Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3769
Peer-review started: November 30, 2015
First decision: December 16, 2015
Revised: January 11, 2016
Accepted: January 17, 2016
Article in press: January 19, 2016
Published online: April 14, 2016
Processing time: 120 Days and 18.5 Hours
AIM: To investigate the effect and mechanism of stimulation of the hypothalamic paraventricular nucleus with glutamate acid in rats with ulcerative colitis (UC).
METHODS: The rats were anesthetized with 10% chloral hydrate via abdominal injection and treated with an equal volume of TNBS + 50% ethanol enema, injected into the upper section of the anus with the tail facing up. Colonic damage scores were calculated after injecting a certain dose of glutamic acid into the paraventricular nucleus (PVN), and the effect of the nucleus tractus solitarius (NTS) and vagus nerve in alleviating UC injury through chemical stimulation of the PVN was observed in rats. Expression changes of C-myc, Apaf-1, caspase-3, interleukin (IL)-6, and IL-17 during the protection against UC injury through chemical stimulation of the PVN in rats were detected by Western blot. Malondialdehyde (MDA) content and superoxide dismutase (SOD) activity in colon tissues of rats were measured by colorimetric methods.
RESULTS: Chemical stimulation of the PVN significantly reduced UC in rats in a dose-dependent manner. The protective effects of the chemical stimulation of the PVN on rats with UC were eliminated after chemical damage to the PVN. After glutamate receptor antagonist kynurenic acid was injected into the PVN, the protective effects of the chemical stimulation of the PVN were eliminated in rats with UC. After AVP-Vl receptor antagonist ([Deamino-penl, val4, D-Arg8]-vasopressin) was injected into NTS or bilateral chemical damage to NTS, the protective effect of the chemical stimulation of PVN on UC was also eliminated. After chemical stimulation of the PVN, SOD activity increased, MDA content decreased, C-myc protein expression significantly increased, caspase-3 and Apaf-1 protein expression significantly decreased, and IL-6 and IL-17 expression decreased in colon tissues in rats with UC.
CONCLUSION: Chemical stimulation of the hypothalamic PVN provides a protective effect against UC injury in rats. Hypothalamic PVN, NTS and vagus nerve play key roles in this process.
Core tip: This study confirms that the chemical stimulation of the paraventricular nucleus with glutamic acid reduces intestinal injury in ulcerative colitis (UC). Furthermore, this study also discusses the possible regulatory mechanism of the central nervous system of rats with UC, so as to provide a theoretical basis for clinical treatment of UC.
- Citation: Deng QJ, Deng DJ, Che J, Zhao HR, Yu JJ, Lu YY. Hypothalamic paraventricular nucleus stimulation reduces intestinal injury in rats with ulcerative colitis. World J Gastroenterol 2016; 22(14): 3769-3776
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3769
Ulcerative colitis (UC) is a common non-specific inflammatory bowel disease that easily recurs, has many complications and has a very poor prognosis[1]. At present, due to the influence of environmental, diet and other factors, UC has increasingly prevailed in the population, but its exact etiology and pathogenesis remain unclear[2,3]. A study carried out by Mascaraque et al[4] revealed that the central and peripheral nervous systems are involved in the regulation of UC. In addition, many studies have confirmed that the hypothalamic paraventricular nucleus (PVN) and nucleus tractus solitarius (NTS) may play an important role in regulating stress-induced gastric mucosal damage (SGMD) and gastric ischemia-reperfusion injury (GI-RI), and that the PVN and NTS may have participated in the regulatory role of the PVN in GI-RI[5-7]. However, the regulatory role of the PVN in UC, as well as its regulatory role in the treatment of UC, has been rarely reported. Therefore, in this study, we observed whether hypothalamic PVN stimulation could reduce intestinal injury in rats with UC through PVN chemical stimulation, chemical damage, injection of chemical antagonists and other methods, and preliminarily explored the related regulatory and molecular mechanisms.
SPF-grade SD rats weighing 200-250 g were purchased from the Shanghai Experimental Animal Center (Shanghai, China). Glutamic acid, kainic acid (KA), kynurenic acid (KYNA) and [Deamino-penl, val4, D-Arg8]-vasopressin (DPVDAV) were purchased from Sigma-Aldrich; 2,4,6-trinitrobenzene sulfonic acid (TNBS) was purchased from Gibco; anti-PCNA antibody kit was purchased from Santa Cruz Biotechnology; rat interleukin-17 (IL-17) ELISA kit and BCA protein assay kit were purchased from Beyotime; C-myc polyclonal antibody and Apaf-1 polyclonal antibody were purchased from Invitrogen; the 680 automatic microplate reader and Western electrophoresis apparatus were purchased from Bio-Rad; the DYY-6B nucleic acid electrophoresis apparatus was purchased from Nanjing Xianou Instrument Factory; the centrifuge was purchased from Eppendorf; and the optical biological microscope was purchased from Olympus.
Preparation of the UC model: Rats were provided with water before establishing the model. After one day of fast, the animals were anesthetized with 10% chloral hydrate via abdominal injection and treated with an equal volume of TNBS + 50% ethanol enema, injected into the upper section of the anus with the tail facing up. The rats were maintained in an inverted position for 5 min. Three days after the successful establishment of the model, rats were sacrificed. Then, the brain and colon were removed and preserved for use.
Colonic damage scoring: After the successful modeling of UC rats in each group, the colon was obtained and cut by careful incision with forceps, damage was carefully observed after the colon was rinsed, and scoring was performed according to the scoring criteria shown in Table 1, with the average score of each group as the final score of the group.
| Characteristic | Score |
| Ulcer | |
| No ulcer | 0 |
| Punctate hyperemia, no ulcer | 1 |
| Ulcer, no hyperemia or bowel wall thickening | 2 |
| Ulcer with inflammation | 3 |
| Ulcer with two or more inflammations | 4 |
| The length of ulcer damage was greater than l cm along the longitudinal axis of the colon | 5 |
| The length of ulcer damage was greater than 2 cm along the longitudinal axis of the colon, each additional 1 cm of the disease, score plus 1 | 6-10 |
| Adhesions | |
| No adhesions | 0 |
| Slight adhesions (the colon can easily be separated with other tissues) | 1 |
| Severe adhesions | 2 |
| Diarrhea | |
| No diarrhea | 0 |
| Have diarrhea | 1 |
| Total score |
Chemical stimulation and damage to the hypothalamic PVN: Glutamic acid and KA were selected as PVN chemical stimuli and chemical damaging agents, and the glutamate receptor antagonist was KYNA, all of which were dissolved in 0.3 μL of 0.9% normal saline for use. For chemical stimulation group (PVN group), after stimulation of the PVN with 3, 6 or 12 μg of glutamic acid, UC was induced and colon injury scoring was performed. For PVN damage group (PVN + KA group), 0.3 μg of KA was injected into the PVN to induce PVN chemical damage. After 3 d, 12 μg of glutamic acid was injected again for hypothalamic PVN stimulation, then UC was induced and the UC injury scoring was performed. For glutamic acid antagonistic group (PVN + KYNA group): glutamic acid antagonist KYNA was injected into the PVN, 12 μg of glutamic acid was injected 10 min later for PVN stimulation, then UC was induced and the UC damage situation was observed. Control groups consist of a normal untreated group (normal group), a UC model group (UC alone group), and a vehicle group, in which the UC model was prepared by enema after 0.3 μL of 0.9% normal saline was microinjected into the cerebral nuclei. There are eight rats in each group.
Chemical damage to the NTS and injection of the AVP-Vl receptor antagonist into the NTS: KA was selected to induce chemical damage to the NTS and the AVP-Vl receptor antagonist agent was DPVDAV, both of which were dissolved in 0.3 μL of 0.9% normal saline for use. In the NTS damage group (NTS + KA group), 0.3 μg of KA was used to induce NTS chemical damage. After 3 d, 12 μg of glutamic acid was used again for hypothalamic PVN stimulation, then UC was induced and the score conditions were observed. In AVP-Vl receptor antagonist group (NTS + DPVDAV group), AVP-Vl receptor antagonist DPVDAV was injected into the NTS. After 10 min, 12 μg of glutamic acid was injected again for PVN stimulation, then UC was induced and UC damage was observed. Control groups included a PVN group, a UC group and a vehicle group. There are eight rats in each group.
Determination of IL-6, IL-17 and C-myc, Apaf-1, caspase-3 protein expression in colon tissue of rats with UC by Western blot assay: Fifteen microliters of the test sample was mixed with 5 μL of 4 × loading buffer and boiled for 10 min. Then, 10 μL of the same sample was placed onto a 12% SDS-PAGE gel for electrophoresis at a voltage of 80-120 V. At the end of electrophoresis, the sample was transferred onto an NC membrane, blocked with 5% milk at room temperature for 2 h, and rinsed with TBST three times at 30-min intervals. Then, primary antibodies were successively added with the following dilutions: IL-6 at 1:1000, IL-17 at 1:1000, Apaf-1 at 1:500, C-myc at 1:600, caspase-3 at 1:750; and incubated overnight at 4 °C. Subsequently, the samples were washed three times with TBST at 10-min intervals, selected alkaline phosphatase-labeled secondary antibodies, and incubated at room temperature for 2 h. After the samples were washed three times with TBST at 30-min intervals, wetted with coloured solution, and pictures were developed and photographed in an ECL developer.
Detection of SOD activity and MDA content in colon tissue of rats with UC: SOD activity was measured in tissues of rats in each group. One hundred microliters of the supernatant and 100 μL of Reagent 7 were mixed. After 5 min, tissues were centrifuged (5000 r/min for 20 min) and the supernatant was obtained. For the control supernatant, the supernatant of tissue homogenate was replaced with normal saline; and other operations were the same. Control tubes and reaction tubes included: SOD control tube (500 μL of the agent- + 100 μL of purified water), SOD measurement tube (500 μL of the agent- + 100 μL of the sample), Cu-Zn SOD control tube (500 μL of the agent- + 100 μL of the control supernatant), and Cu-Zn SOD measurement tube (500 μL of the agent- + 100 μL of the supernatant of samples). Then, 100 μL of Reagents 2, 3 and 4 were added again and incubated for 1 h at 37 °C. After adding the chromogenic reagent for 10 min, SOD activity was determined by colorimetric analysis and calculated in accordance with absorbance values.
MDA content was determined from the same tissue homogenate supernatant selected in each group. Standard tube consisted of 200 μL of standard product + 3 mL of Reagent 2 + 1 mL of Reagent 3; standard blank tube contained 200 μL ethanol + 3 mL of Reagent 2 + 1 mL Reagent 3; measuring tube consisted of 200 μL of the sample + 3 mL of Reagent 2 + 1 mL of Reagent 3; measuring blank tube contained 200 μL of the sample + 3 mL of Reagent 2 + 1 mL of Reagent 3 + 1 mL of acetic acid. After mixing, the tubes were placed in water bath at 95 °C, and centrifuged (5000 r/min, 20 min) after 1 h of cooling, the supernatant was obtained, and absorbance value was measured. MDA content was calculated according to the absorbance value of each tube.
Statistical analysis: SPSS 19.0 software was used for statistical analyses. Quantitative data are expressed as mean ± SD. The t-test was carried out between two groups, and one-way analysis of variance (ANOVA) was used for multiple comparisons. Pearson’s correlation analysis was employed for correlation analysis. P < 0.05 was considered statistically significant.
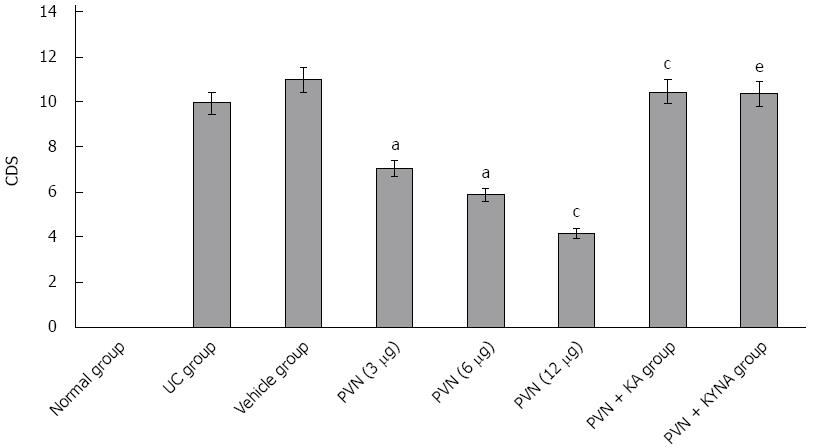
After chemical stimulation of the PVN was induced with 3, 6 or 12 μg of glutamic acid, colonic damage scores (CDS) of rats were 7.25 ± 0.98, 5.34 ± 0.89 and 3.74 ± 0.94, respectively, which significantly decreased compared to the vehicle group (10.24 ± 1.02). As the amount of glutamic acid increased, CDS decreased significantly (P < 0.05). After KA was used to induce chemical damage to the PVN, 12 μg of glutamic acid was used again to induce chemical stimulation in rats, the CDS was 10.05 ± 1.21, which significantly increased compared to simply using 12 μg of glutamic acid to induce chemical stimulation in rats (3.74 ± 0.94, P < 0.05). However, the difference was not statistically significant compared to rats in the UC group (9.86 ± 1.04, P > 0.05). In addition, after injecting glutamic acid antagonist KYNA into the PVN, chemical stimulation was carried out and the CDS was 9.87 ± 1.01, which significantly increased compared to simply using 12 μg of glutamic acid for chemical stimulation (3.74 ± 0.94, P < 0.05; Figure 1).
Influence of chemical damage to the NTS and injection of AVP-Vl receptor antagonist into the NTS on alleviated UC injury in rats by glutamic acid stimulation of the hypothalamic PVN
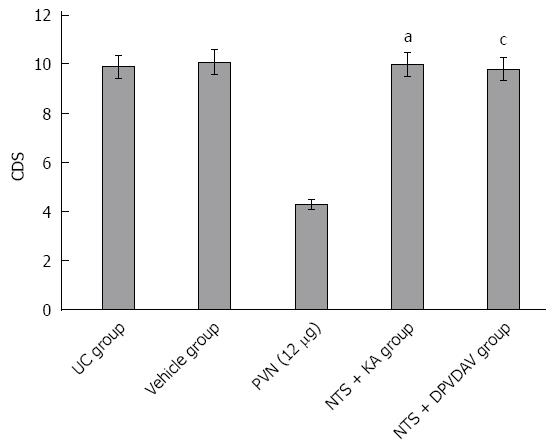
Three days after chemical damage to the NTS, 12 μg of glutamic acid was used again for the chemical stimulation of the hypothalamic PVN. CDS was 10.21 ± 1.09, which significantly increased compared to simply using 12 μg of glutamic acid for chemical stimulation (3.74 ± 0.94, P < 0.05). Ten minutes after DPVDAV was injected into the NTS of rats, 12 μg of glutamic acid was injected again to stimulate the PVN. CDS in rats was 9.67 ± 1.05, which significantly increased compared to simply using 12 μg of glutamic acid for chemical stimulation (3.74 ± 0.94, P < 0.05). When comparing the CDS of the NTS chemical damage group and DPVDAV group with the UC group and vehicle group, the differences were not statistically significant (P > 0.05; Figure 2).
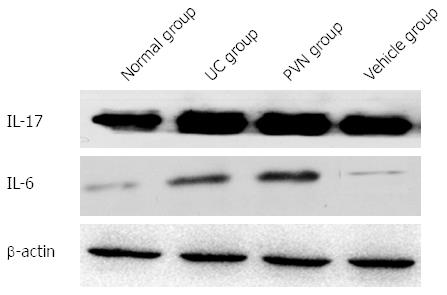
The contents of IL-6 and IL-17 in colon tissues were significantly increased in the UC group compared with the normal group, while the contents of IL-6 and IL-17 were significantly decreased in the 12-μg glutamic acid stimulation PVN group compared to the UC group and vehicle group (P < 0.05; Figure 3).
SOD activity significantly decreased in colon tissues in the UC group compared with the normal group, while SOD activity was significantly higher in the 12-μg glutamic acid stimulation group compared with the UC group (P = 7.964, t = 0.001; P = -7.335, t = 0.002). MDA content was significantly increased in colon tissue in the UC group than in the normal group, while MDA content in the 12-μg glutamic acid chemical stimulation group was significantly decreased than in the UC group (P = -4.604, t = 0.010; P = 5.124, t = 0.007; Table 2).
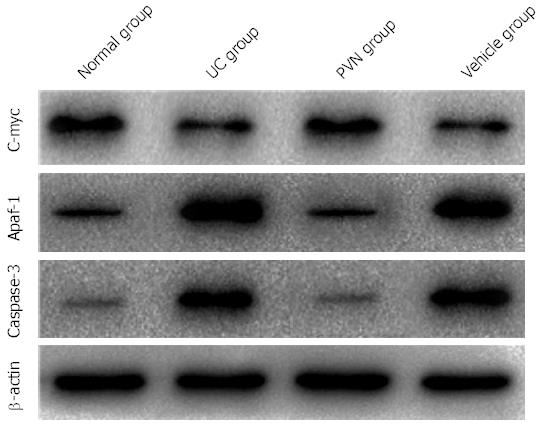
Apaf-1 and caspase-3 protein expression levels were significantly higher in the UC group than in the normal group, while C-myc protein expression levels were significantly lower than the normal group (P < 0.05). After 12 μg of glutamic acid was used for stimulation in rats in the PVN group, Apaf-1 and caspase-3 protein expression levels were significantly decreased compared with the UC and vehicle groups, while C-myc protein expression levels were significantly increased compared with the UC and vehicle groups (P < 0.05; Figure 4).
UC is a common and complex gastrointestinal disease that often brings great suffering to patients[1]. At present, the exact etiology and pathogenesis of UC remain unclear. In this study, we investigated the chemical stimulation of rat hypothalamic PVN, as well as its role in UC in rats and its regulatory mechanisms. On the basis of the establishment of a UC rat model and the stimulation of the hypothalamic PVN with glutamic acid, we observed whether PVN stimulation could alleviate intestinal damage in rats with UC, and investigated related regulatory and molecular mechanisms, to provide a theoretical basis for clinical studies.
As one of the centers of visceral activity, the PVN can extensively regulate various gastrointestinal mucosal injuries and repair processes[8]. In this study, stimulation of the PVN with glutamic acid significantly reduced intestinal damage in rats with UC, and as the dose of glutamic acid increased, the scale of improvement correspondingly increased. This effect disappeared when the PVN was damaged before glutamic acid injection. This may be because the PVN is a central nervous system that plays a key part in the protection and repair of intestinal injuries in rats, and this effect may be limited to the center of the PVN and its related neurons and has nothing to do with its other parts or other neurons.
The studies of A1-Ghoul WM and Mateos JM successively found that mRNAs of two kinds of glutamate receptor subtypes (metabotropic glutamate and ionotropic) are closely associated with the PVN, which are only distributed in the PVN region[9,10]. In this study, glutamic acid receptor antagonist KYNA can block the stimulation of the PVN with glutamic acid for the protection and repair of intestinal injury in rats with UC. Therefore, we conclude that glutamic acid receptors at the molecular level possibly mediate PVN stimulation to alleviate this effect in intestinal injury in rats with UC.
Hypothalamic PVN has a wide range of projection capabilities, which can communicate with many functional areas in the brain by signals, and is most closely associated with the NTS[11]. In this study, stimulation of the PVN with glutamic acid for alleviating intestine injury in rats with UC can be blocked after using KA to damage the NTS, revealing that the NTS may play an important role in the regulation of the PVN in intestinal injury repair[12]. The research team of Hegart found that AVP neurons in the PVN have a direct fiber projection with other functional areas of the brain, and the NTS is one of these. A bidirectional fiber link can be formed between the NTS and PVN, because the NTS is rich in AVP-Vl receptors[13]. In this study, AVP-Vl receptor antagonist DPVDAV was injected into the NTS in order to investigate whether AVP-Vl receptors are involved in the regulation of the PVN in the intestinal repair of rats via the NTS. It was found that the effect of glutamic acid stimulation of the PVN for alleviating intestinal injury in rats with UC disappeared. After KA induced damage to the NTS, similar experimental results were obtained, which partly confirmed the key role that AVP-Vl receptors played.
PVN stimulation regulates the intestinal repair possibly through anti-apoptotic effects, antioxidant effects, as well as the inhibition of inflammatory cytokines and other procedures
UC can secrete large amounts of inflammatory cytokines in the body[14-16], in which TNF-α and IL-1β may play an extremely important role in the inflammatory process[17,18]. This study revealed that after glutamic acid stimulation, the hypothalamic PVN may cause TNF-α and IL-1β expression levels to significantly decrease in colon tissue. This may be because the stimulation of the PVN with glutamic acid can reduce inflammatory effects by inhibiting the expression of inflammatory cytokines, and thus, play a role in repairing damage.
Increased oxygen free radicals can significantly injure body tissues. In order to eliminate such harm, the body can produce a free radical scavenger to scavenge oxygen free radicals. Therefore, the level of free radical scavenger activity can indirectly reflect the degree of injured body tissues, in which SOD is one of the most important members[19-22]. The study of the group of Talero E confirmed that UC can produce large amounts of oxygen free radicals such as reactive oxygen and nitrogen, which can cause great damage to the colonic mucosa[23-27]. In addition, an increase in oxygen free radicals can cause lipid peroxidation, resulting in increased MDA levels. Therefore, the level of MDA content may indirectly reflect the degree of cell damage. In this study, after PVN stimulation with glutamic acid, MDA content within the colonic mucosa significantly decreased in rats with UC, while SOD activity significantly increased. Perhaps, this is because of the regulatory role of the PVN after stimulation, in which the antioxidant capacity of colonic epithelial cells was enhanced so as to alleviate the injury.
The injury degree of UC to the colon depends on the balance between colonic epithelial cell proliferation and apoptosis to some extent. If this balance is broken and apoptosis is more than proliferation, it can result in the exacerbation of intestinal injury, while when proliferation is more than apoptosis, it would likely alleviate UC disease. The studies of the groups of Kararmnolis DG and Limura M revealed that colonic epithelial injury is usually accompanied by C-myc, Apaf-1 and caspase-3 expression changes[28-31], in which C-myc protein can inhibit apoptosis and promote cell proliferation. On the contrary, Apaf-1 and caspase-3 proteins are closely associated with apoptosis, and their overexpression can inhibit C-myc and other anti-apoptotic effects, resulting in increased apoptosis and diminished proliferation capacity. In this study, when glutamic acid was used to stimulate the PVN, Apaf-1 and caspause-3 protein expression levels significantly decreased and C-myc protein expression levels significantly increased in rats with UC. This shows that PVN stimulation can reduce tissue damage by enhancing the anti-apoptotic ability of colonic epithelial cells.
In summary, this study confirms that chemical stimulation of the PVN with glutamic acid can reduce intestinal injury in UC. Furthermore, this study also discussed the possible regulatory mechanism of the central nervous system of rats with UC, so as to provide a theoretical basis for clinical protection and the treatment of UC.
Ulcerative colitis (UC) is a common non-specific inflammatory bowel disease that easily recurs, has many complications and has a very poor prognosis. At present, due to the influence of environmental, diet and other factors, UC has increasingly prevailed in the population, but its exact etiology and pathogenesis remain unclear.
A study carried out by Mascaraque et al revealed that the central and peripheral nervous systems are involved in the regulation of UC.
The hypothalamic paraventricular nucleus (PVN) and nucleus tractus solitarius (NTS) may play an important role in regulating stress-induced gastric mucosal damage and gastric ischemia-reperfusion injury (GI-RI), and they may have participated in the regulatory role of PVN in GI-RI. However, the regulatory role of the PVN in UC, as well as its regulatory role for the treatment of UC, has been rarely reported.
In this study, the authors observed whether hypothalamic PVN stimulation could reduce intestinal injury in rats with UC through PVN chemical stimulation, chemical damage, injection of chemical antagonists and other methods, and preliminarily explored related regulatory and molecular mechanisms.
In this manuscript, the authors investigated the effect and mechanism of glutamate acid stimulation of the hypothalamic paraventricular nucleus in rats with UC. The study is well designed and the results are very interesting.
P- Reviewer: Imai K, Shimizu Y, Sumi K S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Pedersen J, LaCasse EC, Seidelin JB, Coskun M, Nielsen OH. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med. 2014;20:652-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Gothe F, Beigel F, Rust C, Hajji M, Koletzko S, Freudenberg F. Bile acid malabsorption assessed by 7 alpha-hydroxy-4-cholesten-3-one in pediatric inflammatory bowel disease: correlation to clinical and laboratory findings. J Crohns Colitis. 2014;8:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3349] [Article Influence: 186.1] [Reference Citation Analysis (11)] |
| 4. | Mascaraque C, González R, Suárez MD, Zarzuelo A, Sánchez de Medina F, Martínez-Augustin O. Intestinal anti-inflammatory activity of apigenin K in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. Br J Nutr. 2015;113:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Li TT, Zhang JF, Fei SJ, Zhu SP, Zhu JZ, Qiao X, Liu ZB. Glutamate microinjection into the hypothalamic paraventricular nucleus attenuates ulcerative colitis in rats. Acta Pharmacol Sin. 2014;35:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Wang T, Zhou YT, Chen XN, Zhu AX, Wu BH. Remote ischemic postconditioning protects against gastric mucosal lesions in rats. World J Gastroenterol. 2014;20:9519-9527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Bonamin F, Moraes TM, Dos Santos RC, Kushima H, Faria FM, Silva MA, Junior IV, Nogueira L, Bauab TM, Souza Brito AR. The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem Biol Interact. 2014;212:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Radley JJ, Sawchenko PE. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. J Comp Neurol. 2015;523:2769-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Shi Z, Li B, Brooks VL. Role of the Paraventricular Nucleus of the Hypothalamus in the Sympathoexcitatory Effects of Leptin. Hypertension. 2015;66:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Samuelsson AM. New perspectives on the origin of hypertension; the role of the hypothalamic melanocortin system. Exp Physiol. 2014;99:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ong ZY, Alhadeff AL, Grill HJ. Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol. 2015;308:R800-R806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Gao L, Zhu T, Xie G, Lou X, Li S, Zhou Y, Deng Z, Chu D, Lou J, Du D. GABA(A) receptor overexpression in the lateral hypothalamic area attenuates gastric ischemia-reperfusion injury in rats. Mol Med Rep. 2015;11:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Huber MJ, Basu R, Cecchettini C, Cuadra AE, Chen QH, Shan Z. Activation of the (pro)renin receptor in the paraventricular nucleus increases sympathetic outflow in anesthetized rats. Am J Physiol Heart Circ Physiol. 2015;309:H880-H887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Hundorfean G, Neurath MF, Mudter J. Functional relevance of T helper 17 (Th17) cells and the IL-17 cytokine family in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, Sonoda H, Shimizu T, Fujiyama Y, Andoh A. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Pearl DS, Shah K, Whittaker MA, Nitch-Smith H, Brown JF, Shute JK, Trebble TM. Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity. J Crohns Colitis. 2013;7:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Rodríguez-Perálvarez ML, García-Sánchez V, Villar-Pastor CM, González R, Iglesias-Flores E, Muntane J, Gómez-Camacho F. Role of serum cytokine profile in ulcerative colitis assessment. Inflamm Bowel Dis. 2012;18:1864-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Lipinski S, Bremer L, Lammers T, Thieme F, Schreiber S, Rosenstiel P. Coagulation and inflammation. Molecular insights and diagnostic implications. Hamostaseologie. 2011;31:94-102, 104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Hayashi Y, Narumi K, Tsuji S, Tsubokawa T, Nakaya MA, Wakayama T, Zuka M, Ohshima T, Yamagishi M, Okada T. Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: insights from in vitro and in vivo experimental studies. Int J Colorectal Dis. 2011;26:1453-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Ballal SA, Veiga P, Fenn K, Michaud M, Kim JH, Gallini CA, Glickman JN, Quéré G, Garault P, Béal C. Host lysozyme-mediated lysis of Lactococcus lactis facilitates delivery of colitis-attenuating superoxide dismutase to inflamed colons. Proc Natl Acad Sci USA. 2015;112:7803-7808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Wang C, Han Z. Ginkgo Biloba Extract Enhances Differentiation and Performance of Neural Stem Cells in Mouse Cochlea. Cell Mol Neurobiol. 2015;35:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Grimstad T, Bjørndal B, Cacabelos D, Aasprong OG, Janssen EA, Omdal R, Svardal A, Hausken T, Bohov P, Portero-Otin M. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand J Gastroenterol. 2012;47:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Bank S, Skytt Andersen P, Burisch J, Pedersen N, Roug S, Galsgaard J, Ydegaard Turino S, Brodersen JB, Rashid S, Kaiser Rasmussen B. Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS One. 2014;9:e98815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Büning C, von Kraft C, Hermsdorf M, Gentz E, Wirth EK, Valentini L, Haas V. Visceral Adipose Tissue in Patients with Crohn’s Disease Correlates with Disease Activity, Inflammatory Markers, and Outcome. Inflamm Bowel Dis. 2015;21:2590-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Stallhofer J, Friedrich M, Konrad-Zerna A, Wetzke M, Lohse P, Glas J, Tillack-Schreiber C, Schnitzler F, Beigel F, Brand S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm Bowel Dis. 2015;21:2327-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Franzè E, Monteleone I, Cupi ML, Mancia P, Caprioli F, Marafini I, Colantoni A, Ortenzi A, Laudisi F, Sica G. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci (Lond). 2015;129:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Ramonaite R, Skieceviciene J, Kiudelis G, Jonaitis L, Tamelis A, Cizas P, Borutaite V, Kupcinskas L. Influence of NADPH oxidase on inflammatory response in primary intestinal epithelial cells in patients with ulcerative colitis. BMC Gastroenterol. 2013;13:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Leucht K, Caj M, Fried M, Rogler G, Hausmann M. Impaired removal of Vβ8(+) lymphocytes aggravates colitis in mice deficient for B cell lymphoma-2-interacting mediator of cell death (Bim). Clin Exp Immunol. 2013;173:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Zhao QJ, Yu YB, Zuo XL, Dong YY, Li YQ. Milk fat globule-epidermal growth factor 8 is decreased in intestinal epithelium of ulcerative colitis patients and thereby causes increased apoptosis and impaired wound healing. Mol Med. 2012;18:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Schwarte LA, Schwartges I, Scheeren TW, Schober P, Picker O. The differential effects of recombinant brain natriuretic peptide, nitroglycerine and dihydralazine on systemic oxygen delivery and gastric mucosal microvascular oxygenation in dogs. Anaesthesia. 2012;67:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013;47:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |