Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.3892
Peer-review started: January 29, 2016
First decision: February 18, 2016
Revised: March 7, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: April 21, 2016
Processing time: 67 Days and 2.4 Hours
The burden of alcoholic liver disease has rapidly grown in the past two decades and is expected to increase further in the coming years. Alcoholic hepatitis, the most florid presentation of alcoholic liver disease, continues to have high morbidity and mortality, with significant financial and healthcare burden with limited treatment options. Steroids remain the current standard of care in severe alcoholic hepatitis in carefully selected patients. No specific treatments are available for those patients who are steroid ineligible, intolerant or unresponsive. Liver transplant has shown good short-term outcome; however, feasibility, ethical and economic concerns remain. Modification of gut microbiota composition and their products, such as lipopolysaccharide, nutritional interventions, immune modulation, increasing steroid sensitivity, genetic polymorphism and epigenetic modification of alcohol induced liver damage, augmenting hepatic regeneration using GCSF are potential therapeutic avenues in steroid non-responsive/ineligible patients. With better understanding of the pathophysiology, using “Omics” platforms, newer options for patients with alcoholic hepatitis are expected soon.
Core tip: With better treatment options available for other liver diseases like viral hepatitis the proportion of alcoholic liver disease is on the rise. Alcoholic hepatitis is the most serious presentation of alcoholic liver disease with significant morbidity, mortality and health care burden. Treatment options in steroid non-responders and steroid ineligible patients of severe alcoholic hepatitis are limited. Newer treatment options for these patients are the need of the hour. The molecular and cellular targets have been discussed.
- Citation: Shasthry SM, Sarin SK. New treatment options for alcoholic hepatitis. World J Gastroenterol 2016; 22(15): 3892-3906
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/3892.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.3892
Better fortunes and increased stress have led to life style changes across the world. Alcohol has become the commonest and most socially acceptable hepatotoxin worldwide. Additionally, with the availability of better drugs and more treatment options for managing liver disorders related hepatotrophic viruses, the proportion and burden of alcoholic liver disease has grown, and is likely to increase in the coming years. Alcoholic hepatitis (AH) related hospital admissions continue to increase with substantial increase in healthcare cost and utilization[1,2]. The spectrum of alcoholic liver disease varies from fatty liver, steatohepatitis, compensated/ decompensated cirrhosis, to hepatocellular carcinoma. Alcoholic hepatitis is the most florid manifestation of alcoholic liver disease with substantial morbidity, mortality and financial burden. The treatment options of severe alcoholic hepatitis (Maddrey’s discriminant score > 32) however, have not changed in nearly past three decades. Steroids, pentoxifylline and nutrition therapy remain the only accepted options available for the management of severe alcoholic hepatitis[3-5]. There is no advantage of combining or sequencing steroids and pentoxifylline in comparison with either alone[6-10]. No medical options are available to treat severe alcoholic hepatitis patients who are steroid unresponsive (Lille score > 0.45). Similarly no options are available for steroid ineligible patients (upper gastrointestinal bleed, impaired renal functions and/or sepsis) who far outnumber the patients who are eligible and respond to steroids. With advances in basic sciences and better understanding of the pathogenesis of alcoholic liver disease, significant advances in the management of severe alcoholic hepatitis are likely. This review discusses various aspects of the pathogenesis and targeted treatment options in patients with alcoholic liver disease.
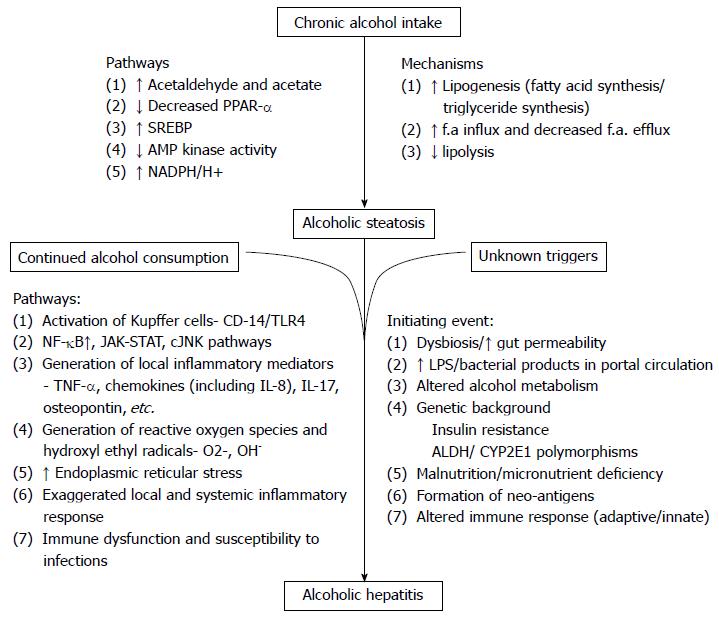
Alcoholic steatosis is a complex process manifested through several mechanisms (Figure 1). The main pathogenetic factors underlying this process are increased fatty acid and triglyceride synthesis, enhanced hepatic influx of free fatty acids from adipose tissue and of chylomicrons from the intestinal mucosa, increased hepatic lipogenesis, inhibited lipolysis, and damaged mitochondria and microtubules, all of which result in accumulation of VLDL[11-16]. Lipogenic enzymes are overexpressed in alcoholics due to downregulation of peroxisome proliferator activated receptor α (PPARα) and the induction of sterol regulatory element binding protein (SREBP)[17,18]. AMP activated protein kinase (alters relative concentrations of intra cellular malonyl coenzyme A and long chain acyl coenzyme A) and the downstream pathways of fatty acid synthesis and degradation[19], the NADH reduction/oxidation potential in the liver and reduced microsomal triglyceride transfer protein activity are all implicated in the chronic alcoholism related fatty liver[20,21].
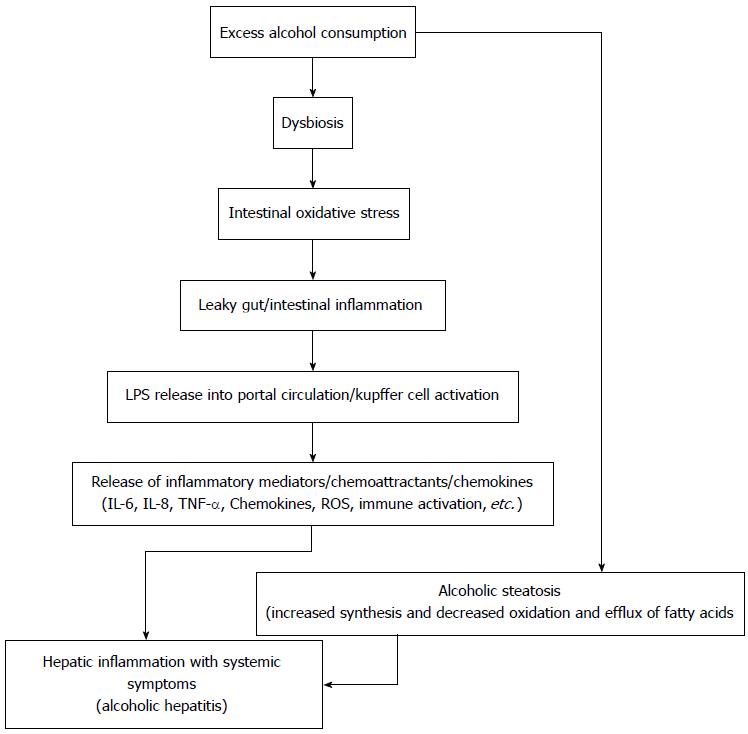
Some unclear triggering event is responsible for the initiation of steatohepatitis, in patients with alcoholic fatty liver who continue to consume excessive alcohol. Gut dysbiosis, increased gut permeability, altered alcohol metabolism, increased lipopolysaccharide release into portal circulation, genetic background, associated malnutrition and micronutrient deficiency have been implicated as the initiating event for inflammation[21,22] and decide the severity of alcoholic hepatitis and cellular injury[23-25]. Acetaldehyde (the major product of alcohol metabolism) adducts forming neoantigens leading to activation of adaptive immune system[26,27], impaired glutathione function, oxidative stress and apoptosis[28-30] lead to inflammation and liver injury.
Prolonged alcohol intake markedly upregulatesCytochrome P450 2E1 (CYP2E1) activity. CYP2E1 dependent microsomal electron transport system of the respiratory chain, NADH dependent cytochrome reductase and xanthine oxidase are involved in the production of reactive oxygen species[31-33] in addition to alcohol dehydrogenase and aldehyde dehydrogenase. Thus alcohol ingestion stimulates the generation of ROS and hydroxyl radicals[34]. These metabolites and ROS activate various downstream inflammatory pathways that involve nuclear factor-κB (NF-κB), signal transducer and activator of transcription (STAT)-Janus kinase (JAK) and cJun N terminal kinase (JNK) in hepatic resident cells, leading to local synthesis of inflammatory mediators, such as tumor necrosis factor (TNF), IL-17, CXC chemokines (including IL-8), as well as osteopontin[21,35-40].
Alcohol intake also induces dysbiosis in the gut and alters the gut permeability leading to increased levels of lipopolysaccharides in the portal circulation. This increase in lipopolysaccharide induces inflammatory activation of Kupffer cells via the CD14-Toll-like receptor (TLR) 4 pathway[41]. Finally, increased endoplasmic reticular stress due to impaired protein degradation forms Mallory Denk bodies (hepatocellular aggregates of cytokeratins)[42].
Robust involvement of innate and adaptive immune disturbances in patients with alcoholic hepatitis is well known but has been only partially explored. Exaggerated systemic inflammatory response but still increased susceptibility to bacterial infections is the characteristic of alcoholic hepatitis[43] meaning to say that their immune cells are stimulated but with impaired antibacterial functions[44]. Although precise immunological mechanisms of alcoholic hepatitis are not clear, both adaptive[45] (neoantigens) and innate immune activation[46] are known to drive the clinical out look of alcoholic hepatitis (Systemic inflammatory response). The precise mechanism or event that triggers the acute event in alcoholic hepatitis is still cryptic.
Some questions like, why only few chronic alcoholics develop liver disease and more so alcoholic hepatitis, why only few patients with alcoholic hepatitis respond to steroids and the standard of care and why only some of the alcoholic hepatitis patients are prone to develop renal impairment/ infections remain unanswered. The potential new treatments and key to better management of alcoholic hepatitis lie in the answers to the above questions.
Metabolomic profiling is one of the recently developed methods for identifying newer biomarkers. A recent study[47] compared serum metabolic profile between severe alcoholic hepatitis patients (n = 25) and alcoholic cirrhotics (n = 25). They have found altered levels of many biochemicals in subjects with severe AH and also demonstrated that metabolomic profiles separated the two cohorts with 100% accuracy. Severe AH was associated with enhanced triglyceride lipolysis, impaired mitochondrial fatty acid beta-oxidation, upregulated omega oxidation and decreased plasma membrane remodeling. While most measured bile acids were increased, low deoxycholate and glycodeoxycholate levels suggested intestinal dysbiosis in severe AH. Several changes in substrate utilization including increased glucose consumption by the pentose phosphate pathway, altered tricarboxylic acid (TCA) cycle activity, and enhanced peptide catabolism were noted. The same group has also demonstrated distinct lipidomic profile[48] in patients with severe alcoholic hepatitis in comparison with alcoholic cirrhosis with higher serum Resistin and plasma activation inhibitor-1 levels in and a decrease in serum Leptin levels in severe alcoholic hepatitis patients. Serum levels of the pro-lipolytic cytokines - tumor necrosis factor α, interleukin (IL)-6; IL-8 and IL-15 were found to be higher in severe alcoholic hepatitis patients in comparison to alcoholic cirrhosis. Serum IL-6 levels ≥ 38.66 pg/mL most precisely identified deaths in severe alcoholic hepatitis patients (levels ≥ 38.66 pg/mL had significantly decreased mean survival). Explosion of knowledge and understanding of metabolomic profiling (transcriptome, proteome and metabolome) in alcoholic hepatitis should lead to discovery of novel weak points in the pathogenesis. Future metabolomics studies should be able to identify novel specific therapeutic targets in the management of alcoholic hepatitis, which is the need of the hour.
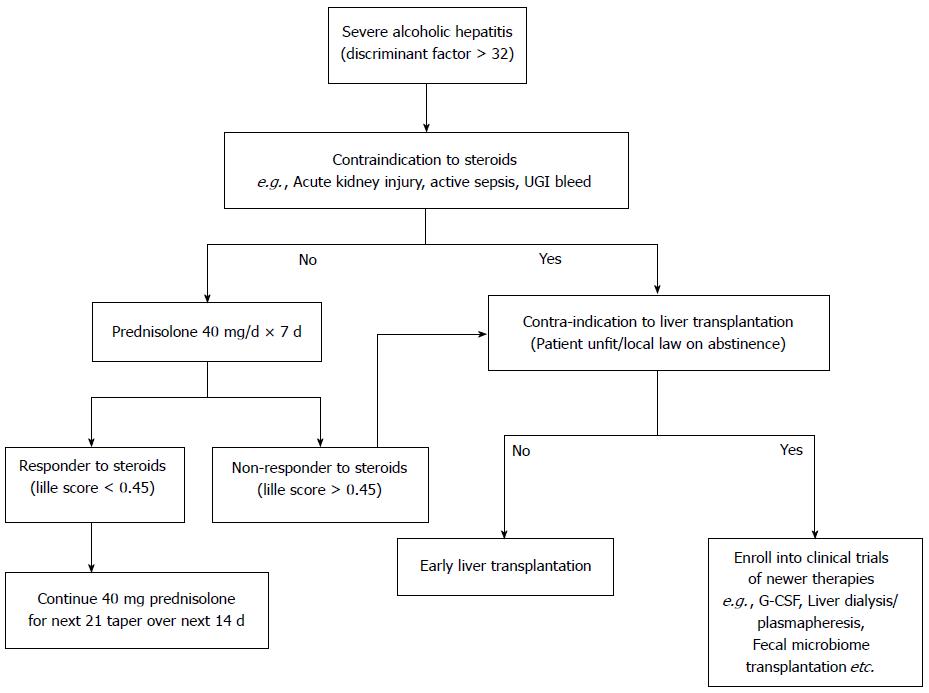
Prednisolone is the treatment of choice in the management of sub group of severe alcoholic hepatitis without any active infections or active UGI bleeding or renal impairment. Prednisolone is conventionally given at a dose of 40 mg per day for 4 wk followed by tapering over next 2 wk, with close monitoring for infections and other side effects of steroids. Despite the best standard of care and steroids, many patients either do not respond to steroids or are not eligible for steroids. Large majority of steroid intolerant, ineligible, unresponsive alcoholic hepatitis patients are thus left with no specific available treatment options. Better understanding of the pathogenesis of alcoholic hepatitis and advances in the basic sciences are opening new avenues for alcoholic hepatitis, as described below (Table 1).
| Gut microbiota modification |
| Antibiotics (luminal, systemic) |
| Prebiotics and probiotics |
| Fecal microbiota transplantation (FMT) |
| Blockade of LPS and its downstream pathways e.g., PD-1 and TIM-3 inhibition |
| Immune modulation |
| Chemokines e.g., CCL20 inhibition |
| IL-8, IL-17 inhibition |
| Recombinant IL-22, recombinant human IL-10 |
| Osteopontin inhibition |
| TNF-alfa superfamily receptor modulation |
| ADAMTS 13 enhancement |
| Inhibition of complement activation |
| Inhibition of inflammasome activation |
| Increasing steroid sensitivity |
| E.g., Basiliximab, Theophylline |
| Modification of genetic polymorphism of alcohol metabolizing enzymes |
| Epigenetic modification of alcohol induced liver damage |
| Liver regeneration and Early liver transplantation |
| Granulocyte colony stimulating factor (G-CSF) |
| Liver transplantation (DDLT/LDLT) |
| Setting up of alcohol units for post transplant support |
| Others |
| Extracorporeal liver support |
| Granulocytopheresis |
| Anti-oxidants - N-Acetyl Cysteine, S-Adenosyl Methionine |
Pre/probiotics and antibiotics: Several studies have shown that patients with liver disease have abnormal bowel flora overgrowth and thus probiotics, which help to restore normal bowel flora, have been proposed as a possible treatment for alcoholic liver disease[49,50]. Alcoholic patients who received probiotics (Bifidobacterium or Lactobacillus) for 5 d had improved AST, ALT and GGT levels in comparison to placebo[51]. Another study on alcoholic patients has shown that 4 wk probiotics (Bifidobacterium or Lactobacillus) improve and normalize neutrophil phagocytic capacity and decrease the endotoxin stimulated levels of soluble TNF-receptor-1, soluble TNF-receptor-2 and interleukin-10 ex vivo at the end of the study[52].
Rifaximin, a derivative of Rifamycin, with low systemic absorption and broad-spectrum activity against gastrointestinal tract micro-organisms that has been previously used to treat hepatic encephalopathy[53], is now being studied with regards to improving liver function in alcoholic cirrhosis. In a study, 28-d course of Rifaximin has been shown to decrease serum endotoxin levels in both systemic and splanchnic circulation along with significant improvement in HVPG in alcoholic cirrhotic patients[54].
Role of intestinal flora in the pathogenesis of alcoholic hepatitis is well known. However, targeting gut micro biome in the management of alcoholic hepatitis has rarely been attempted. This looks to be a promising area.
Lipopolysaccharide: Gut-derived microbial Lipopolysaccharide (LPS), a component of the outer wall of gram-negative bacteria, has been known to have a central role in the pathogenesis of ALD[55,56]. Alcohol has been known to cause dysbiosis and as well disrupt the gut barrier function, consequently, promoting the translocation of microbial LPS from the lumen of the intestines to the portal vein, where it travels to the liver. In the Kupffer cell, LPS binds to CD14, which combines with toll-like receptor 4, ultimately activating multiple pro-inflammatory cytokine genes[57]. Therefore, probiotics, prebiotics, antibiotics, or transplantation of gut-microbiota may be proposed as possible treatment avenues for AH, by attenuation of the increase in LPS or normalising the healthy gut flora. Recently, fecal microbiota transplantation (FMT) has been successfully used in the treatment of life-threatening infections with Clostridium difficile. Gut bacteria being actively involved in the pathogenesis of alcoholic hepatitis, FMT might have a potential role in the management of alcoholic hepatitis. There is however, little data to support this proposition.
Michelena et al[58] have recently shown in 162 alcoholic hepatitis patients that blood LPS levels help in predicting progression to multiorgan failure, mortality and the response to steroids. Severe AH patients also have increased expression of TLRs (TLR 2, 4, and 9) in neutrophils along with impaired phagocytic function and increased secretion of CXC chemokines[44,59] suggesting that increased expression of TLRs can trigger neutrophils to display an inflammatory rather than phagocytic phenotype. Markwick et al[60] have recently shown that 2 known immunoinhibitory factors [i.e., programmed death (PD-1) and T-cell immunoglobulin and mucin domain (TIM-3)] may play a role in the impaired immune function in AH. The authors found that antibacterial innate and adaptive immunity is severely dysfunctional in AH, differentiating these patients from stable alcoholic cirrhotics. Importantly, LPS or gut-derived endotoxin, which are typically elevated in sera of patients with AH, induced the overexpression of PD-1 and TIM-3 and their ligands PD-L1 and galectin-9 in all T-cell subsets. The blockade of these immunoinhibitory pathways restored normal lymphocyte (T-cell, natural killer/natural killer T cells, and T-regulatory cells) immunity and enhanced neutrophil antimicrobial activity. These results strongly suggest that LPS-induced expression of immunoinhibitory factors may play a role in the impaired antibacterial host immunity in AH. They also have shown that reduced interferon-γ/IL-10 ratios had a direct effect on PD1 and Tim3 expression on T-cell subsets. In another study, the soluble CD163, a specific marker of inflammatory macrophage activation was shown to be elevated in severe alcoholic hepatitis patients likely via LPS pathway (10 folds in comparison to controls and 3 folds in comparison to stable alcoholic cirrhotics)[61]. Hepatic macrophages may thus present a target for biological therapy of AH. Inflammatory activation of resident hepatic macrophages (Kupffer cells) by portal-derived lipopolysaccharide (LPS) has a primary role in hepatic inflammation in alcoholic hepatitis.
So, gut dysbiosis leading to increased gut derived bacterial products leads to immune-activation, exhaustion and paralysis in severe alcoholic hepatitis. Thus, changing the gut microbiome and/or altering the down stream inhibitory immune signals might improve outcomes in alcoholic hepatitis (Figure 2).
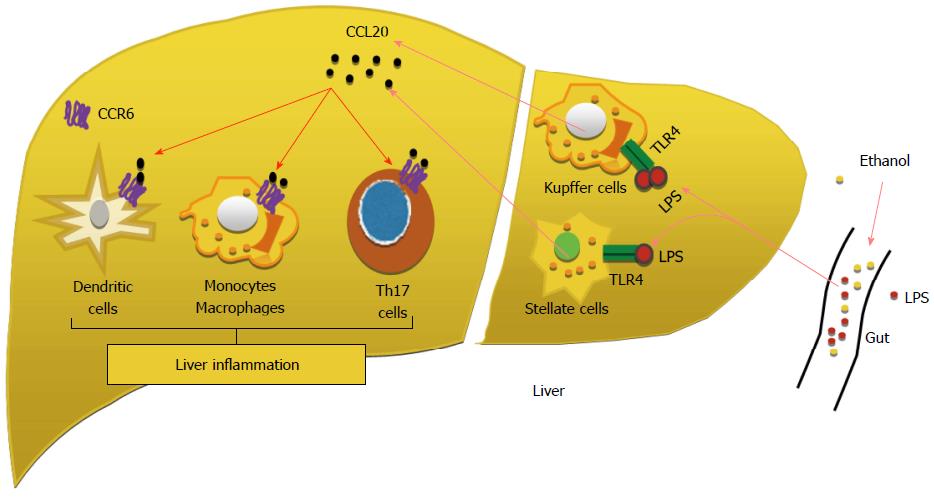
Chemokines: Chemokines are known to play an important role in the pathogenesis of alcoholic hepatitis. Many of the chemokines have been shown to be upregulated in the livers of alcoholic hepatitis. Early studies revealed that the levels of several CXC subfamily members, including IL-8, Gro-α, CXCL5, CXCL6, CXCL10 and platelet factor 4, are significantly elevated in AH livers compared with normal healthy control livers, and correlate with neutrophil infiltration and the severity of portal hypertension and patient survival[36,38,62]. CC chemokine CCL2 is also upregulated[62]. Higher expression levels of IL-8, CXCL5, Gro-γ, and CXCL6 were associated with a worse prognosis[38]. Among these chemokines, CCL20 is one of the most upregulated chemokines in AH liver tissue[63]. Many inflammatory mediators such as LPS, TNF-α, and IL-1β induce CCL20 expression. The mechanism by which CCL20 contributes to the pathogenesis of AH remains unknown. The major function of CCL20 is to attract lymphocytes, dendritic cells, Helper T 17 (Th17) cells and monocytes and to a much lesser extent, attract neutrophils[62]. These cells then produce inflammatory mediators and chemokines that subsequently cause neutrophil infiltration in AH. So CCL20 has an important role in regulating adaptive immunity and autoimmunity (Figure 3). In their study Affò et al[63] have shown that the levels of CCL20 are elevated in patients with alcoholic hepatitis and correlate with the levels of endotoxemia, degree of fibrosis and portal hypertension. Macrophages and hepatic stellate cells (HSCs) were identified as the main CCL20 producing cell types. And also they have shown that silencing CCL20 in vivo reduces - LPS induced aspartate aminotransferase and lactate dehydrogenase serum levels and hepatic proinflammatory and profibrogenic genes.
Further studies in future models and human livers of AH are required to determine if targeting CCL20 is an effective and safe therapeutic strategy to modulate the inflammatory response and liver injury in alcoholic hepatitis[64].
Several chemokine receptor antagonists are being developed. But so for only two drugs have been approved for non-inflammatory diseases: a CCR5 inhibitor used to treat HIV, and a CXCR4 antagonist which serves as a hematopoietic stem cell mobiliser[65]. Most common reason for failure is the receptor redundancy (one chemokine can target several receptors and vice versa). Development of chemokine receptor blockers which can block multiple receptors are needed to counter the receptor redundancy. Better translational and human studies are required to identify further key targets to block chemokine induced hepatic inflammation.
IL-8: Inhibition of neutrophil mediated hepatic injury might also be a potential therapeutic approach in managing AH. A higher level of IL-8 gene expression has been shown to correlate with poorer outcome in patients with alcoholic hepatitis[38]. IL-8 gene expression is also related to neutrophil hepatic infiltration as well as increased portal pressure[38]. IL-8 inhibition should lead to decrease in neutrophil infiltration of liver but the effect on bactericidal function of neutrophils could be a potential concern.
Th17: T cells producing Th17 have a central role in many inflammatory and autoimmune conditions[66]. IL-17 can act as a neutrophilchemotaxinand can also stimulate production of other chemotaxins such as IL-8 and CXCL1[67]. Serum and liver tissue IL-17 and IL-17 producing T cell are elevated in patients with alcoholic hepatitis and the number of infiltrating cells correlate with the Maddrey Discriminant Function. The increased levels of IL-17 within the liver are likely to act on hepatic stellate cells, which when stimulated with IL-17 increase chemotaxis of neutrophils[39].
Secukinumab, a humanised anti-IL-17A monoclonal antibody has shown some success in the phase 1 trials in the treatment of rheumatoid arthritis, psoriasis and uveitis[68]. To date no studies of secukinumab have been reported in patients with liver disease, which could be a potential agent.
IL-22/signal transducer and activator of transcription 3: IL-22 a member of IL-10 family of cytokines (produced by helper T17 and NK cells) has important role in bacterial infections and tissue repair[37]. IL-22 might be used to treat patients with ALD because of its antioxidant, antiapoptotic, antisteatotic, proliferative, and antimicrobial effects and it has been shown to work through activation of STAT3 in animal models of ALD. More over IL-22 receptor 1 expression is upregulated, whereas IL-22 expression is undetectable, in patients with alcoholic hepatitis[40]. Støy et al[69] have found higher frequencies of IL-22 producing T-helper cells in alcoholic hepatitis patients (n = 21) in comparison with stable cirrhotics (n = 10) and healthy controls (n = 10). The frequency of IL-22-producing T helper cells was higher in AH patients and more so in those whose condition seemed to improve with improvement in alcoholic hepatitis severity. A study in a chronic ethanol-fed mouse model showed that treatment with recombinant IL-22 improves liver injury and hepatic oxidative stress[40] and another study in murine model of acute hepatitis, IL-22 receptors have been shown to be upregulated in the hepatocytes and blockade of IL-22 receptors has been shown to exacerbate the disease and administration of IL-22 to ameliorate the same[70].
So, IL-22 augmentation could be a potential therapeutic target in the management of alcoholic hepatitis. As IL-22 is produced by IL-17 cells, which also are involved in the production of pro inflammatory cytokines in the pathogenesis of alcoholic hepatitis, selective augmentation of IL-22 might be useful.
IL-10: IL-10 is a potent anti-inflammatory cytokine, which suppresses the production of many pro-inflammatory cytokines including TNF-α by Kupfer cells, monocytes and T-Helper cells and also from neutrophils[71]. IL-10 levels correlate with response to steroids and survival in patients of alcoholic hepatitis[72,73]. So far, recombinant human IL-10 (rhuIL-10) has failed to show any benefit in clinical trials on patients with Crohn’s disease although well tolerated[74,75]. A pilot open label study of rhuIL-10 in combination with glucocorticoids in 8 patients with severe AH failed to show any changes to neutrophil-derived or serum IL-8 and TNF-α production or improvement in mortality or disease severity in comparison with the control group[76].
Osteopontin: Osteopontin is an extracellular matrix protein and is highly expressed in alcoholic hepatitis patients and the levels of osteopontin (OPN) are found to have very good correlation with the disease severity. OPN is reported to act as an immune modulator in a variety of manners- chemotaxis, immune cell recruitment and activation and modulation of apoptosis. In target cells, OPN binds to integrin and CD44 to promote profibrogenic and inflammatory actions[77]. There is growing evidence to suggest that OPN plays a major role in the wound-healing response to acute and chronic injury in many organs[78]. Hepatic expression and serum levels of OPN are markedly increased in AH, compared to normal livers and other types of chronic liver diseases, and its levels have been shown to correlate with short-term survival. Serum levels of OPN also correlated with hepatic expression and disease severity. OPN was mainly expressed in areas with inflammation and fibrosis. Two proteases that process OPN (thrombin and matrix metalloproteinase 7) and cleave OPN are increased in livers with AH. OPN synthesis is induced by lipopolysaccharide[78]. Fibrogenic mediators such as TGF-β are known to increase the OPN expression and also the alcohol mediated liver injury is attenuated in mice that lack OPN[21]. Human and experimental data suggest a role for OPN in the pathogenesis of AH. Further studies should evaluate OPN as a potential therapeutic target.
TNF superfamily receptors: Anti TNF-α agents like infliximab have been used with only limited clinical success and significant side effects[79,80]. Several members of the TNF receptor superfamily are markedly upregulated in patients with alcoholic hepatitis. TNF receptor superfamily member 12A (also known as Fn14 or the Tweak receptor) is markedly over expressed in these patients and its expression correlates with the severity of alcoholic hepatitis[21]. TNFRSF12A is mainly expressed in hepatic progenitor cells, which accumulate in patients with severe forms of alcoholic hepatitis. Therapeutic utility of this information needs further exploration. With the availability of metabolomics and transcriptomics, new therapeutic targets are expected. Affò et al[81], have identified increased expression of TNF-α superfamily receptors by transcriptome analysis of the liver tissue from alcoholic hepatitis patients. They have also shown that 207 genes are differentially expressed in patients with AH (> 5-fold) and revealed seven pathways differentially regulated including “cytokine-cytokine receptor interaction”. Several tumor necrosis factor (TNF) superfamily receptors, but not ligands, were overexpressed in AH. Fn14, the receptor for TNF-like weak inducer of apoptosis, was selectively upregulated in patients with AH (n = 5) in comparison to normal controls (n = 7).
Intricate linkage analysis of the downstream signaling pathways might provide further insight in to the exact role and as well as therapeutic utility of the TNF and its receptors.
Proapoptotic molecules:Fas and Bcl-2: Oxidative stress stimulates the expression of Fas and Bcl2 in the livers of patients with alcoholic liver disease[82,83]. These targets are very appealing in the short-term management of acute alcoholic hepatitis.
ADAMTS13: von Willebrand factor cleaving protease: Activity of ADAMTS13 (a disintegrin and metalloproteinase produced by stellate cells) is decreased in alcoholic hepatitis due to pro inflammatory cytokines. This leads to accumulation of thrombi of unusually large von Willebrand factor leading to sinusoidal microcirculatory disturbances and subsequent liver injury[84,85]. Therapeutic potential of this fact needs to further exploration.
Complement: Inflammation and systemic inflammatory response (SIRS) are an integral part of alcoholic hepatitis. Alcohol induced activation of complement system contributes to the pathophysiology of alcohol related liver injury[86]. In their study Shen et al[86] have shown significantly higher immunoreactivity intensity of C1q, C3, and C5 as well gene expression of C1q and C5 in patients with alcoholic hepatitis than that seen in normal controls. They have also shown the co-localization of C5a receptor (C5aR) in Mallory-Denk bodies (MDBs) forming balloon hepatocytes. C5aR was focally overexpressed in the MDB forming cells.Inhibition of complement activation could be a potential therapeutic option in the management of severe alcoholic hepatitis.
Inflammasomes: Recent studies indicate that the inflammasome activation plays important roles in the pathogenesis of AH. Nod-like receptor protein 3 (NLRP3) is a key component of the macromolecular complex that is so called the inflammasome that triggers caspase 1-dependent maturation of the precursors of IL-1β and IL-18 cytokines. It is expressed in myeloid cells and is a component of the innate immune system[87]. Inflammasome activation in AH liver biopsy specimen has been shown to correlate with Mallory Denk body (MDB) formation, suggesting that MDB could be an indicator of the extent of inflammasome activation.
So, new studies targeting inhibition of inflammaosome activation might discover some new treatment avenues for managing alcoholic hepatitis.
A 48-h in vitro measure of steroid sensitivity, the dexamethasone inhibition of lymphocyte proliferation assay (DILPA), predicts 6-mo survival with 78% sensitivity[88]. The accuracy of the DILPA in predicting 6-mo survival, assessed by area under the receiver-operating characteristic (AUROC), was 0.86. Addition of the anti-IL-2 receptor (anti-CD25) monoclonal antibody, Basiliximab was shown to reverse the steroid resistance in vitro with improvement in lymphocyte proliferation count in 91% of the tested patients. Suggesting that intrinsic lack of steroid sensitivity may contribute to poor clinical response to steroids in severe AH and IL-2 receptor blockade represents a potential mechanism to overcome this. Basiliximab, the CD25 (IL-2 receptor) inhibitor which is used as single dose therapy to prevent transplant rejection, could reverse glucocorticoid resistance in peripheral blood mononuclear cells from patients with alcoholic hepatitis[88].
As T cells play a role in the recruitment of neutrophils and the perpetuation of inflammation in AH, Basiliximab may prove to be a useful adjunct to glucocorticoid therapy in patients who do not respond to this therapy.
Genetic polymorphism: Discoveries through genomic technologies and genome-wide association studies (GWAS), have increased evidence for genetic determinants of liver damage and progression to cirrhosis, and have implicated novel etiologic pathways. We know that not all heavy drinkers ever progress to cirrhosis. So the question remains why some people progress to cirrhosis, while others who drink to similar levels don’t? Evidence from twin studies, variability in inter-ethnic ALD mortality rates and the recent association of PNPLA3 variant with alcoholic cirrhosis indicate that there is an underlying genetic basis that may account for the variability of liver damage observed in heavy drinkers, independent of alcohol dependence[89]. Genetic polymorphisms of ethanol metabolizing enzymes such as cytochrome p450 (CYP) 2E1 activation may change the severity of ASH.
Epigenetic modification: Ethanol consumption causes epigenetic changes that may contribute to alcohol-induced liver damage. Exposure to ethanol or its metabolite (acetate), up-regulates histone acetylation in macrophages, which causes up-regulation of transcription of several pro-inflammatory cytokines, leading to the development of alcoholic hepatitis. Therefore, epigenetic modifications can be new therapeutic target[90].
Liver regeneration and stem cell therapy are active areas of current research in the field of hepatology. Ineffective liver regeneration has been postulated as one of the major reasons for progressive liver failure and non-recovery with conservative management in patients with alcoholic hepatitis. Recently Dubuquoy et al[91] have shown in explant livers from a small group of steroid non-responsive alcoholic hepatitis (n = 16) that their livers lack cytokine profile conducive for liver regeneration (TNF-α and IL-6) and also have shown insufficient hepatic progenitor cell differentiation tendency towards hepatocyte lineage.
In experimental models of alcoholic hepatitis, the administration of the cytokine granulocyte colony-stimulating factor (G-CSF) was found to mobilize the hematopoietic stem cells, induce liver regeneration, and improve survival[92,93]. In patients of alcoholic hepatitis, a 5 d G-CSF administration, (10 μg/kg/d, subcutaneously) mobilised CD34+ stem cells, increased circulating hepatocyte growth factor and induced proliferation of hepatic progenitor cells in liver biopsy specimens[94]. A randomised placebo-controlled trial from our group using G-CSF (12 doses 5 μg/kg s.c. each over 1 mo) in patients with ACLF (57% had alcoholic hepatitis) has shown mobilization of CD34+ stem cells, with significantly improved survival, and decreased the risk of bacterial infection and kidney failure in G-CSF group[95]. In another recent study, Singh et al[96] have used G-CSF (5 μg/kg every 12 h for 5 d) in patients with severe alcoholic hepatitis and compared with Pentoxifylline (1200 mg/d) and have shown mobilization of CD34+ cells and as well decreased infections, improved Maddrey’s discriminant score and increased survival at 3 mo in patients receiving G-CSF. Other than stem cell mobilization and liver regeneration another potential mechanism of action of G-CSF in alcoholic hepatitis patients is postulated to be by stimulating the bactericidal activity of neutrophils[97-99] thus overcoming the immune paralysis.
It would be worthwhile to assess the role of G-CSF therapy in comparison to corticosteroids as none of the above studies have used steroids in their standard of care. G-CSF might improve the ineffective regeneration seen in those who fail to respond to the standard of care (e.g., steroids) and may as well improve the neutrophil functionality and prevent infections. There is also need to study the role G-CSF in treating alcoholic hepatitis patients who are steroid ineligible or steroid unresponsive, in larger group of patients.
The vast majority of transplant programs (85%) require 6 mo of abstinence[100] prior to transplantation commonly known as “6-mo rule”. But there is a lack of evidence to support a 6-mo sobriety period. Patients who do not respond to steroids have a 6-mo survival of 25%-30%, and patients with hepatorenal syndrome (HRS) have a 3-mo mortality rate above 90%, unless treated with liver transplantation[101]. To date, nobody has been able to establish a certain period of abstinence, which ensures no future alcohol relapses; apart from this fact, in case of SAH, the 3-mo mortality rate is about 70%[102].
Currently there are very few options left in the management of severe alcoholic hepatitis patients, especially once they are unresponsive to steroids with Lille score > 0.45. Despite controversies, liver transplantation remains the sole major hope for such patients. Liver transplantation is more ethical in the case of living related donor transplantation as no other patient is deprived of the limited cadaveric resources and the emotionally attached relative wants to donate part of his liver to save his relative who may not survive to fulfill the current 6-mo abstinence rule. In a recently conducted public survey[103], majority of the respondents were neutral towards donating their organs for an early transplantation of a severe alcoholic hepatitis patient and only minority (26.3%) were hesitant to donate their organs to such patients. The scenario in a severe alcoholic hepatitis with a good family support and in the setting of living donor liver transplantation should be even better. So, early transplantation for carefully selected patients with acute alcoholic hepatitis may not be as controversial to the public as previously thought.
Emerging data has challenged the 6-mo abstinence rule as beneficial effects of early liver transplantation have been shown in select group of steroid un-responsive severe alcoholic hepatitis patients. In an elegant study, by Mathurin et al[104], twenty-six patients (median Lille score, 0.88) were selected and placed on liver transplantation list within a median of 13 d after nonresponse to steroid therapy. The cumulative 6-mo survival rate was higher among patients who received early transplantation than among those who did not (77% vs 23%, P < 0.001). This benefit of early transplantation was maintained through 2 years of follow-up (HR = 6.08; P = 0.004). The authors concluded that there are no major ethical barriers in transplanting patients affected by severe SAH, not responding to medical therapy. Presence of an Alcohol Addiction Unit (Alcohology unit) within a liver transplant center may significantly reduce the risk of alcohol relapse and the recurrence of disease after LT, and may allow liver transplantations in some selected patients, even in case of less than 6 mo of abstention[105]. Singal et al[106] have also shown a 5-year outcome in alcoholic hepatitis patients (n = 11) at par with alcoholic cirrhotic patients (n = 33) undergoing liver transplant from the UNOS database.
There is published data of seven severe alcoholic hepatitis patients with non-response to therapies, and hepatorenal syndrome, who were submitted to transjugular intrahepatic portosystemic stent shunt (TIPS), and then underwent liver transplantation[102,107]. Steroid therapy was contraindicated because of the presence of renal failure. All patients were followed up by the Alcohology Unit, and attended self-help groups. None of them had recidivism over the next 5 years.
Despite the promising results from many studies and over all public and professional concern and intent towards a shorter mandatory abstinence period prior to liver transplant after initial non-response to medical management, too many uncertainties exist. There is a need to define and form guidelines for setting universally suitable and logically acceptable norms to do liver transplant in this group of patients which should also address delicate issues like pre transplantation counseling, deceased vs living related donor liver transplantation, setting up of Alcohology units for post transplant support systems etc.
Extracorporeal liver support: Extracorporeal liver support procedures, which have the ability to remove some potential damaging circulating molecules may, therefore, logically have a role in patients with severe AH. Survival in severe alcoholic hepatitis patients with non-response to medical care and renal impairment is very poor even with best of the available management. Despite great efforts[108,109], no clear benefits have been proved using these complicated liver dialysis devices, which additionally are very expensive and have many issues to be answered before the utilization in the clinical setting. There are some motivating reports concerning albumin dialysis[110] as a support treatment in patients with severe AH which may potentially bridge recovery or liver transplantation who otherwise have no other options available especially those with renal impairment[111].
Granulocytapheresis: Granulocytapheresis, a technique that removes up to 60% of activated granulocytes and monocytes from circulating blood, is well tolerated and many case series exist in literature on its usefulness in severe alcoholic hepatitis patients. Some also mention their benefit in steroid non-responders[112,113]. Role of granulocytapheresis is still not proven in any good quality studies till date and only case series are available.
Anti-oxidants: Although oxidative stress is implicated in the pathogenesis of alcoholic hepatitis[114], several studies have negated any additional benefit of N-Acetyl cysteine in comparison to corticosteroids in the management of alcoholic hepatitis[115-117]. Only few of the studies have shown a short-term benefit of combination therapy with corticosteroid plus N-acetylcysteine with increased 1-mo survival among patients with severe alcoholic hepatitis, but without any improvement in 6 mo survival was noted[117]. A Cochrane review earlier had shown that use of S-Adenosyl-L-Methionine is not of any help in managing alcoholic hepatitis[118]. Conceptually anti-oxidants should potentially have a role in the management of alcoholic hepatitis but we need more data to establish the definitive role of N-Acetyl Cysteine or S-Adenosyl-L-Methionine in the management of severe alcoholic hepatitis. There remains a large void of treatment options for SAH. The ongoing clinical trials in treating alcoholic hepatitis are mentioned in Table 2. A major improvement in our understanding and a paradigm shift in the treatment approaches are required to improve the outcome of these patients.
| Treatment | Type of molecule/intervention | Mechanism of action | Identifier |
| ELAD | Extracorporeal, human cell-based liver support system | Supplement hepatic function | NCT01829347, NCT00973817, NCT01471028 |
| Obeticholic acid | Biliary acid | Affect bile acid abnormalities | NCT02039219 |
| Corticosteroids + Bovine + Colostrum | Protein supplement | Improving immunity | NCT02473341 |
| IMM 124-E | Hyperimmune bovine colostrum | Improving immunity | NCT01968382 |
| G-CSF | Liver regeneration/immunomodulation | improves liver regeneration and immunity in steroid non-responders | NCT02442180, NCT02451033, NCT01820208 |
| Corticosteroids + N-acetyl cysteine | Anti-oxidant | Augments steroid function | NCT00863785 |
| Amoxicillin + corticosteroid | Antibiotic | Decreases infections | NCT02281929 |
| S-adenosyl-l-methionine | Antioxidant | decreasing oxidative stress | NCT02024295 |
| Rifaximin | Luminal antibiotic | Improves gut dysbiosis | NCT02116556, NCT02485106 |
| Ciprofloxacin | Antibiotic | Decreasing infections | NCT02326103 |
| Emricasan/IDN-6556 | Pan-caspase inhibitor | Reduces apoptosis | NCT01912404 |
| Lactobacillus Rhamnosus GG | Probiotic | Improving dysbiosis | NCT01922895 |
| MycophenolateMofetil and Rilonacept | Immunosuppressant and immune modulation | Decreasing hepatic inflammation | NCT01903798 |
| Metadoxine | Anti-oxidant | NCT02161653 | |
| Fecal microbiota transplantation | Healthy microbiome replacement | Correction of dysbiosis | NCT02458079 |
| Early liver transplantation | New liver | Liver transplant in patients unresponsive to medical treatment | NCT01756794 |
| Anakinra | interleukin-1 receptor antagonist | Decreases hepatic inflammation | NCT01809132 |
The burden of alcoholic hepatitis is on the rise and the proportion of patients with alcoholic hepatitis is rapidly increasing with better treatment options of viral liver diseases. The morbidity, mortality and the treatment options for the management of alcoholic hepatitis have not significantly changed in the last many decades. Currently steroids, pentoxifylline and nutrition remain the only acceptable treatment options. A better and newer treatment options for alcoholic hepatitis are the need of the hour.Advances in the basic science, “Omics” platform and translational medicine have given a better insight into the pathogenesis and have opened up many potential new therapeutic avenues in the management of alcoholic hepatitis. Targeting gut microbes and their products, targeting hepatic inflammation and infections through immune modulation, improving liver regeneration by G-CSF and early liver transplantation for those not responding to the standard of care (Figure 4) are the most promising areas for research and future clinical trials should focus on these areas in developing new therapies in the management of alcoholic hepatitis.
P- Reviewer: Abenavoli L, Mach TH, Skrypnyk IN S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Jinjuvadia R, Liangpunsakul S. Trends in Alcoholic Hepatitis-related Hospitalizations, Financial Burden, and Mortality in the United States. J Clin Gastroenterol. 2015;49:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 2. | André T, Tournigand C, Mineur L, Fellague-Chebra R, Flesch M, Mabro M, Hebbar M, Postel Vinay S, Bidard FC, Louvet C. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007;18:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Antar R, Wong P, Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26:463-467. [PubMed] |
| 5. | Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 2015;35:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, Deltenre P, Canva V, Plane C, Mathurin P. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Duseja A. Combination Therapy in Severe Alcoholic Hepatitis-Doesn’t Really Work. J Clin Exp Hepatol. 2013;3:353-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, Diaz E, Thabut D, Moirand R. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | De B, Mandal S, Sau D, Mani S, Chatterjee S, Mondal S, Bhattacharya K, Sil K, Bhattacharya R. Pentoxifylline Plus Prednisolone versus Pentoxifylline Only for Severe Alcoholic Hepatitis: A Randomized Controlled Clinical Trial. Ann Med Health Sci Res. 2014;4:810-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 554] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 11. | Salaspuro MP, Shaw S, Jayatilleke E, Ross WA, Lieber CS. Attenuation of the ethanol-induced hepatic redox change after chronic alcohol consumption in baboons: metabolic consequences in vivo and in vitro. Hepatology. 1981;1:33-38. [PubMed] |
| 12. | Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049-2063. [PubMed] |
| 13. | Coll O, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 476] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 16. | Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem. 2002;277:29342-29347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 413] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 19. | You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798-1808. [PubMed] |
| 20. | Sugimoto T, Yamashita S, Ishigami M, Sakai N, Hirano K, Tahara M, Matsumoto K, Nakamura T, Matsuzawa Y. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. J Hepatol. 2002;36:157-162. [PubMed] |
| 21. | Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491-501. [PubMed] |
| 22. | Bataller R, Rombouts K, Altamirano J, Marra F. Fibrosis in alcoholic and nonalcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2011;25:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | MacSween RN, Burt AD. Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 162] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 342] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 25. | Altamirano J, Higuera-de laTijera F, Duarte-Rojo A, Martínez-Vázquez MA, Abraldes JG, Herrera-Jiménez LE, Michelena J, Zapata L, Perez-Hernández J, Torre A. The amount of alcohol consumption negatively impacts short-term mortality in Mexican patients with alcoholic hepatitis. Am J Gastroenterol. 2011;106:1472-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Svegliati-Baroni G, Baraona E, Rosman AS, Lieber CS. Collagen-acetaldehyde adducts in alcoholic and nonalcoholic liver diseases. Hepatology. 1994;20:111-118. [PubMed] |
| 27. | You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1-G6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 28. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 29. | Román J, Colell A, Blasco C, Caballeria J, Parés A, Rodés J, Fernández-Checa JC. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HEP G2 cells: effect on transcription factors AP-1 and NF-kappaB. Hepatology. 1999;30:1473-1480. [PubMed] |
| 30. | Zhou Z, Sun X, Kang YJ. Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway. Am J Pathol. 2001;159:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Mansouri A, Gaou I, De Kerguenec C, Amsellem S, Haouzi D, Berson A, Moreau A, Feldmann G, Lettéron P, Pessayre D. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181-190. [PubMed] |
| 32. | Yin M, Gäbele E, Wheeler MD, Connor H, Bradford BU, Dikalova A, Rusyn I, Mason R, Thurman RG. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. 2001;34:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488-1499. [PubMed] |
| 34. | Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917-920. [PubMed] |
| 36. | Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballería J. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballería J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 39. | Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 40. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 41. | Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737-4742. [PubMed] |
| 42. | Donohue TM, Cederbaum AI, French SW, Barve S, Gao B, Osna NA. Role of the proteasome in ethanol-induced liver pathology. Alcohol Clin Exp Res. 2007;31:1446-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 44. | Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 45. | Albano E. Role of adaptive immunity in alcoholic liver disease. Int J Hepatol. 2012;2012:893026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 46. | Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30 Suppl 1:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Rachakonda V, Gabbert C, Raina A, Bell LN, Cooper S, Malik S, Behari J. Serum metabolomic profiling in acute alcoholic hepatitis identifies multiple dysregulated pathways. PLoS One. 2014;9:e113860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Rachakonda V, Gabbert C, Raina A, Li H, Malik S, DeLany JP, Behari J. Stratification of risk of death in severe acute alcoholic hepatitis using a panel of adipokines and cytokines. Alcohol Clin Exp Res. 2014;38:2712-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 50. | Zhao HY, Wang HJ, Lu Z, Xu SZ. Intestinal microflora in patients with liver cirrhosis. Chin J Dig Dis. 2004;5:64-67. [PubMed] |
| 51. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 52. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 53. | Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995;49:467-484. [PubMed] |
| 54. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 273] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 56. | Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18:337-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 693] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 58. | Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P, Dominguez M, García-Pagán JC, Fernández J, Arroyo V. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 59. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2162] [Article Influence: 180.2] [Reference Citation Analysis (5)] |
| 60. | Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590-602.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 61. | Sandahl TD, Grønbaek H, Møller HJ, Støy S, Thomsen KL, Dige AK, Agnholt J, Hamilton-Dutoit S, Thiel S, Vilstrup H. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. Am J Gastroenterol. 2014;109:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Gao B, Xu M. Chemokines and alcoholic hepatitis: are chemokines good therapeutic targets? Gut. 2014;63:1683-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Affò S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, Millán C, Coll M, Caviglia JM, Arroyo V. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 64. | Leake I. Alcoholic hepatitis: potential role of cytokine CCL20 in alcoholic hepatitis. Nat Rev Gastroenterol Hepatol. 2014;11:76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Pease J, Horuk R. Chemokine receptor antagonists. J Med Chem. 2012;55:9363-9392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3779] [Article Influence: 236.2] [Reference Citation Analysis (0)] |
| 67. | Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748-753. [PubMed] |
| 68. | Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 692] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 69. | Støy S, Sandahl TD, Dige AK, Agnholt J, Rasmussen TK, Grønbæk H, Deleuran B, Vilstrup H. Highest frequencies of interleukin-22-producing T helper cells in alcoholic hepatitis patients with a favourable short-term course. PLoS One. 2013;8:e55101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 502] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 71. | Fuchs AC, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, Kennedy JS, Rabson AR, Radwanski E, Affrime MB. Clinical, hematologic, and immunologic effects of interleukin-10 in humans. J Clin Immunol. 1996;16:291-303. [PubMed] |
| 72. | Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, Opolon P, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol. 2000;32:579-586. [PubMed] |
| 73. | Taïeb J, Chollet-Martin S, Cohard M, Garaud JJ, Poynard T. The role of interleukin-10 in severe acute alcoholic hepatitis. Clin Biochem. 2001;34:237-238. [PubMed] |
| 74. | Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473-1482. [PubMed] |
| 75. | Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461-1472. [PubMed] |
| 76. | Dhanda AD, Lee RW, Collins PL, McCune CA. Molecular targets in the treatment of alcoholic hepatitis. World J Gastroenterol. 2012;18:5504-5513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 78. | Morales-Ibanez O, Domínguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, Houchi H, Affò S, Sancho-Bru P, Altamirano J. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Sharma P, Kumar A, Sharma BC, Sarin SK. Infliximab monotherapy for severe alcoholic hepatitis and predictors of survival: an open label trial. J Hepatol. 2009;50:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broët P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 81. | Affò S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millán C, Loaeza-del-Castillo A, Altamirano J. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2013;62:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 82. | Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 83. | Ramalho RM, Cortez-Pinto H, Castro RE, Solá S, Costa A, Moura MC, Camilo ME, Rodrigues CM. Apoptosis and Bcl-2 expression in the livers of patients with steatohepatitis. Eur J Gastroenterol Hepatol. 2006;18:21-29. [PubMed] |
| 84. | Matsuyama T, Uemura M, Ishikawa M, Matsumoto M, Ishizashi H, Kato S, Morioka C, Fujimoto M, Kojima H, Yoshiji H. Increased von Willebrand factor over decreased ADAMTS13 activity may contribute to the development of liver disturbance and multiorgan failure in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2007;31:S27-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Ishikawa M, Uemura M, Matsuyama T, Matsumoto M, Ishizashi H, Kato S, Morioka C, Fujimoto M, Kojima H, Yoshiji H. Potential role of enhanced cytokinemia and plasma inhibitor on the decreased activity of plasma ADAMTS13 in patients with alcoholic hepatitis: relationship to endotoxemia. Alcohol Clin Exp Res. 2010;34 Suppl 1:S25-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Shen H, French BA, Liu H, Tillman BC, French SW. Increased activity of the complement system in the liver of patients with alcoholic hepatitis. Exp Mol Pathol. 2014;97:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1457] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 88. | di Mambro AJ, Parker R, McCune A, Gordon F, Dayan CM, Collins P. In vitro steroid resistance correlates with outcome in severe alcoholic hepatitis. Hepatology. 2011;53:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Neuman MG, French SW, French BA, Seitz HK, Cohen LB, Mueller S, Osna NA, Kharbanda KK, Seth D, Bautista A. Alcoholic and non-alcoholic steatohepatitis. Exp Mol Pathol. 2014;97:492-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Kendrick SF, O’Boyle G, Mann J, Zeybel M, Palmer J, Jones DE, Day CP. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 91. | Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, Maggiotto F, Gantier E, Buob D, Leteurtre E. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 92. | Theocharis SE, Papadimitriou LJ, Retsou ZP, Margeli AP, Ninos SS, Papadimitriou JD. Granulocyte-colony stimulating factor administration ameliorates liver regeneration in animal model of fulminant hepatic failure and encephalopathy. Dig Dis Sci. 2003;48:1797-1803. [PubMed] |
| 93. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 94. | Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, Hadengue A. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 95. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 96. | Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 97. | Tato CM, Cua DJ. SnapShot: Cytokines IV. Cell. 2008;132:1062.e1-1062.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Rolas L, Makhezer N, Hadjoudj S, El-Benna J, Djerdjouri B, Elkrief L, Moreau R, Périanin A. Inhibition of mammalian target of rapamycin aggravates the respiratory burst defect of neutrophils from decompensated patients with cirrhosis. Hepatology. 2013;57:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Moreau R, Rautou PE. G-CSF therapy for severe alcoholic hepatitis: targeting liver regeneration or neutrophil function? Am J Gastroenterol. 2014;109:1424-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 100. | Testino G, Burra P, Bonino F, Piani F, Sumberaz A, Peressutti R, Giannelli Castiglione A, Patussi V, Fanucchi T, Ancarani O. Acute alcoholic hepatitis, end stage alcoholic liver disease and liver transplantation: an Italian position statement. World J Gastroenterol. 2014;20:14642-14651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 101. | Testino G, Ferro C, Sumberaz A, Messa P, Morelli N, Guadagni B, Ardizzone G, Valente U. Type-2 hepatorenal syndrome and refractory ascites: role of transjugular intrahepatic portosystemic stent-shunt in eighteen patients with advanced cirrhosis awaiting orthotopic liver transplantation. Hepatogastroenterology. 2003;50:1753-1755. [PubMed] |
| 102. | Testino G, Sumberaz A, Borro P. Comment to “liver transplantation for patients with alcoholic liver disease: an open question”. Dig Liver Dis. 2013;45:80-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Stroh G, Rosell T, Dong F, Forster J. Early liver transplantation for patients with acute alcoholic hepatitis: public views and the effects on organ donation. Am J Transplant. 2015;15:1598-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 104. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 654] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 105. | Addolorato G, Mirijello A, Leggio L, Ferrulli A, D’Angelo C, Vassallo G, Cossari A, Gasbarrini G, Landolfi R, Agnes S. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 106. | Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology. 2012;55:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 107. | Testino G, Leone S, Sumberaz A, Borro P. Liver transplantation in alcoholic patients. Alcohol Clin Exp Res. 2014;38:1800-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van Vlierberghe H, Escorsell A. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782-789.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 109. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 110. | Parés A, Mas A. Extracorporeal liver support in severe alcoholic hepatitis. World J Gastroenterol. 2014;20:8011-8017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003;38:24-31. [PubMed] |
| 112. | Morris JM, Dickson S, Neilson M, Hodgins P, Forrest EH. Granulocytapheresis in the treatment of severe alcoholic hepatitis: a case series. Eur J Gastroenterol Hepatol. 2010;22:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Kamimura K, Imai M, Sakamaki A, Mori S, Kobayashi M, Mizuno K, Takeuchi M, Suda T, Nomoto M, Aoyagi Y. Granulocytapheresis for the treatment of severe alcoholic hepatitis: a case series and literature review. Dig Dis Sci. 2014;59:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1491] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 115. | Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 116. | Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, Record C, Day CP. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 117. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 118. | Rambaldi A, Gluud C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst Rev. 2001;CD002235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |