Published online Apr 7, 2016. doi: 10.3748/wjg.v22.i13.3644
Peer-review started: November 10, 2015
First decision: December 11, 2015
Revised: December 31, 2015
Accepted: January 30, 2016
Article in press: January 30, 2016
Published online: April 7, 2016
Processing time: 138 Days and 21.9 Hours
AIM: To investigate feasibility and accuracy of near-infrared fluorescence imaging using indocyanine green: nanocolloid for sentinel lymph node (SLN) detection in gastric cancer.
METHODS: A prospective, single-institution, phase I feasibility trial was conducted. Patients suffering from gastric cancer and planned for gastrectomy were included. During surgery, a subserosal injection of 1.6 mL ICG:Nanocoll was administered around the tumor. NIR fluorescence imaging of the abdominal cavity was performed using the Mini-FLARE™ NIR fluorescence imaging system. Lymphatic pathways and SLNs were visualized. Of every detected SLN, the corresponding lymph node station, signal-to-background ratio and histopathological diagnosis was determined. Patients underwent standard-of-care gastrectomy. Detected SLNs outside the standard dissection planes were also resected and evaluated.
RESULTS: Twenty-six patients were enrolled. Four patients were excluded because distant metastases were found during surgery or due to technical failure of the injection. In 21 of the remaining 22 patients, at least 1 SLN was detected by NIR Fluorescence imaging (mean 3.1 SLNs; range 1-6). In 8 of the 21 patients, tumor-positive LNs were found. Overall accuracy of the technique was 90% (70%-99%; 95%CI), which decreased by higher pT-stage (100%, 100%, 100%, 90%, 0% for respectively Tx, T1, T2, T3, T4 tumors). All NIR-negative SLNs were completely effaced by tumor. Mean fluorescence signal-to-background ratio of SLNs was 4.4 (range 1.4-19.8). In 8 of the 21 patients, SLNs outside the standard resection plane were identified, that contained malignant cells in 2 patients.
CONCLUSION: This study shows successful use of ICG:Nanocoll as lymphatic tracer for SLN detection in gastric cancer. Moreover, tumor-containing LNs outside the standard dissection planes were identified.
Core tip: Sentinel lymph node (SLN) detection using indoyanine green adsorbed to nanocolloid (ICG:Nanocoll) was investigated in 26 patients with gastric cancer. Adsorption of ICG to nanocolloid results in better retention in SLNs and staining of less 2nd tier nodes. After subserosal injection, fluorescent SLN detection using the Mini-FLARE™ system was performed. A mean number of 3.1 SLNs per patient were found and overall accuracy was 90%. In 8 patients, SLNs outside the standard resection planes were identified, that contained malignant cells in 2 patients. To conclude, NIR fluorescence imaging using ICG:Nanocoll as lymphatic tracer identified SLNs in- and outside standard dissection planes.
- Citation: Tummers QRJG, Boogerd LSF, de Steur WO, Verbeek FPR, Boonstra MC, Handgraaf HJM, Frangioni JV, van de Velde CJH, Hartgrink HH, Vahrmeijer AL. Near-infrared fluorescence sentinel lymph node detection in gastric cancer: A pilot study. World J Gastroenterol 2016; 22(13): 3644-3651
- URL: https://www.wjgnet.com/1007-9327/full/v22/i13/3644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i13.3644
Gastric cancer is still one of the most frequent causes of cancer deaths worldwide with an incidence rate varying between countries[1,2]. The highest estimated mortality rates are in Eastern Asia (24 per 100000 in men, 9.8 per 100000 in women), the lowest in North America (2.8 and 1.5, respectively)[3].
Surgical resection of the tumor is the only curative treatment option. Depending on the size, infiltration depth, and location of the tumor, surgery can be performed endoscopically, or by partial or total gastrectomy. In addition to resection of the affected part of the stomach, a lymph node (LN) dissection is typically performed. This can either be done by extensive lymphadenectomy or by a sentinel lymph node (SLN) procedure, depending on T status and size of the tumor. Nodal involvement in gastric cancer occurs in only 2%-18% when the depth of cancer invasion is limited to the mucosal or submucosal layer (T1), and in about 50% when tumors invade the subserosal layer (T2)[4]. In patients with tumor-negative lymph nodes, a SLN procedure could avoid the risk of morbidity and mortality of an unnecessary lymphadenectomy. Additionally, in patients who are undergoing a partial or total gastrectomy combined with lymphadenectomy, identification of potentially involved LNs outside the standard plane of resection is possible by detecting the SLN. In this way, also in tumors with higher T stages, one can find the true first tumor draining LN(s), and not leaving them in situ. As the lymphatic drainage route of gastric cancer is generally multidirectional and complicated[5], intraoperative assistance in identification of potentially involved lymph nodes could improve gastric cancer treatment.
SLN detection in gastric cancer was first described by Kitagawa et al[6] Since then, multiple studies were performed. A prospective multicenter trial in 433 patients with T1 or T2 stadium tumors showed an accuracy rate of 99% for identification of metastasis in SLNs with the use of a dual tracer consisting of radiolabeled tin colloid and blue dye[7].
Near-infrared (NIR) fluorescence imaging is an innovative technique to visualize tumors, vital structures, lymphatic channels, and LNs[8]. Soltesz et al[9] in a preclinical setting and Kusano et al[10] in a clinical setting were the first to report the SLN procedure in gastric cancer using NIR fluorescence imaging. Since then, multiple studies confirmed the feasibility of this technique for both open and laparoscopic surgery[11-15]. All clinical studies reported to date utilized indocyanine green (ICG) as the lymphatic tracer. However, the use of ICG resulted in detection of more fluorescent lymph nodes per patient than expected due to migration through the SLN to second tier nodes. Consequently, resection and pathological assessment of multiple nodes was still needed. Adsorption of ICG to a nanocolloid (ICG:Nanocoll) increases its hydrodynamic diameter, which may result in better retention of the lymphatic tracer in the SLN, and thereby staining less second-tier nodes. This results in intraoperative identification of true SLNs, and avoids analyzing non-SLNs during pathological assessment for tumor-status of the SLN. This principle was already successfully described for breast cancer[16] and skin melanoma[17].
The aim of this study was to investigate feasibility of ICG adsorbed to nanocolloid as a lymphatic tracer for the intraoperative detection of the SLN in gastric cancer patients with different pT stages, and to determine the prognostic utility of the detected SLN.
ICG:Nanocoll was prepared by diluting 25 mg ICG (Pulsion Medical Systems, Munich, Germany) in 5 mL water and diluting 0.5 mg Nanocoll (GE Healthcare, Eindhoven, the Netherlands) in 3 mL saline. Portions of these solutions were mixed to obtain 1.6 mL ICG:Nanocoll containing 0.05 mg ICG and 0.1 mg Nanocoll. Preparation was performed in the operating room, following preparation instructions of the institutional pharmacist.
The trial was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. Registration within the Netherlands Trial Register was performed (NTR4280).
Twenty-six patients with different T stages of gastric cancer, planned for a partial or total gastrectomy, were included between February 2013 and March 2015. Patients underwent standard-of-care preoperative imaging using a computed tomography (CT) scan. No standard endoscopic ultrasound or staging laparoscopy was performed. All procedures were performed by surgeons with broad experience in gastric cancer surgery.
After opening of the abdominal cavity the tumor was exposed without causing damage to lymphatic vessels around the tumor as much as possible. When no metastasized disease was found, 1.6 mL ICG:Nanocoll was administered subserosally in 4 quadrants around the tumor. Directly after injection NIR fluorescence images of lymphatic pathways were acquired using the Mini-FLARETM NIR fluorescence imaging system[18]. Fluorescence imaging was performed on multiple time points during surgery. A SLN was defined as fluorescent hotspot that appeared after injection of the tracer. When multiple fluorescent hotspots appeared in the same LN basin all fluorescent LNs were defined as SLNs. The anatomical location of the fluorescent hotspots was determined using the lymph node stations as defined by the Japanese Research Society for the Study of Gastric Cancer[19]. Patients underwent a standard-of-care partial or total gastrectomy with modified D2 resection, consisting of resection of the peri-gastric LNs and LN station 7, 8 and 9. After resection, the specimen was analyzed ex vivo using the FLARETM NIR fluorescence imaging system at the Pathology Department. The marked fluorescent hotspots were resected from the specimen, transected, fixed in formalin and embedded in paraffin for routine hematoxylin and eosin staining, and analyzed for tumor status.
The in vivo signal-to-background ratio (SBR) of the SLN was calculated by dividing the fluorescence intensity of the SLN by the fluorescence intensity of the directly surrounding fatty tissue. Accuracy rate was defined by the number of patients in which tumor-negative SLNs were found when no tumor-positive lymph nodes were found in the entire specimen and the number of patients in which tumor-positive SLNs were found when tumor-positive lymph nodes were found in the whole specimen divided by the total number of patients. Accuracy rate was expressed as percentage with a 95%CI. A false-negative patient was defined as a patient in whom tumor-negative SLNs were found, while tumor-positive LNs were found in the resection specimen.
Confidence intervals for the binomial proportions were calculated using exact binomial confidence intervals. Numerical data were summarized with median (range).
Intraoperative imaging procedures were performed using the Mini-Fluorescence-Assisted Resection and Exploration (Mini-FLARETM) image-guided surgery system, as described earlier[18]. Briefly, the system consists of 2 wavelength isolated light sources: a “white” light source, generating 26600 lx of 400 to 650 nm light, and a “near-infrared” light source, generating 1.08 mW/came of about 760 nm light. Color video and NIR fluorescence images are simultaneously acquired and displayed in real time using custom optics and software that separate the color video and NIR fluorescence images. A pseudo-colored (lime green) merged image of the color video and NIR fluorescence images is also displayed. The imaging head is attached to a flexible gooseneck arm, which permits positioning of the imaging head at extreme angles virtually anywhere over the surgical field. For intraoperative use, the imaging head and imaging system pole stand are wrapped in a sterile shield and drape (Medical Technique Inc., Tucson, AZ).
Twenty-six patients with gastric cancer undergoing partial or total gastrectomy were included in this study (Table 1). Median age was 64 years (range 30-82) and 19 patients were male. T-stadium of tumors was pTx, pT1, pT2, pT3, and pT4 in respectively 2, 5, 5, 10, and 4 patients. Median tumor size was 31 mm (range 10-90 mm). Tumors were located in the cardia in 8, corpus in 6 and antrum in 12 patients. Eleven patients underwent a total gastrectomy, 14 patients underwent a partial gastrectomy and in 1 patient no resection was performed due to metastasized disease. Twenty-three patients received neoadjuvant chemotherapy consisting of Epirubicine, Oxaliplatin and Capecitabine or Epirubicine, Cisplatine and Capecitabine.
| Characteristic | Median | Range |
| Age (yr) | 64 | 30-82 |
| Tumor size (mm) | 35 | 10-90 |
| N (n = 26) | ||
| Gender | ||
| M | 19 | 73% |
| F | 7 | 27% |
| Tumor location | ||
| Cardia | 8 | 31% |
| Corpus | 6 | 23% |
| Antrum | 12 | 46% |
| Tumor pT stage | ||
| pTx | 2 | 8% |
| pT1 | 5 | 19% |
| pT2 | 5 | 19% |
| pT3 | 10 | 39% |
| pT4 | 4 | 15% |
| Type of resection | ||
| Total gastrectomy | 11 | 42% |
| Partial gastrectomy | 14 | 54% |
| No resection | 1 | 4% |
| Preoperative CTx | 23 | 88% |
Three patients (No.3, No.12, and No.16) did not receive an injection of ICG:Nanocoll because metastatic disease was found during surgery. In 1 patient (No.8), ICG:Nanocoll was injected through the wall of the stomach. After this technical failure, this patient was excluded for further analysis.
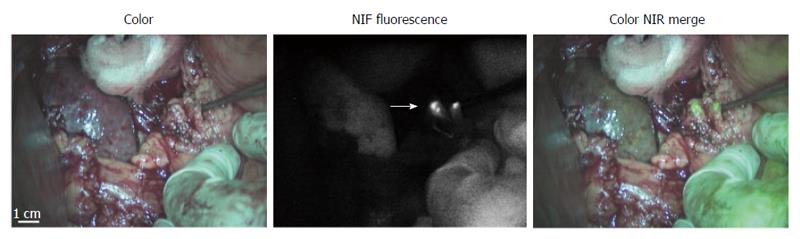
Table 2 shows the characteristics of the intraoperatively detected SLNs in each patient. In 21 of the remaining 22 patients (95%, 77%-100%, 95%CI interval), at least 1 SLN was found during surgery (mean of 3.1 SLNs per patient; range 1-6). SLNs were identified as bright fluorescent spots in the surrounding tissue of the stomach. Figure 1A shows a bright fluorescent spot, which was found histologically to be a tumor-negative lymph node. Figure 1B shows an example of a tumor-positive lymph node and visualization of lymphatic vessels running from the injection site to the lymph node. The mean SBR of the SLNs was 4.4 (range 1.4-19.8). In total, 533 LNs were identified in the resection specimens by the pathologist, resulting in a mean number of 24 resected LNs (range 11-44) per patient.
| PatientNo. | T stage | Tumorlocation | Pre-operativeCTx | Number of detected SLNs | Location of detected SLNs by LN St | Mean SBRof SLNs | Tumorstatus inSLNs | SLNIdentificationaccurate? | SLN outsideStandarddissection plane? |
| 1 | 1 | M | No | 3 | 3 | 5.4 | Neg | Yes | No |
| 2 | 4 | M | Yes | 3 | 3; 3 and 6 | 1.9 | Pos | No | No |
| 3 | 4 | D | Yes | No tracer injected because of metastasized disease | |||||
| 4 | 1 | D | Yes | 3 | 4; 6 and 6 | 4.3 | Neg | Yes | No |
| 5 | 2 | U | Yes | 4 | 3; 3; 4 and 4 | 3.9 | Neg | Yes | No |
| 6 | 3 | M | Yes | 3 | 4; 4 and 7 | 5.3 | Neg | Yes | No |
| 7 | 1 | M | No | 4 | 3; 3; 6 and 6 | 3.1 | Neg | Yes | No |
| 8 | 2 | U | Yes | Technical failure of tracer administration | |||||
| 9 | 3 | U | Yes | 2 | 1 and 1 | 8.7 | Neg | Yes | No |
| 10 | 3 | D | No | 3 | 6; 6 and 12 | 4.7 | Pos | No | Yes |
| 11 | 3 | D | Yes | 4 | 5; 6; 6 and 12 | 4.9 | Pos | Yes | Yes |
| 12 | 4 | U | Yes | No tracer injected because of metastasized disease | |||||
| 13 | 2 | U | Yes | 3 | 1; 7 and 9 | 3.5 | Neg | Yes | No |
| 14 | 2 | D | Yes | 4 | 6 and 12 | 5.1 | Pos | Yes | Yes |
| 15 | 2 | M | Yes | 0 | NA | NA | NA | NA | No |
| 16 | 4 | M | Yes | No tracer injected because of metastasized disease | |||||
| 17 | 3 | U | Yes | 2 | 8 and 12 | 2.6 | Neg | Yes | Yes |
| 18 | 3 | D | Yes | 2 | 5 and 6 | 11.4 | Neg | Yes | No |
| 19 | x | D | Yes | 2 | 3 and 3 | 3.6 | Neg | Yes | No |
| 20 | x | D | Yes | 2 | 3 and 14 | 3.6 | Neg | Yes | Yes |
| 21 | 3 | M | Yes | 5 | 3; 3; outside standard planes of 9 (3x) | 2.8 | Pos | Yes | Yes |
| 22 | 3 | D | Yes | 4 | 3; 6; 6 and 14 | 3.1 | Pos | Yes | Yes |
| 23 | 3 | U | Yes | 1 | 1 | 6.2 | Pos | Yes | No |
| 24 | 3 | D | Yes | 6 | 3; 3; 6; 6; 6 and 11 | 4.0 | Pos | Yes | Yes |
| 25 | 1 | D | Yes | 4 | 3; 3; 4 and 6 | 4.6 | Neg | Yes | No |
| 26 | 1 | D | Yes | 4 | 5; 6; 6 and 6 | 5.6 | Neg | Yes | No |
In 19 out of 21 patients, an accurate SLN was found. The overall accuracy of the SLN procedure was 90% (95%CI: 70%-99%) and a higher pT-stadium was associated with a lower accuracy rate. Accuracy rates for pTx, pT1, pT2, pT3, and pT4 were respectively 100%, 100%, 100%, 90%, and 0%.
Histological analysis of the SLNs showed lymph node metastases in 8 out of 21 patients. In 6 patients, the SLNs that were identified using NIR fluorescence imaging were tumor-positive (true positive). In the other 2 patients, tumor-positive lymph nodes were not identified using NIR fluorescence imaging (false-negative). One false-negative patient (No.2) had a T4 tumor. The tumor positive lymph nodes (3 out of 33 LNs) in this patient were found in the peripancreatic fatty tissue and in lymph node station 3, where a SLN was also detected. The second false-negative patient (No.10) had a T3 tumor. Four out of 11 LNs that contained tumor cells were not detected by NIR fluorescence imaging. Of particular importance, all 7 tumor-positive LNs that were not detected by NIR fluorescence imaging were completely effaced by tumor tissue and no lymphatic tissue could be identified.
In 8 patients, SLNs outside the standard resection plane were identified. In 4 patients these were located in the hepatoduodenal ligament (LN station 12), in 2 patients near the border of the pancreas (LN station 14), in 1 patient outside the standard plane near LN station 9 and in 1 patient in LN station 11. In 2 patients, the extra-detected lymph nodes outside the standard plane of resection contained tumor cells (No.21 and No.22) (Figure 2).
No adverse events regarding the use of ICG:Nanocoll or NIR fluorescence imaging were encountered.
The current feasibility study demonstrates that the SLN detection in gastric cancer, using ICG adsorbed to nanocolloid as the lymphatic tracer, is feasible and safe. In 21 out of 22 patients at least 1 SLN was identified, and in 19 out of 21 patients an accurate SLN was found.
In fewer than 50 percent of patients with a T1 or T2 tumor, lymph nodes show tumor involvement. In these patients, the SLN procedure has the potential to avoid an unnecessary lymphadenectomy, and its associated potential morbidity and mortality. Kitagawa et al[7] reported an accuracy of nodal evaluation for metastasis of 99 percent, underlining the clinical applicability of the technique in this selected patient group. The SLN procedure in gastric cancer was validated for previously untreated cT1-T2 tumors with a diameter of less than 4 mm. However, in the Western world patients often present with a higher T stadium, and are often pretreated with chemotherapy. In these patients who need an extensive lymphadenectomy, the described technique could assist in identifying potentially involved lymph nodes located outside the standard plane of resection. Moreover, morbidity and mortality rates increase when a more extended lymph node dissection is performed[20]. A more targeted and personalized treatment, including identification and dissection of truly or potentially involved lymph nodes, could result in improved gastric cancer treatment. Therefore, in both early gastric cancer and in resectable cases of advanced gastric cancer, accurate identification of true SLNs is of great importance.
Many different lymphatic tracers have been reported for SLN identification. The largest prospective multicenter trial until now used a combination of blue dye and radiolabeled tin colloids[7]. However, both tracers have disadvantages. Blue dye could alter the surgical field by dark staining, and only permits identification of superficially located lymph nodes. Moreover, in previous studies comparing radiolabeled colloids, blue dye, and NIR fluorescence for SLN detection in breast cancer patients, only 84%-88% of the identified SLNs stained blue compared to 100% that were NIR fluorescent[16,21]. For the SLN procedure in skin melanoma blue dye staining was successful in only 73%[22]. Radiolabeled colloids only permit acoustic guidance during SLN identification, but no visual guidance. Besides, radioactive isotopes are scarce in many areas of the world. NIR fluorescence imaging could overcome these limitations as it only needs an imaging system and fluorescent tracer, and allows real-time optical identification of lymph nodes in up to about 6 mm of tissue, for example in visceral dense fat tissue[8].
Since Kusano et al[10] reported the first SLN procedure in gastric cancer using NIR fluorescence imaging, multiple studies confirmed the feasibility of this technique for both open and laparoscopic surgery[11-15]. All reported studies to date used ICG alone as lymphatic tracer, which resulted in detection of many fluorescent lymph nodes per patient. For example, Tajima et al[14] reported a mean number of 7.2 ± 7 SLN per patient and Fujita et al[11] a mean number of 9.3 ± 6.4 SLN per patient when using ICG as lymphatic tracer. This was possibly due to migration through the SLN to second tier nodes, and resection and pathological assessment of multiple nodes was still needed.
By combining ICG with nanocolloid, its hydrodynamic diameter increases from ≤ 1 nm (ICG) to 20-80 nm (ICG:Nanocoll). It has been shown that the hydrodynamic diameter of a lymphatic tracer has a major impact on the lymphatic migration and accumulation in lymph nodes. Molecules with a hydrodynamic diameter less than approximately 10 nm (for example ICG) have the potential to migrate through the SLN to second tier nodes, while larger molecules with a hydrodynamic diameter of < 100 nm (ICG:Nanocoll) are retained in the SLN[23]. In the current study, a mean number of 3.1 SLN per patient was found. This lower number of detected SLNs is in accordance with our hypothesis that better retention in SLNs is obtained when a lymphatic tracer with a higher hydrodynamic diameter is used. This highly improves the clinical applicability of SLN detection using NIR fluorescence imaging in gastric cancer.
Although the number of patients was limited in the current study, an excellent accuracy rate was obtained in lower pT stages, in which the clinical value of a SLN procedure is becoming more and more accepted. These data are consistent with previous studies where the SLN procedure was performed for T1 and T2 tumors. However, even for advanced gastric cancer, identifying the first draining lymph nodes can be of added value. In the current study, LNs from 8 patients were identified outside the standard resection margin using NIR fluorescence imaging; they would otherwise not have been resected. In 2 patients, the extra-detected LNs outside the standard plane of resection contained tumor cells. These LNs were only resected because NIR fluorescence imaging identified them. Larger studies are needed to determine the additional value of the described technique in advanced gastric cancer.
In 2 patients, tumor-positive LNs were not identified using NIR fluorescence imaging. One explanation for the false positivity of these LNs might be the fact that these tumors were relatively large, respectively 60 and 45mm diameter, and consequently, adequate injection of the tracer in four quadrants around the tumor might be hampered. However, other fluorescent LNs that that did contain tumor cells, were found in tumors with a median size of 52mm (range 27-90). Moreover, all 7 of these LNs were completely effaced by tumor tissue. Such LNs lose function, lymph doesn’t flow in or out, and no lymphatic tissue is present to trap the fluorescent tracer. Subsequently, these tumor-positive LNs can’t be identified, a principle that counts for the SLN procedure in all solid cancers. In one of these patients however, the identified SLN was found in the same LN basin as one of the tumor-involved LNs. This underlines the theory that whenever SLNs are visualized, the entire lymph node basin should be resected instead of only the SLN by lymph node picking, because it is shown that most of the metastatic non-SLNs are positioned in the same basin as the detected SLNs[24,25].
A well-known difficulty in SLN mapping in gastric cancer is the presence of skip metastasis: involvement of extra-perigastric lymph nodes without the detection of perigastric lymph node metastasis. The incidence of skip metastasis among patients with gastric cancer and metastasis is reported to be as high as 11%[26]. The described technique could assist in identifying these potentially involved extra-perigastric lymph nodes.
One of the limitations of this study is the administration technique, which is performed during surgery in the subserosal layer of the gastric wall. Opening the abdominal cavity, and exposing the affected part of the stomach could damage lymphatic vessels. This potentially hampers lymphatic flow to SLNs and was overcome as much as possible by avoiding dissections near the primary tumor. Besides, injecting the tracer in the submucosal layer seems more appropriate in case of tumor invasion limited to the mucosa or submucosa. However, it is shown that subserosal injection leads to drainage of the tracer to the same lymph nodes as submucosal injection, because of communication through vertical connections of lymphatic vessels in the gastric wall[27]. These limitations could be overcome by submucosal endoscopic administration of the lymphatic tracer before surgery. One of the additional advantages of this administration technique is that it allows visual tumor demarcation during surgery through the stomach wall, which assists in intraoperative tumor identification, and determination of resection margins. Especially in patients who experience good response on neoadjuvant chemotherapy this could be of added value.
Another limitation of the current feasibility study is that no true SLN procedure was performed, but instead intraoperative detection of SLNs. However, all fluorescent hotspots that were detected during surgery were directly marked using sutures. After the surgical procedure, they were mapped from the specimen for pathological assessment. By doing this, the same LNs were analyzed as if they would have been resected directly during surgery, and feasibility of ICG:Nanocoll as lymphatic tracer could still be showed.
Finally, pathological assessment of the LNs consisted of standard-of-care transection and hematoxylin and eosin staining. Multiple transections and additional keratin staining could possibly have resulted in the detection of tumor-tissue in the SLNs of the 2 false-negative patients, and thereby increasing accuracy rate. However, small tumor deposits in the detected SLNs were unlikely to be present as the tumor-positive LNs in the specimen were completely effaced by tumor.
In conclusion, this is the first study using ICG combined with nanocolloid as lymphatic tracer for the detection of the SLN in gastric cancer patients by NIR fluorescence imaging. In T1 and T2 gastric tumors, an excellent accuracy was observed. Moreover, this technique allowed identification of tumor-positive lymph nodes outside the standard dissection planes.
We thank David J. Burrington, Jr. for editing.
Treatment of gastric cancer consists of surgical resection of the tumor and identification and resection of potentially involved lymph nodes. The latter can either be done by extensive lymphadenectomy or by a sentinel lymph node (SLN) procedure. Identification of these lymph nodes can be challenging, and assistance in identification of potentially involved lymph nodes could improve gastric cancer treatment. Near-infrared (NIR) fluorescence imaging is an innovative technique to visualize lymph nodes and lymphatic channels, and could therefore improve identification of SLNs.
NIR fluorescence imaging is gaining more and more scientific and clinical attention worldwide, and physicians are exploring the possibilities and capabilities of clinically available contrast agents like indocyanine green (ICG). Optimizing optical and accuracy properties of lymphatic tracers, as performed in the current study, can have major impact on its clinical relevance.
Several studies to date are reported on the use of ICG as fluorescent tracer for identification of SLNs. However, the use of ICG alone resulted in detection of more fluorescent lymph nodes per patient than expected due to migration through the SLN to second tier nodes. Consequently, resection and pathological assessment of multiple nodes was still needed. Adsorption of ICG to a nanocolloid (ICG:Nanocoll) increases its hydrodynamic diameter, which may result in better retention of the lymphatic tracer in the SLN, and thereby staining less second-tier nodes. To the best of our knowledge, this is the first study reported on the use of ICG:Nanocoll as lymphatic tracer in gastric cancer.
Gastric cancer is one of the most frequent causes of cancer deaths worldwide, and optimizing its treatment can have major impact on patient outcome. The described agents for NIR fluorescence imaging (ICG and Nanocoll) are commercially available. Therefore this technique can be easily applied to the treatment of gastric cancer.
NIR fluorescence imaging is an optical imaging technique using light in the NIR spectrum (700-900 nm). Indocyanine green is a cyanine dye with fluorescence properties in the NIR spectrum.
In this manuscript, the author investigated the usefulness of the indoyanine green adsorbed to nanocolloid in gastric cancer patients. The manuscript is well written.
P- Reviewer: Handa O, Yang F S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1245] [Cited by in RCA: 1401] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 2. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 795] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalance worldwide in 2012. Accessed 30th June 2014. Available from: http://globocan.iarc.fr/Default.aspx. |
| 4. | Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346-351. [PubMed] |
| 5. | Tokunaga M, Ohyama S, Hiki N, Fukunaga T, Yamada K, Sano T, Yamaguchi T. Investigation of the lymphatic stream of the stomach in gastric cancer with solitary lymph node metastasis. World J Surg. 2009;33:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Kitagawa Y, Fujii H, Mukai M, Kubota T, Otani Y, Kitajima M. Radio-guided sentinel node detection for gastric cancer. Br J Surg. 2002;89:604-608. [PubMed] |
| 7. | Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1020] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 9. | Soltesz EG, Kim S, Kim SW, Laurence RG, De Grand AM, Parungo CP, Cohn LH, Bawendi MG, Frangioni JV. Sentinel lymph node mapping of the gastrointestinal tract by using invisible light. Ann Surg Oncol. 2006;13:386-396. [PubMed] |
| 10. | Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. 2008;25:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Fujita T, Seshimo A, Kameoka S. Detection of sentinel nodes in gastric cancer by indocyanine green fluorescence imaging. Hepatogastroenterology. 2012;59:2213-2216. [PubMed] |
| 12. | Miyashiro I, Miyoshi N, Hiratsuka M, Kishi K, Yamada T, Ohue M, Ohigashi H, Yano M, Ishikawa O, Imaoka S. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol. 2008;15:1640-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Miyashiro I, Kishi K, Yano M, Tanaka K, Motoori M, Ohue M, Ohigashi H, Takenaka A, Tomita Y, Ishikawa O. Laparoscopic detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging. Surg Endosc. 2011;25:1672-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Tajima Y, Yamazaki K, Masuda Y, Kato M, Yasuda D, Aoki T, Kato T, Murakami M, Miwa M, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Tajima Y, Murakami M, Yamazaki K, Masuda Y, Kato M, Sato A, Goto S, Otsuka K, Kato T, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol. 2010;17:1787-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Schaafsma BE, Verbeek FP, Rietbergen DD, van der Hiel B, van der Vorst JR, Liefers GJ, Frangioni JV, van de Velde CJ, van Leeuwen FW, Vahrmeijer AL. Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg. 2013;100:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Brouwer OR, Klop WM, Buckle T, Vermeeren L, van den Brekel MW, Balm AJ, Nieweg OE, Valdés Olmos RA, van Leeuwen FW. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann Surg Oncol. 2012;19:1988-1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol. 2011;18:2483-2491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127-139. [PubMed] |
| 20. | Giuliani A, Miccini M, Basso L. Extent of lymphadenectomy and perioperative therapies: two open issues in gastric cancer. World J Gastroenterol. 2014;20:3889-3904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW, Liefers GJ, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99(m) technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol. 2012;19:4104-4111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | van der Vorst JR, Schaafsma BE, Verbeek FP, Swijnenburg RJ, Hutteman M, Liefers GJ, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Dose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanoma. Br J Dermatol. 2013;168:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | van Leeuwen AC, Buckle T, Bendle G, Vermeeren L, Valdés Olmos R, van de Poel HG, van Leeuwen FW. Tracer-cocktail injections for combined pre- and intraoperative multimodal imaging of lymph nodes in a spontaneous mouse prostate tumor model. J Biomed Opt. 2011;16:016004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Ajisaka H, Miwa K. Micrometastases in sentinel nodes of gastric cancer. Br J Cancer. 2003;89:676-680. [PubMed] |
| 25. | Miyashiro I. What is the problem in clinical application of sentinel node concept to gastric cancer surgery? J Gastric Cancer. 2012;12:7-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596-602. [PubMed] |
| 27. | Yaguchi Y, Ichikura T, Ono S, Tsujimoto H, Sugasawa H, Sakamoto N, Matsumoto Y, Yoshida K, Kosuda S, Hase K. How should tracers be injected to detect for sentinel nodes in gastric cancer--submucosally from inside or subserosally from outside of the stomach? J Exp Clin Cancer Res. 2008;27:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |