Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.3937
Peer-review started: December 30, 2015
First decision: January 28, 2016
Revised: February 15, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: April 21, 2016
Processing time: 96 Days and 18.5 Hours
In oncosurgical approach to colorectal liver metastases, surgery remains considered as the only potentially curative option, while chemotherapy alone represents a strictly palliative treatment. However, missing metastases, defined as metastases disappearing after chemotherapy, represent a unique model to evaluate the curative potential of chemotherapy and to challenge current therapeutic algorithms. We reviewed recent series on missing colorectal liver metastases to evaluate incidence of this phenomenon, predictive factors and rates of cure defined by complete pathologic response in resected missing metastases and sustained clinical response when they were left unresected. According to the progresses in the efficacy of chemotherapeutic regimen, the incidence of missing liver metastases regularly increases these last years. Main predictive factors are small tumor size, low marker level, duration of chemotherapy, and use of intra-arterial chemotherapy. Initial series showed low rates of complete pathologic response in resected missing metastases and high recurrence rates when unresected. However, recent reports describe complete pathologic responses and sustained clinical responses reaching 50%, suggesting that chemotherapy could be curative in some cases. Accordingly, in case of missing colorectal liver metastases, the classical recommendation to resect initial tumor sites might have become partially obsolete. Furthermore, the curative effect of chemotherapy in selected cases could lead to a change of paradigm in patients with unresectable liver-only metastases, using intensive first-line chemotherapy to intentionally induce missing metastases, followed by adjuvant surgery on remnant chemoresistant tumors and close surveillance of initial sites that have been left unresected.
Core tip: Surgery is considered as the only potentially curative option for patients with colorectal liver metastases, while chemotherapy alone is considered as a palliative treatment. Recent data shown that colorectal liver metastases disappearing after chemotherapy, so-called missing metastases, could not reappear on the long-term, suggesting that systemic treatments might be curative in selected cases. Accordingly, we propose that classical recommendation to limit surgery only when all initial tumor sites could be resected might have become partially obsolete. Furthermore, when missing liver metastases have been induced, adjuvant surgery targeting the resistant part of the disease could represent a new strategy.
- Citation: Lucidi V, Hendlisz A, Van Laethem JL, Donckier V. Missing metastases as a model to challenge current therapeutic algorithms in colorectal liver metastases. World J Gastroenterol 2016; 22(15): 3937-3944
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/3937.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.3937
The current oncosurgical approach in patients with isolated colorectal liver metastases (CRLM) is primarily driven by the concept that surgery represents the only potentially curative option. Retrospective series regularly report 5-year overall survival (OS) rates superior to 50% after surgical resection[1-5], reaching 75% in selected groups[6,7], while rates of cure, defined as disease-free survival (DFS) greater than 10 years[8], may reach 35%[9]. In contrast, in non-resected patients treated with chemotherapy only, median OS is still limited to 30 mo[10,11] and survival exceeding 10 years remains exceptional[12-14]. Such a dichotomy in the respective potentials of surgery and chemotherapy has major implications for establishment of strategic management plans in which resectability plays a central role in therapeutic decisions. Currently, surgical resection is the standard of care in patients with resectable CRLM (defined as patients in whom radical R0 resection is possible), irrespective of tumor load and tumor biology[15]. At the opposite end of the spectrum, in patients with diffuse metastatic liver infiltration that will never be amenable to surgery, chemotherapy is given in a strict palliative setting which aims to provide global cancer control and maintain an acceptable quality of life rather than induce an optimal tumor response. However, it can be argued that this vision is overly simplified, and is, at least partially, dogmatic rather than evidence-based. For example, it is clear that technical resectability does not optimally categorize all patients with CRLM. In some patients, rapid postoperative recurrence despite curative-intent resection casts doubt upon the benefits of surgery[16]. Moreover, no randomized study is currently available comparing surgery and chemotherapy in similar patients and the interpretation is massively biased when comparing the results of surgery in patients with limited disease and favorable prognostic factors to those of chemotherapy in patients with massive tumor burden. For obvious ethical reasons, it is not possible to conduct a trial that randomizes surgery and chemotherapy in patients with resectable CRLM and, therefore, arguments to challenge current therapeutic algorithms can only arise from indirect observations. The constant improvement in survival rates in patients receiving surgery for CRLM in recent years may represent the first evidence that challenges the current view. This has been achieved in the context of 2 major evolutionary changes. At the surgical level, sophisticated techniques now allow curative-intent surgery in patients with advanced metastatic disease who would have been previously ineligible for surgery. In parallel, the efficacy of chemotherapeutic regimens has continuously improved, resulting in significantly increased tumor response rates[11]. It is postulated that it is the combination of these 2 factors that is responsible for major improvements in post-surgical outcomes. A paradox is that the chances of long-term survival after resection have increased despite extension of the oncological indications. From a surgical point of view, it is unlikely that technical progress has improved the oncological efficacy of liver resection. This brings up the possibility that better results observed in these patients may be attributable to improved performance of chemotherapy, usually combined with surgery in complex cases. One could hypothesize that these results in patients with advanced CRLM may rely on the capacity of modern systemic regimens to clear occult metastatic disease[17-19]. Along this same line, the prognostic factors for postoperative outcomes have changed notably in the last few years. Previously, significant prognostic factors were mainly related to tumor stage, at primary and secondary levels, and to the possibility for radical surgery[20,21]. In the current era of efficient multimodal treatments, the predictive value of these factors has substantially decreased, replaced by prognostic markers that define the intrinsic tumor biology and potential interactions between the cancer and systemic treatments. Accordingly, several retrospective studies have indicated that the response to preoperative chemotherapy has a major prognostic impact and, particularly, that the chances of cure are significantly increased in patients with complete pathological response (CPR) in resected metastases as compared with patients with minor tumor response or progressive disease[5-7,16,22-24]. These data were not systematically confirmed[25] and should be interpreted with caution due to the retrospective nature of the studies and the variability of treatment regimens. Moreover, these observations do not constitute proof of the benefit of preoperative chemotherapy, as CPR may represent a surrogate marker of favorable tumor biology and/or genetics. Therefore, excellent outcomes in these patients may be related to surgery only in individuals with favorable tumor biology, identified by their positive response to chemotherapy. Still, and despite these reservations, if preoperative chemotherapy may contribute to improved postoperative survival, its effect is not expected to be dependent on its efficacy at the level of the responding metastasis itself, as it will be subsequently resected, but rather to active tumoricidal effects on occult disease. The potential capacity of modern therapeutic agents to eliminate microscopic disease could also explain why the predictive value of surgical margins regularly decreases. Classically, resectability was defined as the possibility of achieving a 1 cm negative margin and surgery was considered to be beneficial only when radical[26-28]. These principles are now challenged by several works showing that neither margin width, nor R1 resection have a significant impact on long-term survival in multivariate analyses and, therefore, an anticipated R1 resection should not be considered to be an isolated contraindication for surgery anymore[17,29-35]. Modern surgical transection methods, using ultrasonic dissectors and aspiration devices and high energy coagulation systems, may play a role in this phenomenon, transforming macroscopic R0 resections into R1 pathological resections[30] and R1 anatomical resections into R0 resections in situ. Perioperative chemotherapy may also play a role, potentially through elimination of residual cancer cells at resection margins. This is suggested by retrospective studies showing that the differences in survival between R0 and R1 resection are abolished in patients with optimal responses to chemotherapy[17,18,36], while R1 resection still carries a poor prognosis in patients with suboptimal responses[37]. Taken together, these observations support the hypothesis that new chemotherapeutic regimens might have become potentially curative at a cellular level. If this is true, the next step would be to evaluate whether chemotherapy might be curative on a macroscopic tumor and how to integrate this concept into new therapeutic strategies. Taking into account current established therapeutic algorithms, this simple hypothesis is extremely difficult to verify in a clinical model. However, the particular situation where liver metastases disappear after chemotherapy represents a unique opportunity to address this question. When these so-called missing liver metastases (MLMs) are left unresected, their long-term surveillance provides the chance to verify whether such complete radiological response (CRR) could correspond to a cure.
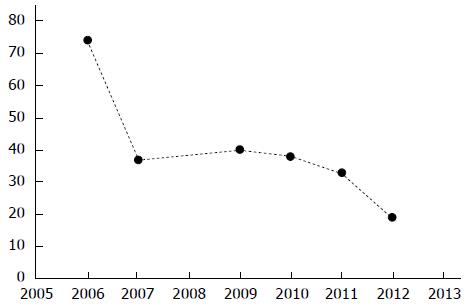
MLMs, or disappearing or vanishing metastases, refer to liver metastases that become undetectable upon imaging after administration of chemotherapy. As radiological response to chemotherapy can be inhomogeneous[38] and as MLMs may be reported as the percentage of patients having at least 1 MLM or as the ratio of MLMs compared to the total number of liver metastases, the exact incidence of this phenomenon remains difficult to evaluate. Yet, consistent with the increased response rates to modern chemotherapies and despite the increasing sensitivity of liver imaging techniques, a trend toward an increasing rate of MLMs has been described in the last years, ranging from 6% to 10% in initial studies to 10% to 24% in more recent works (Figure 1)[39-47]. The definition of MLM critically depends on imaging technique performance. Currently, most authors prefer magnetic resonance imaging (MRI) over computed tomography scans[48] but optimally use both techniques to confirm the disappearance of the lesions[49,50]. Additionally, in 10% to 50% of the cases, MLMs on preoperative imaging are still found at surgery, using visual and manual inspection and intraoperative ultrasound (IOUS)[39,40,42-46,51,52], this rate reaches 80% when contrast-enhanced IOUS is used[51]. After accurate preoperative imaging and intraoperative exploration, some MLMs remain undetectable and should therefore be considered to be true MLMs. The main predictors for development of MLMs are small initial tumor size, low carcinoembryonic antigen (CEA) level, rapid normalization of CEA after chemotherapy, duration of chemotherapy, and use of hepatic arterial infusion of chemotherapy (HAI)[40-43]. The central question concerning MLMs is whether they correspond to a false negative result of preoperative and intraoperative staging or represent a true complete response and, potentially, a cure of the lesion. This question is critical for its strategic implications in cases when MLMs were accidentally induced but also for evaluation of whether intentional induction of MLMs could become part of a therapeutic plan. One possible answer to this question comes from pathologic analysis when the site of an MLM was resected during surgery, either when included in the planned resection or when a blind hepatectomy was performed. Among resected MLMs, the rate of complete pathologic response (CPR), defined as the absence of residual viable cancer cells, varies widely in the literature, from 20% to 100% (Table 1)[39-47]. In different series, the predictive factors for a correlation between CRR and CPR are the use of HAI, the absence of steatosis, low body mass index, an MRI-based diagnosis, the normalization of CEA level during chemotherapy, and the use of a modern chemotherapy regimen[41-43,47]. A second, and more convincing answer, is provided by the long-term local follow-up of MLMs when they were left unresected. When such specific follow-up could be performed, most of the local recurrences appear rapidly, within 20 mo after chemotherapy withdrawal[43]. Among unresected MLMs, the rate of sustained clinical response (SCR), defined as the absence of local recurrence on follow-up, varies massively in literature, from 25% to 80% (Table 1)[39-47]. Interestingly, this rate tends to progressively increase in recent reports, potentially related to improved sensitivity of imaging and improved efficacy of perioperative chemotherapies (Table 1)[39-47]. Particularly, adjuvant chemotherapy and adjuvant HAI could be determinants, as re-growth of MLMs appears substantially increased in patients who do not receive postoperative treatments[41,43]. In an early study, Benoist et al[40] reported that, in the large majority of cases, residual cancer cells were still present at pathology when original sites of MLMs were resected and that local recurrences were almost certain to occur when they were left unresected. This led to the view that MLMs are an undesirable event resulting from preoperative overtreatment and precluding the chances for radical surgical resection. Accordingly, the consensus recommendation was, and remains, to systematically resect all original sites of MLM whenever technically feasible[40,49,53]. Currently however, apart from the surgical difficulty of such blind resections, this recommendation should probably be reevaluated, as recent studies suggest that a significant proportion of MLMs would not reappear in the long term.
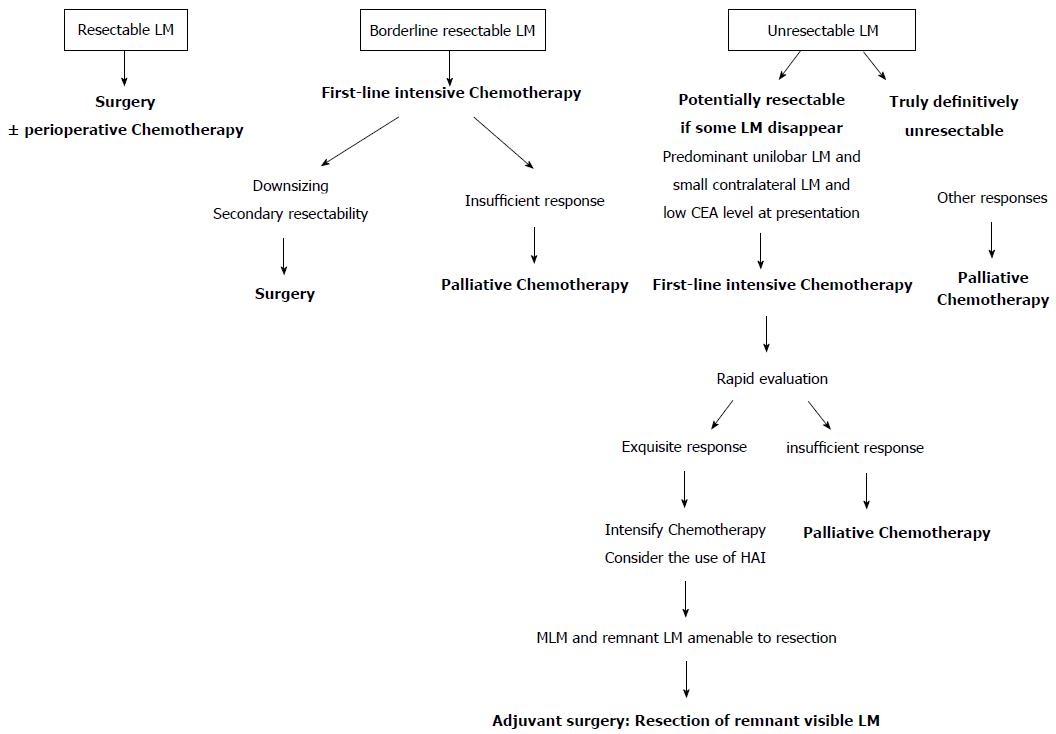
There are 3 categories of patients at the time of presentation of CRLM: Patients with resectable metastases, patients with borderline or potentially resectable metastases, and patients with metastases considered to be definitively unresectable. The therapeutic options are well-defined for the 2 first categories. Patients with resectable CRLM should undergo surgery, giving them, most probably, the best chance for cure (Figure 2). In these cases, the place for perioperative systemic treatments remains under discussion and is currently decided according to associated risk factors. Patients with borderline CRLM are those potentially amenable to radical surgical resection, provided significant downsizing after neoadjuvant chemotherapy. In these cases, first-line intensive chemotherapy is indicated, to maximize the chances of response, followed by surgery with curative intent when allowed by tumor-response (Figure 2). It is in the third group of patients that new therapeutic options can be developed. In the current therapeutic algorithm, these patients receive chemotherapy in a palliative setting, favoring long-term tolerance. If we can concede that disappearance of CRLM after chemotherapy corresponds to a cure in selected cases, a new option in these patients could be to intentionally induce MLMs. In this objective, the initial decision should be, therefore, to modify the first-line chemotherapy from a palliative to an intensive regimen, including possibly HAI, to elicit a maximal tumor response and to enhance the chances of obtaining MLMs. Under these conditions, if MLMs are induced, adjuvant surgery and/or local destruction with radiofrequency, targeted to remnant visible disease could represent an acceptable option when safely feasible (Figure 2). To reasonably develop such an exploratory approach, patient selection is pivotal. First, selection should rely on surgical aspects, reserving this type of approach for patients with tumor distributions that are potentially amenable to surgical resection if some lesions would disappear after chemotherapy (Figure 2). Other selection criteria may include the predictive factors for development of MLMs, such as low CEA level and small tumor size. In these cases, rapid evaluation of tumor chemosensitivity, using evolution of CEA levels and metabolic imaging[54], would be critical, to reinforce first-line chemotherapy or to shift to a palliative regimen in cases of poor initial response. In addition, in such an approach, the use of adjuvant systemic chemotherapy or HAI should be considered to enhance the chances of long-term SCR[41,43].
Classically, MLMs were judged to be false negative results of imaging and recurrence at those sites was considered to be a near certainty. Now, however, taking into account the increased sensitivity of imaging and the improved efficacy of chemotherapy, this view should be reevaluated. Better identification of the factors which may lead to disappearance of liver metastases and better knowledge of their long-term evolution may allow for consideration of the induction of MLMs as a potential therapeutic option, to convert unresectable into macroscopically resectable disease. In patients with initially resectable CRLM, the accidental generation of MLMs during preoperative treatment remains a globally unfavorable event as it may complicate a subsequent curative-intent surgery. However, in these cases, the classical recommendation to resect all initial metastatic sites[40,49,53] might have become partially obsolete with regard to the substantial chances for SCR in these cases. Therefore, when such resections are hazardous, a watch-and-wait strategy now appears to be a reasonable alternative. In patients with unresectable CRLM, the recent demonstration that some MLMs are cured challenges the dogma that curative potential is exclusively reserved to surgery, and may lead to new therapeutic options. In these strategies, the classical roles of chemotherapy and surgery would be modified, using first-line intensive chemotherapy to obtain maximal tumor response, followed, if MLMs were induced, by resection and/or RF destruction of the chemoresistant part of the disease. This may lead, in fact, to the identification of a new subgroup of patients, defined as those with initially unresectable but potentially resectable metastases if MLMs are induced by chemotherapy, and to the new concept of adjuvant surgery, defined as surgery targeting the remnant visible metastases after CRR to chemotherapy of other lesions. The future development of such complex strategies will critically depend upon the identification of accurate predictive factors for response to chemotherapy at the single-tumor level, on the determination of the best chemotherapeutic regimen and on close, multidisciplinary collaborations in therapeutic decisions at the presentation of the disease and during follow-up.
The authors acknowledge the contribution of a medical writer, Sandy Field, PhD.
P- Reviewer: Bester L, Jean-Marc R, Ramia JM, Sonoda H S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1118] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 2. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825; discussion 825-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1364] [Cited by in RCA: 1291] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 3. | Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, Kennamer DL, Ellis LM, Curley SA. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722-730; discussion 730-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, Lodge JP. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. Br J Surg. 2014;101:856-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H, Chikamoto A, Watanabe M, Ishiko T, Baba H. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol. 2014;21 Suppl 3:S405-S413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Blazer DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344-5351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 475] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-4580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 895] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 9. | van Dam RM, Lodewick TM, van den Broek MA, de Jong MC, Greve JW, Jansen RL, Bemelmans MH, Neumann UP, Olde Damink SW, Dejong CH. Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB (Oxford). 2014;16:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Schmiegel W, Reinacher-Schick A, Arnold D, Kubicka S, Freier W, Dietrich G, Geißler M, Hegewisch-Becker S, Tannapfel A, Pohl M. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group. Ann Oncol. 2013;24:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 797] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 12. | Ferrarotto R, Pathak P, Maru D, Agarwal A, Overman M, Hoff PM, Kopetz S. Durable complete responses in metastatic colorectal cancer treated with chemotherapy alone. Clin Colorectal Cancer. 2011;10:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1033] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 14. | Dy GK, Hobday TJ, Nelson G, Windschitl HE, O’Connell MJ, Alberts SR, Goldberg RM, Nikcevich DA, Sargent DJ. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: a North Central Cancer Treatment Group review of 3811 patients, N0144. Clin Colorectal Cancer. 2009;8:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, Sobrero A, Ychou M. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 16. | Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, Ijzermans JN, Mirza DF, Elias D, Adam R. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21:1276-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Truant S, Séquier C, Leteurtre E, Boleslawski E, Elamrani M, Huet G, Duhamel A, Hebbar M, Pruvot FR. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB (Oxford). 2015;17:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Tanaka K, Nojiri K, Kumamoto T, Takeda K, Endo I. R1 resection for aggressive or advanced colorectal liver metastases is justified in combination with effective prehepatectomy chemotherapy. Eur J Surg Oncol. 2011;37:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Parikh AA, Gentner B, Wu TT, Curley SA, Ellis LM, Vauthey JN. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318; discussion 318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254-1262. [PubMed] |
| 22. | Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052-1061; discussion 1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 760] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 23. | Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Small RM, Lubezky N, Shmueli E, Figer A, Aderka D, Nakache R, Klausner JM, Ben-Haim M. Response to chemotherapy predicts survival following resection of hepatic colo-rectal metastases in patients treated with neoadjuvant therapy. J Surg Oncol. 2009;99:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Gallagher DJ, Zheng J, Capanu M, Haviland D, Paty P, Dematteo RP, D’Angelica M, Fong Y, Jarnagin WR, Allen PJ. Response to neoadjuvant chemotherapy does not predict overall survival for patients with synchronous colorectal hepatic metastases. Ann Surg Oncol. 2009;16:1844-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 209] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Shirabe K, Takenaka K, Gion T, Fujiwara Y, Shimada M, Yanaga K, Maeda T, Kajiyama K, Sugimachi K. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, Nordlinger B. Long-term survival following resection of colorectal hepatic metastases. Association Française de Chirurgie. Br J Surg. 1997;84:977-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 236] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715-722, discussion 722-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 812] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 31. | Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1cm rule. Eur J Surg Oncol. 2006;32:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Lordan JT, Karanjia ND. ‘Close shave’ in liver resection for colorectal liver metastases. Eur J Surg Oncol. 2010;36:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, Codina-Barreras A, Mojal S. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Muratore A, Ribero D, Zimmitti G, Mellano A, Langella S, Capussotti L. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol. 2010;17:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Ng JK, Urbanski SJ, Mangat N, McKay A, Sutherland FR, Dixon E, Dowden S, Ernst S, Bathe OF. Colorectal liver metastases contract centripetally with a response to chemotherapy: a histomorphologic study. Cancer. 2008;112:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Ayez N, Lalmahomed ZS, Eggermont AM, Ijzermans JN, de Jonge J, van Montfort K, Verhoef C. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol. 2012;19:1618-1627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | van Kessel CS, Samim M, Koopman M, van den Bosch MA, Borel Rinkes IH, Punt CJ, van Hillegersberg R. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer. 2013;49:2486-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Elias D, Youssef O, Sideris L, Dromain C, Baton O, Boige V, Ducreux M. Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol. 2004;86:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939-3945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 385] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 41. | Elias D, Goere D, Boige V, Kohneh-Sharhi N, Malka D, Tomasic G, Dromain C, Ducreux M. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol. 2007;14:3188-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg. 2009;250:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Auer RC, White RR, Kemeny NE, Schwartz LH, Shia J, Blumgart LH, Dematteo RP, Fong Y, Jarnagin WR, D’Angelica MI. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer. 2010;116:1502-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Goèré D, Gaujoux S, Deschamp F, Dumont F, Souadka A, Dromain C, Ducreux M, Elias D. Patients operated on for initially unresectable colorectal liver metastases with missing metastases experience a favorable long-term outcome. Ann Surg. 2011;254:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg. 2010;14:1691-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Ferrero A, Langella S, Russolillo N, Vigano’ L, Lo Tesoriere R, Capussotti L. Intraoperative detection of disappearing colorectal liver metastases as a predictor of residual disease. J Gastrointest Surg. 2012;16:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Ono T, Ishida H, Kumamoto K, Okada N, Ishibashi K. Outcome in disappearing colorectal cancer liver metastases during oxaliplatin-based chemotherapy. Oncol Lett. 2012;4:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis--meta-analysis. Radiology. 2005;237:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 49. | Gaujoux S, Goéré D, Dumont F, Souadka A, Dromain C, Ducreux M, Elias D. Complete radiological response of colorectal liver metastases after chemotherapy: what can we expect? Dig Surg. 2011;28:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Fowler KJ, Linehan DC, Menias CO. Colorectal liver metastases: state of the art imaging. Ann Surg Oncol. 2013;20:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Fiorentini G, Del Conte A, De Simone M, Guadagni S, Mambrini A, D’Alessandro M, Aliberti C, Rossi G, Cantore M. Complete response of colorectal liver metastases after intra-arterial chemotherapy. Tumori. 2008;94:489-492. [PubMed] |
| 52. | Arita J, Ono Y, Takahashi M, Inoue Y, Takahashi Y, Saiura A. Usefulness of contrast-enhanced intraoperative ultrasound in identifying disappearing liver metastases from colorectal carcinoma after chemotherapy. Ann Surg Oncol. 2014;21 Suppl 3:S390-S397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Bischof DA, Clary BM, Maithel SK, Pawlik TM. Surgical management of disappearing colorectal liver metastases. Br J Surg. 2013;100:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Hendlisz A, Golfinopoulos V, Garcia C, Covas A, Emonts P, Ameye L, Paesmans M, Deleporte A, Machiels G, Toussaint E. Serial FDG-PET/CT for early outcome prediction in patients with metastatic colorectal cancer undergoing chemotherapy. Ann Oncol. 2012;23:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |