Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3813
Peer-review started: October 12, 2015
First decision: December 11, 2015
Revised: December 17, 2015
Accepted: January 17, 2016
Article in press: January 18, 2016
Published online: April 14, 2016
Processing time: 170 Days and 14.9 Hours
AIM: To compare the short- and long-term outcomes of vascularizing lymph node dissection (VLND) and non-vascularizing lymph node dissection (NVLND) from a single institution.
METHODS: Data of 315 patients with advanced gastric cancer who underwent standard D2 lymphadenectomy with curative intent was collected between January 1994 and December 2006. One hundred and fifty-two patients received VLND while 163 patients received NVLND. Short- and long-term clinical outcomes were compared between the two groups.
RESULTS: The median followed-up time was 82 mo. The rate of postoperative complications in the VLND group was 13.2%, while that in the NVLND group was 11.7% (P = 0.686). The overall 5-year survival rate was 64% in the VLND group and 59% in the NVLND group (P = 0.047). When subgroup analyses were performed according to Bormann type, type of differentiation and lymph node status, survival benefit was demonstrated in patients with Bormann type III or IV (59% vs 50%, P = 0.032), undifferentiated type (63% vs 49%, P = 0.021) or presence of lymph node metastasis (53% vs 38%, P = 0.010) in the VLND group.
CONCLUSION: D2 VLND in advanced gastric cancer treatment allows survival benefit with acceptable morbidity and mortality. VLND for patients with potentially curable advanced gastric cancer is feasible and safe when performed by a well-trained surgical team.
Core tip: This study investigates the short- and long-term outcomes of vascularizing lymph node dissection (VLND) and non-vascularizing lymph node dissection (NVLND) from a single institution. The overall 5-year survival rate was 64% in the VLND group and 59% in the NVLND group (P = 0.047). We draw a conclusion that VLND with D2 lymphadenectomy has overall survival benefit for patients with advanced gastric cancer without significant operative complications and mortality if performed by a well-trained and experienced surgical team. If undifferentiated adenocarcinoma is confirmed by endoscopic biopsy preoperatively or macroscopically enlarged regional lymph nodes are found intraoperatively in advanced gastric cancer, VLND with D2 lymphadenectomy may be a considerable alternative.
- Citation: Han FH, Zhou SN, Li HM, He YL, Zhan WH. Vascularizing lymph node dissection for advanced gastric cancer: A single-institution experience. World J Gastroenterol 2016; 22(14): 3813-3820
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3813.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3813
Gastric cancer (GC) is the third leading cause of cancer-related deaths in China[1]. Although current therapeutic practice includes neoadjuvant and adjuvant chemotherapy or radiotherapy into the treatment protocol[2], curative gastrectomy with radical lymphadenectomy is the only curative therapy for gastric cancer. An Italian multicenter randomized controlled trial[3,4] showed that subtotal and total gastrectomies, with second-level lymphadenectomy, had similar postoperative complication rate and survival probability, provided that the surgical margin of the resection fell in healthy tissue. The 10-year and the 15-year survival outcomes of Dutch gastric cancer trial showed that D2 dissection may be of benefit, especially for patients with N2 disease[5,6]. A Taiwanese single-institution trial (No. ClinicalTrials.gov NCT00260884) showed[7] that D3 dissection showed better survival outcome for gastric cancer patients compared with D1 lymphadenectomy if performed by experienced surgeons. Japanese MRCT showed[8] that more extended resection (D2+ para-aortic lymph node dissection) cannot provide survival benefit in curable gastric cancer patients when compared with D2 lymph node dissection alone. Nowadays in China, the majority (about 90%) of gastric cancer patients are diagnosed in advanced stages. Hence, D2 dissection has become the standard procedure for advanced gastric cancer. Numerous studies have shown that surgical specialization and experience as well as surgery volume may contribute to differences in outcomes; the morbidity and mortality vary among different surgeons and in different hospitals[9-11]. However, it has not been reported whether different lymph node dissection techniques would affect the outcome of gastric cancer. Two methods have been applied to dissect the lymph nodes around the artery; one was classified as vascularizing lymph node dissection (VLND) where the dissection is carried out along the plane between the artery adventitia and the vascular sheath, and the other is non-vascularizing lymph node dissection (NVLND) in which the lymph nodes are retrieved outside the vascular sheath with the vascular sheath preserved. So far no randomized controlled trial has been found on the comparison of the two methods. VLND has been performed in our department since the early 1990s. Data of all the patients were collected in the gastric cancer database of our department. The postoperative follow-up data were also included. The present study therefore investigated the long- and short-term clinical outcomes of VLND and NVLND from a single institution.
This study was approved by Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. All patients provided verbal informed consent prior to study enrollment, because all study participants were out of hospital and we contacted them by telephone, message, email, etc.
Three hundred and fifteen patients with primary GC who underwent radical gastrectomy with D2 lymph node dissection in the Department of Gastrointestinal Surgery, the Affiliated Hospital of Sun Yat-sen University between 1994 and 2006 were studied. Among these patients, 152 received VLND, and 163 received NVLND.
In this study, the inclusion criteria before surgical procedure were as follows: (1) histologically proven primary gastric cancer; (2) potentially curable by radial gastrectomy with D2 lymphadenectomy [Japanese Gastric Cancer Association (JGCA) guidelines]; (3) physical tolerance to radical operation; (4) age younger than 75 years; (5) no neoadjuvant chemotherapy or radiotherapy before surgery; (6) no concomitant other cancer; and (7) no previous gastrectomy.
Exclusion criteria were as follows (intraoperative findings): (1) early gastric cancer (T0 or T1); (2) enlarged lymph nodes were found around para-aortic or the hepatoduodenal ligament regions; (3) esophageal involvement; and (4) distant metastasis.
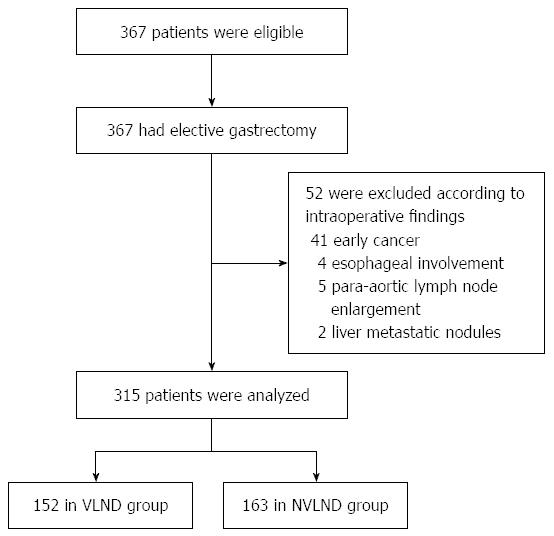
Three hundred and sixty-seven patients met the inclusion criteria, and 52 of them were excluded because they did not meet the exclusion criteria as Figure 1 shows: 41 cases were found with early gastric cancer, 4 cases were found with esophageal involvement, 5 cases were found with para-aortic lymph nodes enlargement and 2 cases were found with liver metastatic nodules intraoperatively. Finally, data of 315 cases underwent statistic analysis (152 cases in the VLND group and 163 cases in the NVLND group).
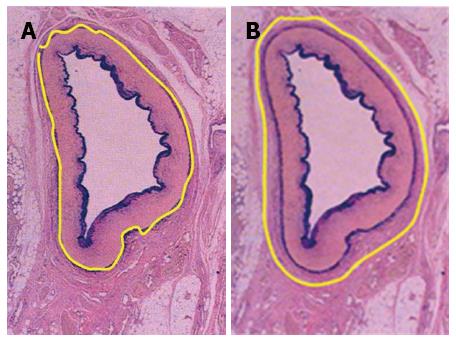
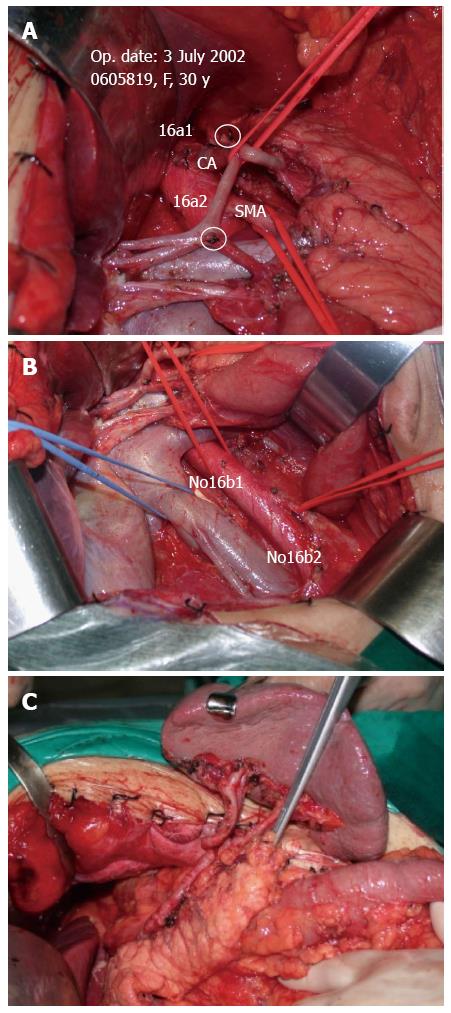
Lymph node dissection was standardized according to the JGCA Guidelines[12]. All patients underwent radical gastrectomy with D2 lymph node resection for curative intent. The stomach, lesser omentum, greater omentum, anterior leaf of transverse mesocolon, capsula pancreatis and lymph nodes were included in the resection. When proximal gastrectomies were performed, Nos. 1, 2, 3a, 4sa, 4sb, 7, 8a, 9, and 11p lymph nodes were removed. A total gastrectomy included the dissection of Nos. 1, 2, 3, 4, 5, 6, 7, 8a, 9, 10, 11p, 11d, and 12a lymph nodes. For distal gastrectomy, Nos. 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 10, 11p and 12a lymph nodes were removed. At least 15 lymph nodes were retrieved and examined. After the surgery, perigastric lymph nodes were dissected immediately and sent for histopathological examination. Tumors were staged according to the latest version of the pathologic classification of the Union for International Cancer Control (UICC). When the cases underwent VLND, the vascular sheath of the artery and the dense fibrous tissue were peeled to expose the tunica adventitia. The loose layer between the vascular sheath and the tunica adventitia was accessed, and the lymph nodes around the artery together with the vascular sheath could be dissected promptly and easily (Figure 2A). In this way, all the anatomic structures in the upper abdominal floor were skeletonized, so that at the end of the procedure the surgical field resembled that seen in an anatomic atlas (Figure 3). As for NVLND, however, dissection was performed outside the vascular sheath, leaving the vascular sheath unopened (Figure 2B).
The standard procedure was set up by Zhan WH.
All surgeons had completed at least 20 gastrectomies with VLND and 20 gastrectomies with NVLND independently before the beginning of this study (Before January 1994).
The UICC TNM staging system was used[13].
The median followed-up time was 82 mo (83 mo in the VLND group and 80 mo in the NVLND group, P = 0.475). Assessments were performed in a follow-up program every 3 mo during the first 3 years after surgery, then every 6 mo during the next 2 years, and annually thereafter until the patient’s death. Statistical analyses were performed with software SPSS 16.0 G for Windows. The clinical and pathological variables were compared using the Fisher’s exact test and Wilcoxon test, and P < 0.05 was considered statistically significant. The primary endpoints were recurrence free survival and overall survival. Comparisons were made by the log-rank test when using the Kaplan-Meier method.
Three hundred and sixty-seven patients met the inclusion criteria, and 52 of them were excluded. Finally, data of 315 cases were analyzed (152 cases in the VLND group and 163 cases in the NVLND group). Among the 315 patients with gastric cancer who underwent standard D2 lymphadenectomy, 64.1% were male (202/315) and 35.9% were female (113/315). The mean ages of the VLND and NVLND groups were 55.72 ± 11.73 years and 56.97 ± 12.19 years, respectively. The clinicopathological characteristics of all patients are showed in Table 1. No statistical difference was found between the two groups in terms of sex, age, macroscopic histological type, tumor site or size. No significant differences were demonstrated in operative procedures or postoperative pathological parameters either. The number of harvested lymph nodes was 29.94 ± 11.70 in the VLND group and 27.85 ± 12.05 in the NVLND group (P = 0.307). The number of involved lymph nodes in the VLND and NVLND groups were 4.41 and 5.30, respectively (P = 0.717). Total gastrectomy was performed in 58 patients who underwent VLND and in 76 patients who underwent NVLND. The median operation time was 271.91 ± 65.58 min in the VLND group and 272.52 ± 57.57 min in the NVLND group, and there was no significant difference between the two groups (P = 0.931).
| Group | VLND | NVLND | P value |
| No. of cases | 152 | 163 | |
| Sex | |||
| Male | 101 | 101 | 0.414 |
| Female | 51 | 62 | |
| Age (yr), mean ± SD | 55.72 ± 11.73 | 56.97 ± 12.19 | 0.163 |
| Tumor location | |||
| Upper stomach | 38 | 46 | 0.679 |
| Middle stomach | 36 | 42 | |
| Lower stomach | 75 | 70 | |
| Whole stomach | 3 | 5 | |
| Tumor size (cm), mean ± SD | 4.59 ± 2.84 | 4.38 ± 3.29 | 0.456 |
| Bormann type | |||
| I or II | 40 | 57 | 0.063 |
| III or IV | 112 | 106 | |
| Postoperative stay (d) | 13.24 | 13.96 | 0.473 |
| Blood transfusion | 0.982 | ||
| Absent | 94 | 101 | |
| Present | 58 | 62 | |
| Mean operation time | 271.91 ± 65.58 | 272.52 ± 57.57 | 0.931 |
| Gastrectomy | 0.129 | ||
| Total | 58 | 76 | |
| Subtotal | 94 | 87 | |
| Type of reconstruction | |||
| Roux-en-Y | 58 | 76 | |
| Billroth I | 7 | 9 | |
| Billroth II | 87 | 78 | |
| Pathological classification and differentiation | 0.466 | ||
| Differentiated | 53 | 47 | |
| Undifferentiated | 99 | 115 | |
| Retrieved nodes, mean ± SD | 29.94 ± 11.70 | 27.85 ± 12.05 | 0.307 |
| Metastatic nodes, mean ± SD | 4.41 ± 7.54 | 5.30 ± 7.33 | 0.717 |
| pT stage | 0.406 | ||
| T1 | 25 | 22 | |
| T2 | 21 | 14 | |
| T3 | 70 | 84 | |
| T4 | 36 | 43 | |
| pN stage | 0.546 | ||
| N0 | 61 | 53 | |
| N1 | 57 | 66 | |
| N2 | 22 | 28 | |
| N3 | 12 | 16 |
No significant difference was observed in postoperative complications between the VLND and NVLND groups (13.2% vs 11.7%, P = 0.686). Table 2 lists all the postoperative complications. As shown, intra-abdominal infection, pulmonary complications, ileus, catheter-related infection and abdominal hemorrhage were the most common complications. There were no deaths during hospital stay.
| Complication | VLND | NVLND | P value |
| Anastomotic leakage | 1 | 1 | |
| Duodenal stump leak | 1 | 2 | |
| Biliary fistula | 2 | 0 | |
| Pancreatic fistula | 1 | 0 | |
| Ileus | 2 | 4 | |
| Lymphorrhea | 2 | 0 | |
| Abdominal hemorrhage | 3 | 1 | |
| Intra-abdominal infection | 3 | 5 | |
| Pulmonary complications | 3 | 4 | |
| Catheter-related infection | 2 | 2 | |
| Total | 20 | 19 | 0.686 |
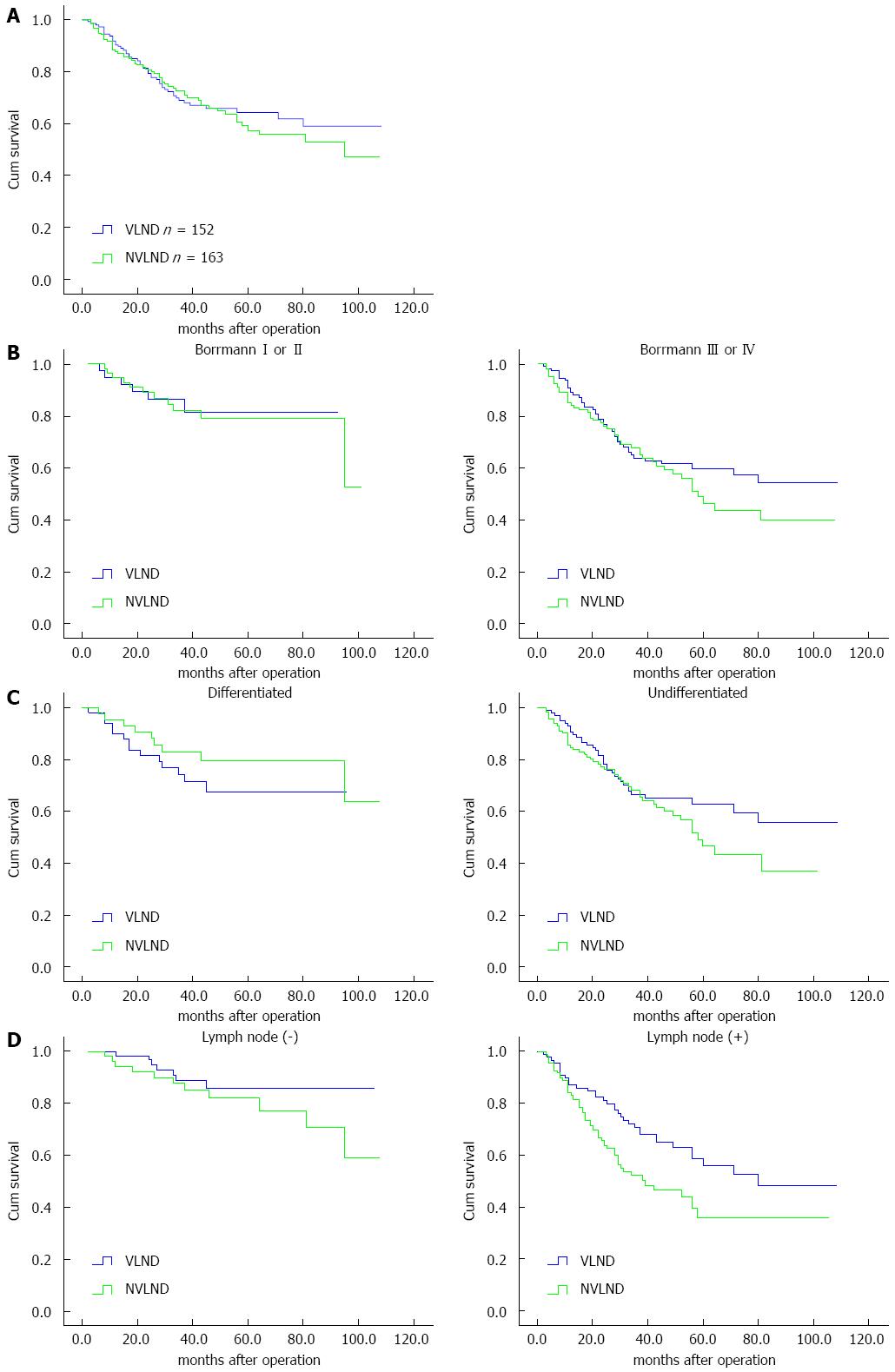
The overall 5-year survival rate was 64% in the VLND group and 59% in the NVLND group (log-rank test P = 0.047; Figure 4A).
Among patients with Bormann type I or II cancer, the overall 5-year survival rate was 81% in the VLND group and 79% in the NVLND group (log-rank test, P = 0.902). However, a significant difference was found between the two groups among patients with Bormann type III or IV cancer. The overall 5-year survival rates of the VLND and NVLND groups were 59% and 50%, respectively (log-rank test, P = 0.032; Figure 4B).
For differentiated GC, the overall 5-year survival rate was 81% for the VLND group and 79% for the NVLND group (log-rank test, P = 0.282). For patients with undifferentiated GC, however, VLND could improve the overall 5-year survival rate by more than 10% (63% for the VLND group vs 49% for the NVLND group, log-rank test, P = 0.021; Figure 4C).
Among patients without lymph node metastasis, no significant difference was found between the two groups. The overall 5-year survival rate was 86% in the VLND group and 82% in the NVLND group (log-rank test, P = 0.222). However, the overall 5-year survival rate was 53% in the VLND group and 38% in the VLND group among patients with regional lymph node metastasis, thus a significant difference was observed (log-rank test, P = 0.010; Figure 4D).
Long-term clinical outcomes of GC were variable according to several published studies[14-16], which can be affected by the physical condition, tumor growth, treatment protocol, and the scrutiny of pathological assessment. The results of the surgical treatment will differ if the patient is old, obese, or with co-morbid conditions such as diabetes and cardiovascular illness. The knowledge, skill and experience of the surgical team, the extent of lymph node dissection, pathological sampling and pathological diagnosis all affect the pathological staging[17-20]. The extent as well as the technique of nodal clearance is of significant influence on surgical quality of lymph node dissection[21-24]. However, the prognostic impact of different lymph node dissection techniques has not been reported in the literature.
In our study, the impact of different lymph node dissection techniques on the short- and long-term clinical outcomes in patients with advanced gastric cancer who underwent radical operation was investigated on the basis of strict surgical quality control. In this setting, the application of VLND to advanced gastric cancer requires a surgical team who has rich experience in radical gastrectomy, familiarity with vascular surgery technique and a good understanding of anatomical structure. The surgical learning period for VLND extends to at least 20 cases. When NVLND was employed, the lymph nodes in the upper abdominal floor were removed leaving the vascular sheath intact and unopened.
Our hospital has a high volume of GC surgery in China. Radical gastrectomy with D2 lymphadenectomy has commonly been carried out here. All involved surgeons in our department were experienced and had accomplished at least 20 gastrectomies with VLND and 20 gastrectomies with NVLND independently before the study. The standard procedure was set up and all surgeons did both VLND and NVLND. Hence, there were no deaths for postoperative complications in both groups, although the overall morbidity rate was slightly higher in the VLND group than in the NVLND group (13.2% vs 11.7%, P = 0.686). When complications were considered independently, no complication could reach a statistically significant difference between the two groups.
The limitation of this study may be as follows: (1) the vascular sheath and the soft tissue between the vascular sheath and the tunica adventitia may be involved in some patients with advanced gastric cancer, thus VLND could reach better regional control. However, the lack of pathological data which can provide evidence of metastasis makes it less compelling; and (2) all the surgical procedures in our study were performed by several experienced surgeons in a single institution. Some more prospective randomized controlled trials are needed to support our results.
In conclusion, the short- and long-term clinical outcomes of VLND and NVLND were studied and reported in a single institution. Data from our study demonstrated that VLND with D2 lymphadenectomy has overall survival benefit for patients with advanced gastric cancer without significant operative complications and mortality if performed by a well-trained and experienced surgical team. If undifferentiated adenocarcinoma is confirmed by endoscopic biopsy preoperatively or macroscopically enlarged regional lymph nodes are found intraoperatively in advanced gastric cancer, VLND with D2 lymphadenectomy may be a considerable alternative.
Gastric cancer is the third leading cause of cancer-related deaths in China. Nowadays in China, the majority (about 90%) of gastric cancer patients are diagnosed in advanced stages. Hence, D2 dissection has become the standard procedure for advanced gastric cancer. However, it has not been reported whether different lymph node dissection techniques would affect the outcome of gastric cancer. Two methods have been applied to dissect the lymph nodes around the artery, and one was classified as vascularizing lymph node dissection (VLND) where the dissection is carried out along the plane between the artery adventitia and the vascular sheath. Non-vascularizing lymph node dissection (NVLND) is a technique in which the lymph nodes are retrieved outside the vascular sheath with the vascular sheath preserved. So far no randomized controlled trial has been found on the comparison of the two methods from a single institution.
The appropriate degree of curative lymph node dissection remains controversial between Western and Eastern countries. There is still ambiguity in comparison between laparoscopic and open operation for gastric cancer.
It has not been reported whether different lymph node dissection techniques would affect the outcome of gastric cancer. This study found that D2 VLND in advanced gastric cancer treatment allows survival benefit and acceptable mortality and morbidity.
VLND with D2 lymphadenectomy has overall survival benefit for patients with advanced gastric cancer if performed by a well-trained and experienced surgical team. In radical operation for advanced gastric cancer, VLND with D2 lymphadenectomy may be a considerable alternative.
VLND: Lymph nodes around the vessels, as well as the vascular sheath of the artery and the dense fibrous tissue are peeled to expose the tunica adventitia.
The authors investigated the short- and long-term outcomes of VLND and NVLND. They show that D2 VLND in advanced gastric cancer treatment allows survival benefit and acceptable morbidity and mortality. They emphasize that if undifferentiated adenocarcinoma is confirmed by endoscopic biopsy preoperatively or macroscopically enlarged regional lymph nodes are found intraoperatively in advanced gastric cancer, VLND with D2 lymphadenectomy would be a considerable alternative. Their findings are interesting and useful for the future treatment of advanced gastric cancer.
P- Reviewer: Ando T S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | IARC GLOBOCAN 2008: Cancer Incidence, Mortality and Prevalence Worldwide. Available from: http://www-dep.iarc.fr. |
| 2. | Lacueva J, Gallego J, Díaz-González JA. Updating controversies on the multidisciplinary management of gastric cancer. Clin Transl Oncol. 2010;12:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Crose N, Gennari L. Total versus subtotal gastrectomy: surgical morbidity and mortality rates in a multicenter Italian randomized trial. The Italian Gastrointestinal Tumor Study Group. Ann Surg. 1997;226:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 653] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1308] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 7. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 9. | Sasako M. Clinical trials of surgical treatment of malignant diseases. Int J Clin Oncol. 2005;10:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Peeters KC, van de Velde CJ. Surgical quality assurance in breast, gastric and rectal cancer. J Surg Oncol. 2003;84:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Hartgrink HH, van de Velde CJ. Status of extended lymph node dissection: locoregional control is the only way to survive gastric cancer. J Surg Oncol. 2005;90:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1942] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 13. | Page DL, Fleming ID, Fritz A. AJCC Cancer Staging Manual. 6th ed. Philadelphia: Lippincott-Raven 2002; . |
| 14. | Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3695] [Cited by in RCA: 3773] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 15. | Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 16. | Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2462] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 17. | Lee JH, Ryu KW, Lee JH, Park SR, Kim CG, Kook MC, Nam BH, Kim YW, Bae JM. Learning curve for total gastrectomy with D2 lymph node dissection: cumulative sum analysis for qualified surgery. Ann Surg Oncol. 2006;13:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Weitz J, Koch M, Friess H, Büchler MW. Impact of volume and specialization for cancer surgery. Dig Surg. 2004;21:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg. 2007;94:145-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 447] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | Kim JP. Surgical results in gastric cancer. Semin Surg Oncol. 1999;17:132-138. [PubMed] [DOI] [Full Text] |
| 21. | Harrison LE, Choe JK, Goldstein M, Meridian A, Kim SH, Clarke K. Prognostic significance of immunohistochemical micrometastases in node negative gastric cancer patients. J Surg Oncol. 2000;73:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Cai J, Ikeguchi M, Maeta M, Kaibara N. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000;127:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Takeuchi H, Kitagawa Y. Is lymphadenectomy a predictor or savior for patients with gastric cancer? Ann Surg Oncol. 2010;17:1257-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 418] [Article Influence: 19.0] [Reference Citation Analysis (0)] |