume of the individual surgeon and the volume of the hospital are related to mortality[4-8]. For example, one study showed that the 30-d mortality rate in centers performing > 21 resections per year was lower compared to centers performing ≤ 10 gastrectomies per year (4.4% vs 6.7%, P = 0.047)[9]. Interestingly, more than half of the respondents from Asia perform > 60 gastrectomies.
The preferred method for gastric cancer surgery for most surgeons is an open gastrectomy. Only for early gastric cancer requiring a distal gastrectomy, the majority of surgeons preferably use a minimally invasive method. This is supported by recent short term results of the KLASS-01 trial, which found that the complication rate was significantly lower after laparoscopic distal gastrectomy compared to the open distal gastrectomy (13% vs 20%, P = 0.001)[10]. Evidence for all other types of gastric cancer are from small randomized trials and retrospective studies only, but suggests minimally invasive gastrectomy to be safe[11-15]. The absence of long-term results might explain the lack of generalized usage of these minimally invasive techniques. Randomized studies in both Asian and Western populations are awaited before worldwide implementation can take place. In South Korea, the KLASS-02 trial and KLASS-03 (NCT01584336) trial are investigating the use of laparoscopy for distal gastrectomy for advanced cancer and total gastrectomy for early cancer respectively[16]. Recently, in Europe two randomized controlled trials (LOGICA-trial and STOMACH-trial) started comparing open with laparoscopic gastrectomy[17,18]. Regarding the current developments in minimally invasive surgery, one can expect the use of these techniques to increase. Since minimally invasive gastrectomy is associated with a considerable learning curve, expert training and proctoring is essential to provide for a safe implementation of this technique[19,20].
This survey showed that the majority of surgeons do not construct a jejunal pouch after total gastrectomy. Although literature remains scarce, studies have shown possible benefits of jejunal pouch reconstruction. Two studies demonstrated an improved quality of life in patients with a jejunal pouch, measured with the Gastro-Intestinal Quality of Life Index. In addition, they found no increase in postoperative morbidity after jejunal pouch reconstruction[21-24]. Several trials on the use of a jejunal pouch are currently running to further investigate this possibly beneficial technique. This study demonstrated the preferable technique for constructing the anastomosis was by means of a mechanical stapler. Studies support these results, since a mechanical anastomosis is constructed significantly quicker compared to the hand-sewn method (11.4 min vs 38.7 min, P < 0.001) with a comparable complication rate[25,26].
For early cancers the majority of surgeons perform a D1+ dissection worldwide. For advanced tumors, the majority of surgeons perform a D2 dissection. The minority of surgeons performs resection of the spleen. These findings are in compliance with literature. In Western countries, pancreas and spleen preserving D2 dissection has been the preferred resection technique since two large trials from the Netherlands and the United Kingdom[27,28]. These trials demonstrated D2 dissection with preserving the pancreas and spleen along with the associated lymph nodes (stations 10 and 11) to results in similar morbidity and mortality as D1 dissection and with better long-term results[27,29-31]. In addition, it was argued that the reason for resecting station 10 and 11 is questionable since metastasis in these lymph nodes confers a poor prognosis (11 year survival: positive station 10 = 8%; negative station 10 = 27%; positive station 11 = 11%; negative station 11 = 35%)[32]. In Asia, surgeons perform a more tailored lymph node dissection. This survey showed that for early gastric cancer, a D2-dissection is performed less frequently in Asia compared to Europe and South America. On the other hand, the Japanese Gastric Cancer Guidelines still advise considering complete clearance of lymph node stations 10 by splenectomy for potentially curable T2-T4 tumors invading the greater curvature of the upper stomach[3]. Some surgeons in Asia also perform a D3 dissection, since a Taiwanese trial showed an improved survival compared to D1 dissection[33]. However, a D3 dissection did not improve survival compared to a D2 dissection in a Japanese trial[34]. Evidence for a D1+ dissection is scarce. Only one small randomized trial demonstrated that D1+ dissection could be a safe alternative to D2 dissection for locally advanced non-junctional tumors. Therefore it seems justifiable that the majority of surgeons perform a D1+ dissection for early gastric cancer[35]. More studies are needed to clarify the role of D1+ dissection for all types of gastric tumors.
The survey reveals that resection of the greater omentum was preferred by 88.5% of the participating surgeons. The value of resection of the greater omentum is currently debated. Advocates of its resection underline the importance of dissection of possible tumor deposits, whereas opponents argue that it is a time consuming procedure associated with additional morbidity. Literature on this topic is scarce and international guidelines vary. A retrospective cohort study in an Asian patient population demonstrated that the 3- and 5-year survival rates were not significantly different between gastrectomy with and without resection of the greater omentum for advanced gastric cancer[36].
Lastly, the majority of surgeons (84.4%) report the application of neoadjuvant chemotherapy or chemoradiation prior to gastrectomy. Literature presents various possible treatment strategies before and after surgery for gastric cancer. In Western countries, perioperative chemotherapy has shown the most beneficial, with an increase in survival around 13%[37]. Interestingly, the percentage of surgeons in Europe using neo-adjuvant therapy in this study (2013-2014) is higher compared to the period of 2011-2012, where many patients did not receive neoadjuvant therapy[38]. In Asia, adjuvant chemotherapy alone demonstrated better results compared to surgery alone with an increase in 3-year survival of 5%-10%[39-41] adjuvant radiation in addition to perioperative chemotherapy can possibly increase survival and is currently investigated in Western countries[42,43]. In Asia however, adjuvant radiation in addition to adjuvant chemotherapy did not increase 7-year overall survival (75% vs 73%, P = 0.484)[39-41,44].
A limitation of this study is that it only evaluates expert opinions rather than objective measurements, which should be taken into account before generalizing these findings. However, its international design provides a unique insight in the current practice of gastric cancer surgeons. Its discussion in the light of contemporary literature can be used for further improvement of gastric cancer surgery worldwide. Lastly, these results can be used for future evaluation of worldwide gastric cancer surgery.
In conclusion, this study is unique in its international design revealing the expert opinion on gastric cancer surgery. Minimally invasive gastrectomy is still not widely adapted and variations between continents are present. Minimally invasive gastrectomy is most popular in Asia to treat patients with early gastric cancer. Neoadjuvant chemotherapy is used by the majority of surgeons worldwide. A D1+ lymphadenectomy is preferred for early gastric cancer and a D2 lymphadenectomy is preferred for advanced gastric cancer.
The authors greatly appreciate the cooperation of the International Gastric Cancer Association. Also, we would like to thank the individual respondents for their valuable contribution.
Gastric cancer is the fifth most common type of cancer worldwide. Its treatment consists of (neo)adjuvant chemotherapy and/or chemoradiation and surgical resection of the tumor and lymph nodes.
The worldwide surgical practices may vary between surgeons, countries and continents. Current topics of debate in gastric cancer surgery are the use of (neo)adjuvant treatment, preferred surgical approach, extent of the lymphadenectomy and preferred anastomotic technique.
Since the introduction of minimally invasive surgery for gastric cancer in 1994, the number of surgeons performing minimally invasive gastrectomy is rising. Although the procedure is technically demanding, the evidence for advantages of laparoscopic total gastrectomy is increasing. It is therefore expected that the majority of surgeons will adopt this technique in the future. (Neo)adjuvant treatment has increased survival of patients with gastric cancer. The preferred treatment type and regimen differs amongst countries worldwide.
The majority of surgeons start performing minimally invasive gastrectomy for early gastric cancer. Early gastric cancer is predominantly seen in Asia. Western countries often see advanced gastric cancer. It is unknown if minimally invasive techniques result in at least comparable outcomes to open surgery for patients with advanced gastric cancer.
This is an interesting paper describing the current trend of surgical treatment of gastric cancer. The authors performed an international cross-sectional survey and concluded that surgical preferences for gastric cancer surgery vary between surgeons worldwide; and the results are informative.
P- Reviewer: Fujiwara Y, Li GX, Shinohara H, Yildiz B S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 4. | Callahan MA, Christos PJ, Gold HT, Mushlin AI, Daly JM. Influence of surgical subspecialty training on in-hospital mortality for gastrectomy and colectomy patients. Ann Surg. 2003;238:629-636; discussion 636-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Bachmann MO, Alderson D, Edwards D, Wotton S, Bedford C, Peters TJ, Harvey IM. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. 2002;89:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3695] [Cited by in RCA: 3773] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 7. | Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Jensen LS, Nielsen H, Mortensen PB, Pilegaard HK, Johnsen SP. Enforcing centralization for gastric cancer in Denmark. Eur J Surg Oncol. 2010;36 Suppl 1:S50-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Dikken JL, van Sandick JW, Allum WH, Johansson J, Jensen LS, Putter H, Coupland VH, Wouters MW, Lemmens VE, van de Velde CJ. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg. 2013;100:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park do J, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016;263:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 494] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 11. | Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 12. | Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Jiang L, Yang KH, Guan QL, Cao N, Chen Y, Zhao P, Chen YL, Yao L. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc. 2013;27:2466-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Wang W, Zhang X, Shen C, Zhi X, Wang B, Xu Z. Laparoscopic versus open total gastrectomy for gastric cancer: an updated meta-analysis. PLoS One. 2014;9:e88753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, Kim W, Han SU. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Haverkamp L, Brenkman HJ, Seesing MF, Gisbertz SS, van Berge Henegouwen MI, Luyer MD, Nieuwenhuijzen GA, Wijnhoven BP, van Lanschot JJ, de Steur WO. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer. 2015;15:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Straatman J, van der Wielen N, Cuesta MA, Gisbertz SS, Hartemink KJ, Alonso Poza A, Weitz J, Mateo Vallejo F, Ahktar K, Diez Del Val I. Surgical techniques, open versus minimally invasive gastrectomy after chemotherapy (STOMACH trial): study protocol for a randomized controlled trial. Trials. 2015;16:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Jeong O, Ryu SY, Choi WY, Piao Z, Park YK. Risk factors and learning curve associated with postoperative morbidity of laparoscopic total gastrectomy for gastric carcinoma. Ann Surg Oncol. 2014;21:2994-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Jung do H, Son SY, Park YS, Shin DJ, Ahn HS, Ahn SH, Park do J, Kim HH. The learning curve associated with laparoscopic total gastrectomy. Gastric Cancer. 2016;19:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Zong L, Chen P, Chen Y, Shi G. Pouch Roux-en-Y vs No Pouch Roux-en-Y following total gastrectomy: a meta-analysis based on 12 studies. J Biomed Res. 2011;25:90-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Kalmár K, Cseke L, Zámbó K, Horváth OP. Comparison of quality of life and nutritional parameters after total gastrectomy and a new type of pouch construction with simple Roux-en-Y reconstruction: preliminary results of a prospective, randomized, controlled study. Dig Dis Sci. 2001;46:1791-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 887] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 25. | Kim T, Yu W, Chung H. Handsewn versus stapled gastroduodenostomy in patients with gastric cancer: long-term follow-up of a randomized clinical trial. World J Surg. 2011;35:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Seufert RM, Schmidt-Matthiesen A, Beyer A. Total gastrectomy and oesophagojejunostomy--a prospective randomized trial of hand-sutured versus mechanically stapled anastomoses. Br J Surg. 1990;77:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 28. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 997] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 29. | Bonenkamp JJ, van de Velde CJ. Lymph node dissection in gastric cancer. Br J Surg. 1995;82:867-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G, Morino M; Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 31. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1308] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 32. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 653] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 33. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 34. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 35. | Galizia G, Lieto E, De Vita F, Castellano P, Ferraraccio F, Zamboli A, Mabilia A, Auricchio A, De Sena G, De Stefano L. Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery. 2015;157:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Hasegawa S, Kunisaki C, Ono H, Oshima T, Fujii S, Taguri M, Morita S, Sato T, Yamada R, Yukawa N. Omentum-preserving gastrectomy for advanced gastric cancer: a propensity-matched retrospective cohort study. Gastric Cancer. 2013;16:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 38. | Messager M, de Steur WO, van Sandick JW, Reynolds J, Pera M, Mariette C, Hardwick RH, Bastiaannet E, Boelens PG, van deVelde CJ, Allum WH; EURECCA Upper GI Group. Variations among 5 European countries for curative treatment of resectable oesophageal and gastric cancer: A survey from the EURECCA Upper GI Group (EUropean REgistration of Cancer CAre). Eur J Surg Oncol. 2016;42:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1291] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 40. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 41. | GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 42. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2437] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 43. | Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, Boot H, van Grieken NC, van de Velde CJ, Verheij M. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer. 2011;11:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 44. | Park SH, Sohn TS, Lee J, Lim do H, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |