Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3845
Peer-review started: December 22, 2015
First decision: January 13, 2016
Revised: February 9, 2016
Accepted: March 1, 2016
Article in press: March 2, 2016
Published online: April 14, 2016
Processing time: 101 Days and 22.2 Hours
AIM: To investigate whether autofluorescence imaging (AFI) endoscopy can distinguish non-erosive reflux disease (NERD) from functional heartburn (FH).
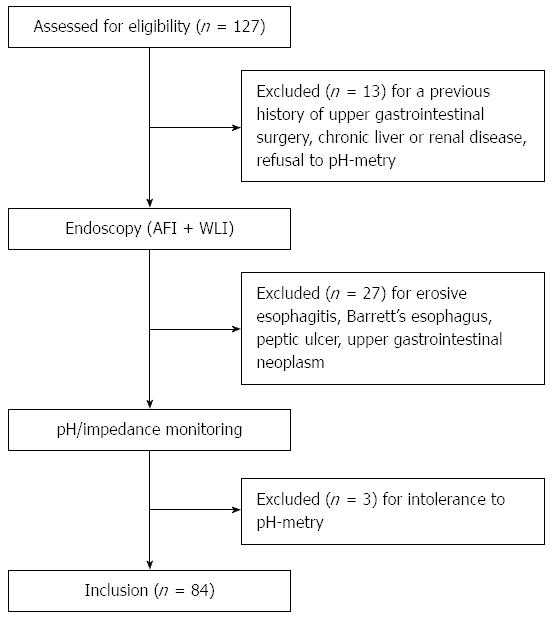
METHODS: In this prospective observational trial, 127 patients presenting with typical reflux symptoms for > 6 mo were screened. All the participants underwent endoscopy, during which white light imaging (WLI) was followed by AFI. Finally 84 patients with normal esophageal appearance on WLI were enrolled. It was defined as being suggestive of NERD if one or more longitudinal purple lines longer than one centimeter were visualized in the distal part of the esophagus during AFI endoscopy. Ambulatory 24-h multichannel intraluminal impedance and pH monitoring was also performed. After standard proton-pump inhibitor (PPI) tests, subjects were divided into an NERD group and an FH group and the diagnostic performance of AFI endoscopy to differentiate NERD from FH was evaluated.
RESULTS: Of 84 endoscopy-negative patients, 36 (42.9%) had a normal pH/impedance test. Of these, 26 patients with favorable responses to PPI tests were classified as having NERD. Finally 10 patients were diagnosed with FH and the others with NERD. Altogether, 68 (81.0%) of the 84 patients were positive on AFI endoscopy. In the NERD group, there were 67 (90.5%) patients with abnormal esophageal findings on AFI endoscopy while only 1 (10%) patient was positive on AFI endoscopy in the FH group. The sensitivity and specificity of AFI in differentiating NERD from FH were 90.5% (95%CI: 81.5%-96.1%) and 90.0% (95%CI: 55.5%-99.7%), respectively. Meanwhile, the accuracy, positive predictive value and negative predictive value of AFI in differentiating between NERD and FH were 90.5% (95%CI: 84.2%-96.8%), 98.5% (95%CI: 92.1%-99.9%) and 56.3% (95%CI: 30.0%-80.2%), respectively.
CONCLUSION: Autofluorescence imaging may serve as a complementary method in evaluating patients with NERD and FH.
Core tip: To date, few efforts have been put on the application of autofluorescence imaging (AFI) endoscopy in patients with non-malignant conditions such as gastrointestinal reflux disease (GERD). Our data showed that endoscopic features on AFI can distinguish non-erosive reflux disease (NERD) from functional heartburn (FH). Its real-time characteristics and simple endoscopic criteria may enhance the use of AFI as a complementary tool in the differentiation of NERD and FH. We believe that these findings have important implications for future research on the application of AFI endoscopy in patients with GERD.
- Citation: Luo X, Guo XX, Wang WF, Peng LH, Yang YS, Uedo N. Autofluorescence imaging endoscopy can distinguish non-erosive reflux disease from functional heartburn: A pilot study. World J Gastroenterol 2016; 22(14): 3845-3851
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3845.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3845
Functional heartburn (FH) and non-erosive reflux disease (NERD) share common manifestations, including the presence of reflux symptoms and the absence of esophageal abnormalities on conventional endoscopy. These common manifestations make it difficult to distinguish between FH and NERD without invasive diagnostic tests[1,2]. Autofluorescence imaging (AFI) is capable of identifying indistinct mucosal lesions[3,4] invisible on conventional endoscopy. Tri-modal endoscopy, which combines AFI with white light imaging (WLI) and narrow band imaging, has been used to screen for early stage gastrointestinal cancer. Recently, AFI endoscopy was shown to be useful in predicting acid reflux[5]. As is known to all, NERD is characterized etiologically by pathologic reflux, whereas FH is not. Whether AFI endoscopy is capable of differentiating NERD from FH has not yet been determined. This study aimed to investigate the diagnostic performance of AFI video endoscopy to distinguish NERD from FH in patients with typical reflux symptoms but no mucosal breaks in the esophagus on WLI.
The study protocol was approved by the Ethics Committee of Chinese PLA General Hospital and conformed to the principles of the Declaration of Helsinki. This trial has been registered at ClinicalTrials.gov (ID: NCT01504971). Written informed consent was obtained before each participant was enrolled.
In this prospective observational trial, consecutive patients with typical heartburn and/or regurgitation for > 6 mo were screened in Chinese PLA General Hospital between 2012 and 2014 (Figure 1). All patients underwent gastroscopy with both WLI and AFI functioning. Ambulatory impedance and pH monitoring was also performed. All participants were subsequently assessed by standard proton-pump inhibitor (PPI) tests. Patients with any symptom suggestive of esophageal motility disorders other than gastroesophageal reflux disease (GERD) were assessed by esophageal manometry.
Patients aged 18-75 years and with negative esophageal findings on WLI were eligible for this study. Patients would not be included if they had any known esophageal disease, including esophagitis or Barrett’s esophagus; gastric or duodenal ulcer (except scarring); a previous history of thoracic or upper gastrointestinal (GI) surgery; clinically significant heart, lung, liver, or kidney disease; or pregnancy.
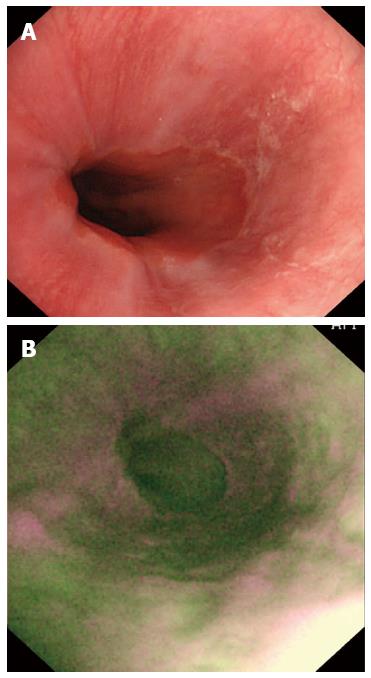
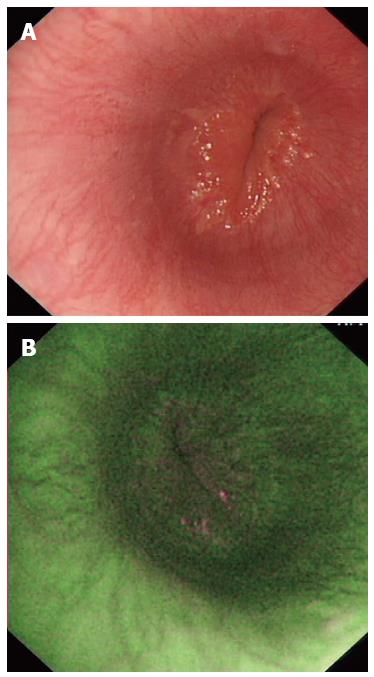
Before endoscopic examination, antisecretory therapy, including PPIs and histamine-2 receptor antagonists, was discontinued for no less than 1 mo. Oral antacid was allowed as rescue medication during wash-out period. Gastroscopy was performed using a FQ260Z endoscope (Olympus Inc., Tokyo, Japan), equipped with multiple charged coupled devices for both high-definition WLI and AFI. During endoscopic examination, the upper GI tract was carefully visualized using WLI, with the presence of a normal or abnormal esophagus documented. The video endoscope was subsequently switched to AFI mode and the esophagus again examined. It was defined as being suggestive of NERD if one or more longitudinal purple lines longer than one centimeter were visualized in the distal part of the esophagus during AFI endoscopy.
Ambulatory 24-h multichannel intraluminal impedance and pH monitoring was performed using the routine protocol of our department[5]. Briefly, a catheter (Sierra Scientific Instruments Inc., Los Angeles, CA, United States) was inserted transnasally and the pH sensor was sited 5 cm above the lower esophageal sphincter (LES) with the impedance recording segments positioned at 3, 5, 7, 9, 15 and 17 cm above the upper border of the LES. The catheter was connected to a data storage device programmed by an AccuTrac pH-Z System (Sierra Scientific Instruments Inc, Los Angeles, CA, United States). Patients were asked to record a diary of their symptoms and activity, including the time of rising in the morning, times in the supine position and meal times, as well as the onset of symptoms. AccuView analysis software (Sierra Scientific Instruments Inc, Los Angeles, CA, United States) was used to identify acid and non-acid episodes. Symptom association probability (SAP) and symptom index (SI) were assessed to determine the relationship of symptoms with acid, weakly acid or weakly alkaline reflux during monitoring. A positive pH/impedance test was defined as (1) acid exposure time more than 4.2% of monitoring time; (2) SAP ≥ 95%; or (3) SI > 50%[6,7].
A diagnosis of NERD was reached when the endoscopy-negative patients presented a positive pH/impedance or PPI test[8,9]. Consistent with Rome III criteria[8], patients with normal esophageal findings on WLI were diagnosed with FH if they had normal pH/impedance monitoring results and negative PPI tests.
Data are expressed as mean and standard deviation. The subjects were divided into an NERD group and an FH group. The diagnostic performance of AFI for differentiating NERD from FH was calculated, using the sensitivity, specificity, accuracy, positive predictive value and negative predictive value with 95%CI. SPSS software (SPSS version 11.5, Chicago, IL, United States) was applied for statistical analyses. The diagnostic performance of different measures was evaluated using the McNemar test.
Of the 127 consecutive patients with typical reflux symptoms screened for this study, 43 were excluded due to a previous history of upper gastrointestinal surgery, erosive esophagitis, Barrett’s esophagus, peptic ulcer, upper gastrointestinal neoplasm, chronic liver or renal disease, or intolerance to pH-metry. Finally, 84 patients with negative esophageal findings on WLI were enrolled; their demographic and clinical characteristics are shown in Table 1.
| Characteristic | Patients (n = 84) |
| Mean age (yr) | 49.0 ± 12.6 |
| Gender (male:female) | 39:45 |
| BMI (kg/m2) | 24.1 ± 3.7 |
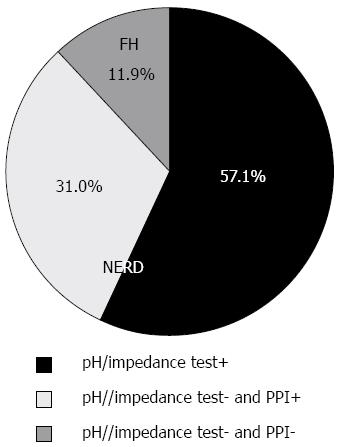
Of 84 eligible participants, 48 (57.1%) were positive on pH/impedance tests (Figure 2), suggesting a diagnosis of NERD. Of 36 patients (42.9%) with normal pH/impedance results, 26 benefitted from PPI tests and were classified as having NERD and the remaining 10 patients were classified as having FH.
Altogether, 68 (81.0%) of the 84 patients were positive on AFI endoscopy (Figure 3) and the others were negative (Figure 4). Of 74 patients diagnosed with NERD, 67 had abnormal AFI findings (Table 2), giving this test a sensitivity of 90.5% (95%CI: 81.5%-96.1%) and a specificity of 90.0% (95%CI: 55.5%-99.7%). Meanwhile, the accuracy, positive predictive value and negative predictive value of AFI in differentiating between NERD and FH were 90.5% (95%CI: 84.2%-96.8%), 98.5% (95%CI: 92.1%-99.9%) and 56.3% (95%CI: 30.0%-80.2%), respectively.
| AFI | NERD | FH | Total |
| Positive | 67 | 1 | 68 |
| Negative | 7 | 9 | 16 |
| Total | 74 | 10 | 84 |
In the present study, patients with typical reflux symptoms were investigated using AFI endoscopy as well as 24-h pH and impedance tests. Our results showed that AFI was able to identify differences in endoscopic features between NERD and FH. Thus, AFI endoscopy may have potential in distinguishing between these two diseases.
AFI, which was incorporated into a Tri-modal Imaging Endoscope system, has been increasingly used in the assessment of Barrett’s esophagus[10-12] and the appearance of purple areas on a green background indicates the neoplastic changes that occur in Barrett’s esophagus. Changes in coloration of the gastrointestinal tract revealed by AFI, however, do not represent one type of neoplasia-specific manifestations, as AFI cannot identify the direct features of gastrointestinal neoplasms, such as loss of micro-architecture regularity and/or disruption of normal capillary patterns in the superficial layer of lesions. It was found that changes in tissue components, regardless of whether they are caused by neoplasia or inflammation, alter the density of autofluorescence emitted from lesions[13-15]. These findings suggest that AFI may also be useful to evaluate diseases caused by inflammation, such as esophagitis[5].
Differentiating NERD from FH remains challenging[8], as both disorders share similar clinical manifestations, such as reflux symptoms and normal esophageal appearance on traditional endoscopy. The responsiveness to PPI tests is diagnostic of GERD and excludes the possibility of FH, but the converse is not necessarily true because the response rate of NERD to PPI therapy was reported to be low, around 37%-73%[16-18]. These findings indicate that more than one quarter of patients with NERD are refractory to PPIs, making it difficult to distinguish between NERD and FH. Routine methods of evaluating patients with persistent reflux symptoms after PPI tests include esophagogastroduodenoscopy and esophageal pH monitoring. Various new techniques have been introduced, including esophageal histopathological analysis and esophageal impedance monitoring, in order to improve the clinical management of patients suspicious for NERD and FH. This study describes a new method using AFI to differentiate between NERD and FH. The presence of purple lines in the distal esophagus on AFI, which are indistinct on standard endoscopy, is considered diagnostic of GERD, including erosive esophagitis and NERD. Moreover, the endoscopic features identified by AFI were found to correlate with pathologic reflux[5]. GERD is characteristic of reflux from the stomach to the esophagus, but no reflux underlies FH. Therefore positive findings on AFI may help distinguish NERD from FH.
Our results showed the diagnostic value of AFI in distinguishing between NERD and FH was promising, with a sensitivity of 90.5% and a specificity of 90%. These findings were comparable to results obtained with other new diagnostic modalities, such as identification of microscopic esophagitis and analysis of esophageal baseline impedance[19,20]. Microscopic esophagitis, which is considered a histological marker of both erosive esophagitis and NERD[21,22], can be observed in the distal esophagus of almost all patients with erosive esophagitis and in 70%-76% of those with NERD[21,23]. Recently, it was reported that histological evaluation of biopsy specimens from the distal esophagus of patients with reflux symptoms to show the presence or absence of microscopic esophagitis was capable of differentiating NERD from FH, with a sensitivity of 74% and an accuracy of 79%[19]. Also, change in baseline impedance was found to be a marker of pathological reflux, which can distinguish NERD from FH with a sensitivity of 78% and a specificity of 71%[20]. In addition, prolonged wireless esophageal pH monitoring was found to have a higher sensitivity in identifying NERD than 24-h pH monitoring. Nearly one-third of patients who fulfilled the Rome III criteria for FH were found to have NERD after esophageal pH monitoring for > 48 h[24].
Although symptom-based approaches are favored in the initial diagnosis of GERD, endoscopic examination is always recommended for patients who do not respond to PPI tests, and patients suspicious for Barrett’s esophagus[9,25]. AFI may improve the diagnostic yield of dysplasia or early stage malignancy in the esophagus. Recent studies showed both second and third generation AFI systems were more effective than first generation systems in detecting early neoplastic lesions[26-29]. Our findings suggest that, in addition to neoplastic disease, AFI may also be helpful in the diagnosis of NERD. Its advantages, including real-time evaluation and simple diagnostic criteria, imply that AFI may serve as a complementary method in differentiating NERD from FH. As stated above, other new diagnostic methods, such as prolonged wireless esophageal pH monitoring[24] and identification of microscopic esophagitis[22], may also be useful in distinguishing between NERD and FH. However, esophageal impedance and wireless pH monitoring cannot be performed simultaneously[24], reducing the ability to diagnose non-acid reflux[30]. In addition, microscopic esophagitis has shown limitations in identifying NERD, as nearly 20% of patients with NERD showed no evidence of microscopic esophagitis[22]. As none of the above diagnostic modalities is perfect, how to choose these methods is needed to be optimized for patients with suspicion of NERD or FH.
One limitation of this study is the lack of esophageal biopsies. Microscopic esophagitis in the distal esophagus due to reflux may change tissue components, such as collagen and other fluorescent substances[31], attenuating AFI. However, we had no histopathological evidence to support this hypothesis, suggesting the need for further studies combining endoscopic and histopathological methods. Another limitation was that repeated AFI was not performed after PPI treatment in patients positive on AFI. It is unclear whether standard PPI treatment reverses abnormal findings on AFI. Previous data showed that treatment with omeprazole for 6 mo completely restored dilated intercellular spaces[32], which are considered characteristic of microscopic esophagitis in NERD[22]. It implied that positive AFI findings are likely to diminish after PPI therapy. Further studies are needed to clarify the duration of PPI treatment required to reverse positive AFI findings.
In summary, this prospective observational study showed that endoscopic features on AFI can distinguish NERD from FH. Its real-time characteristics and simple endoscopic criteria may enhance the use of AFI as a complementary tool in the differentiation of NERD and FH.
The authors would like to thank Professor Enqiang Linghu, Wen Li, Ying-Di Liu, Gang Sun and Qi-Yang Huang for their support. We are also grateful to Jie Zhang, Xiao-Xiao Wang, Jie Ai from GI Endoscopy Center and GI Motility Center for technical assistance.
It is difficult to differentiate between functional heartburn (FH) and non-erosive reflux disease (NERD) endoscopically. Autofluorescence imaging (AFI) was recently shown to reveal indistinct mucosal lesions invisible on conventional endoscopy. Moreover, endoscopy with AFI was shown to predict acid reflux in patients with gastroesophageal reflux disease (GERD). NERD is characterized etiologically by the presence of pathologic reflux, while FH is not. This study aimed to assess whether AFI endoscopy could distinguish NERD from FH.
Reflux symptoms are common in the general population. GERD (including NERD) and FH may underlie these symptoms. In order to differentiate NERD from FH, esophagogastroduodenoscopy, esophageal pH monitoring and proton-pump inhibitor (PPI) test are often needed. Presently, various new techniques have been introduced, including esophageal histopathological analysis and esophageal impedance monitoring, in order to improve the clinical management of patients suspicious for NERD and FH.
To date, few efforts have been put on the application of AFI endoscopy in patients with GERD. In this pilot study, the authors found that endoscopic features on AFI can distinguish NERD from FH.
This study suggested that AFI may serve as a complementary tool in the differentiation of NERD from FH. It provided a new method to improve the clinical management of patients with reflux symptoms.
AFI is a kind of digital imaging technique that detects autofluorescence that is emitted in response to light by endogenous fluorophores and cannot be observed by conventional endoscopy. NERD is a distinct pattern of GERD. It is caused by the reflux of gastric contents into the esophagus, but no mucosal damage is found at conventional endoscopy. FH is defined as retrosternal burning in the absence of GERD or other factors that can be detected in an objective manner.
The authors did an excellent job of assessing AFI endoscopy for its utility in the evaluation of NERD vs FH. The flow diagram explaining patient recruitment and exclusions is extremely helpful and the color images are exceptionally well done, and very instructive.
P- Reviewer: Dumitrascu DL, Parker W, Savarino E S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma S
| 1. | Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013;10:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Savarino E, Marabotto E, Zentilin P, Frazzoni M, Sammito G, Bonfanti D, Sconfienza L, Assandri L, Gemignani L, Malesci A. The added value of impedance-pH monitoring to Rome III criteria in distinguishing functional heartburn from non-erosive reflux disease. Dig Liver Dis. 2011;43:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Song LM, Banerjee S, Desilets D, Diehl DL, Farraye FA, Kaul V, Kethu SR, Kwon RS, Mamula P, Pedrosa MC. Autofluorescence imaging. Gastrointest Endosc. 2011;73:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Uedo N, Iishi H, Tatsuta M, Yamada T, Ogiyama H, Imanaka K, Sugimoto N, Higashino K, Ishihara R, Narahara H. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005;62:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Wang W, Uedo N, Yang Y, Peng L, Bai D, Lu Z, Fan K, Wang J, Wang X, Zhao Y. Autofluorescence imaging endoscopy for predicting acid reflux in patients with gastroesophageal reflux disease. J Gastroenterol Hepatol. 2014;29:1442-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107:1741-1745. [PubMed] |
| 7. | Savarino E, Tutuian R, Zentilin P, Dulbecco P, Pohl D, Marabotto E, Parodi A, Sammito G, Gemignani L, Bodini G. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol. 2010;105:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Galmiche JP, Clouse RE, Bálint A, Cook IJ, Kahrilas PJ, Paterson WG, Smout AJ. Functional esophageal disorders. Gastroenterology. 2006;130:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1120] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 10. | Kara MA, Peters FP, Ten Kate FJ, Van Deventer SJ, Fockens P, Bergman JJ. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc. 2005;61:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Curvers WL, Alvarez Herrero L, Wallace MB, Wong Kee Song LM, Ragunath K, Wolfsen HC, Prasad GA, Wang KK, Subramanian V, Weusten BL. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett’s esophagus. Gastroenterology. 2010;139:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Curvers WL, Singh R, Song LM, Wolfsen HC, Ragunath K, Wang K, Wallace MB, Fockens P, Bergman JJ. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Moriichi K, Fujiya M, Ijiri M, Tanaka K, Sakatani A, Dokoshi T, Fujibayashi S, Ando K, Nomura Y, Ueno N. Quantification of autofluorescence imaging can accurately and objectively assess the severity of ulcerative colitis. Int J Colorectal Dis. 2015;30:1639-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Osada T, Arakawa A, Sakamoto N, Ueyama H, Shibuya T, Ogihara T, Yao T, Watanabe S. Autofluorescence imaging endoscopy for identification and assessment of inflammatory ulcerative colitis. World J Gastroenterol. 2011;17:5110-5116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Chey WD. Erosive esophagitis and NERD: Can we really classify patients with heartburn by endoscopic findings? Clin Gastroenterol Hepatol. 2004;2:654-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24:747-757, e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Savarino E, Zentilin P, Mastracci L, Dulbecco P, Marabotto E, Gemignani L, Bruzzone L, de Bortoli N, Frigo AC, Fiocca R. Microscopic esophagitis distinguishes patients with non-erosive reflux disease from those with functional heartburn. J Gastroenterol. 2013;48:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. 2015;13:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Zentilin P, Savarino V, Mastracci L, Spaggiari P, Dulbecco P, Ceppa P, Savarino E, Parodi A, Mansi C, Fiocca R. Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol. 2005;100:2299-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 22. | Fiocca R, Mastracci L, Riddell R, Takubo K, Vieth M, Yerian L, Sharma P, Fernström P, Ruth M. Development of consensus guidelines for the histologic recognition of microscopic esophagitis in patients with gastroesophageal reflux disease: the Esohisto project. Hum Pathol. 2010;41:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Mastracci L, Spaggiari P, Grillo F, Zentilin P, Dulbecco P, Ceppa P, Baccini P, Mansi C, Savarino V, Fiocca R. Microscopic esophagitis in gastro-esophageal reflux disease: individual lesions, biopsy sampling, and clinical correlations. Virchows Arch. 2009;454:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Penagini R, Sweis R, Mauro A, Domingues G, Vales A, Sifrim D. Inconsistency in the Diagnosis of Functional Heartburn: Usefulness of Prolonged Wireless pH Monitoring in Patients With Proton Pump Inhibitor Refractory Gastroesophageal Reflux Disease. J Neurogastroenterol Motil. 2015;21:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (2)] |
| 26. | Tamai N, Saito S, Aihara H, Kato T, Tajiri H. Evaluation of the effectiveness of color intensity analysis using a second-generation autofluorescence imaging system for diminutive colorectal polyp differentiation. Dig Endosc. 2014;26 Suppl 2:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Tamai N, Takeuchi Y, Tajiri H. Second-generation autofluorescence imaging for colorectal neoplasia. Dig Endosc. 2015;27 Suppl 1:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Ide D, Tamai N, Inomata H, Ohya TR, Aihara H, Saito S, Kato T, Tajiri H. Visualization of colorectal neoplasia by a second-generation autofluorescence imaging system. Scand J Gastroenterol. 2013;48:1302-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Boerwinkel DF, Holz JA, Aalders MC, Visser M, Meijer SL, Van Berge Henegouwen MI, Weusten BL, Bergman JJ. Third-generation autofluorescence endoscopy for the detection of early neoplasia in Barrett’s esophagus: a pilot study. Dis Esophagus. 2014;27:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Tutuian R, Vela MF, Shay SS, Castell DO. Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol. 2003;37:206-215. [PubMed] |
| 31. | Wang TD, Triadafilopoulos G. Autofluorescence imaging: have we finally seen the light? Gastrointest Endosc. 2005;61:686-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenacchi G, Scialpi C, Miglioli M, Di Febo G. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol. 2005;100:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |