Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2777
Peer-review started: June 24, 2014
First decision: August 6, 2014
Revised: September 4, 2014
Accepted: November 18, 2014
Article in press: November 19, 2014
Published online: March 7, 2015
Processing time: 259 Days and 0.9 Hours
AIM: To assess the efficacy of immunotherapy with expanded activated autologous lymphocytes (EAALs) in gastric cancer.
METHODS: An observational study was designed to retrospectively analyze the clinical data of 84 gastric cancer patients, of whom 42 were treated by EAAL immunotherapy plus conventional treatment and another 42 only received conventional treatment (control group). EAALs were obtained by proliferation of peripheral blood mononuclear cells from patients followed by phenotype determination. Clinical data including age, gender, clinical stage, chemotherapeutic regimens, hospitalization, surgical, radiotherapy, and survival data were collected along with EAAL therapy details and side effects. Patients were followed and the relationship between treatment and overall survival (OS) data obtained for the immunotherapy and control groups were compared retrospectively. The safety of EAAL immunotherapy was also evaluated.
RESULTS: After in vitro culture and proliferation, the percentages of CD3+, CD3+CD8+, CD8+CD27+, CD8+CD28+, and CD3+CD16+/CD56+ cells increased remarkably (P < 0.05), while the percentages of CD3+CD4+, CD4+CD25+, and CD3-CD16+/CD56+ (natural killer cells) were overtly decreased (P < 0.05); no significant change was observed in CD4+CD25+CD127- cells (P = 0.448). Interestingly, OS in the immunotherapy group was significantly higher than that in the control group, with 27.0 and 13.9 mo obtained for the two groups, respectively (P = 0.028, HR = 0.573, 95%CI: 0.347-0.945). These findings indicated a 42.7% decrease in the risk of death. In addition, we found that clinical stage and application of EAAL immunotherapy were independent prognostic factors for gastric cancer patients. Indeed, the OS in stage IIIc and IV patients that had received surgery was prolonged after EAAL immunotherapy (P < 0.05). Importantly, in vitro induction and proliferation of EAAL were easy and biologically safe.
CONCLUSION: Overall, EAAL adoptive immunotherapy might prolong the OS in gastric cancer patients.
Core tip: We undertook a retrospective analysis of patients with gastric cancer to perform an observational study on whether expanded activated autologous lymphocytes (EAALs) improved treatment outcomes. The results provide the first evidence for EAALs being an effective treatment regimen in gastric cancer. The therapy was straightforward and showed good safety, and overall survival may be improved with EAAL treatment in gastric cancer patients.
- Citation: Zhang GQ, Zhao H, Wu JY, Li JY, Yan X, Wang G, Wu LL, Zhang XG, Shao Y, Wang Y, Jiao SC. Prolonged overall survival in gastric cancer patients after adoptive immunotherapy. World J Gastroenterol 2015; 21(9): 2777-2785
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2777
Adoptive cellular immunotherapy has been considered an important antitumor treatment for many years. In this process the patient’s own peripheral immune cells are collected and proliferated in vitro to produce larger quantities by the thousands with enhanced antitumor ability. The cells are then infused back into the patient, in this way the patient’s active or passive immunity is strengthened and tumor cells are killed. Adoptive cellular immunotherapy is suitable for immunocompromised patients, such as those who have received high-dose chemotherapy, radiotherapy, and/or bone marrow transplantation[1,2].
Interestingly, various in vitro proliferated effector cells, such as lymphokine-activated killer (LAK) cells[3,4], anti-CD3 induced activated killer (CD3AK) cells[5], activated natural killer (NK) cells[6-9], dendritic cells (DCs)[10,11], tumor-infiltrating lymphocytes (TILs)[12,13], and cytokine-induced killer (CIK) cells[14-16], have shown some anti-tumor effects. However, subsequent large scale clinical trials have failed to demonstrate any advantage in the use of LAK cells in combination with large IL-2 doses over IL-2 monotherapy for the treatment of melanoma and renal cell carcinoma[17,18]. Although TILs have been shown to improve IL-2 therapeutic responses in about one third of patients with metastatic melanoma[19], the rather complex preparation process of autologous TILs limits their application in routine clinical practice. It is known that CIK cells exhibit potent cytotoxicity against a variety of tumor cells, including autologous and allogeneic acute myeloid leukemic (AML) targets[20] as well as various types of solid cancers[21].
Interestingly, a method was developed to isolate T lymphocytes from cancer patients with immobilized anti-CD3 monoclonal antibodies; the resulting expanded activated autologous lymphocytes (EAALs, all CD3+ and HLA-DR+) were shown to be a heterogeneous cell population containing about 30% of CD4+ and 60% of CD8+ cells[22]. Importantly, the same research group demonstrated that EAALs can reduce postsurgical recurrence rates of hepatocellular carcinoma (HCC) in a randomized clinical trial, indicating that EAAL adoptive immunotherapy is a safe and feasible treatment that can improve outcomes for HCC after surgery[23]. These findings demonstrated the superiority of EAALs over other immune cells used in adoptive immunotherapy: in addition to decreasing the frequency of recurrence by 18% compared with controls, EAALs showed a mean expansion index of 1560-fold[22,23].
The potential benefits of EAALs in gastric cancer have not been explored. Therefore, this study aimed to further define EAAL phenotypic traits and examine the clinical effects of EAALs in a case-control observational study where overall survival time of gastric cancer patients was assessed retrospectively.
Activated lymphocytes were generated using an anti-CD3 monoclonal antibody (OKT3) and IL-2 as described previously[24]. Briefly, 50 to 100 mL of peripheral blood was collected from each patient, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient centrifugation. The isolated PBMCs were washed and resuspended in RPMI-1640 culture medium (Gibco, Grand Island, United States) containing 10% serum from human blood group AB plasma supplemented with 700 U/mL of IL-2. The PBMC suspension was then placed in a flask coated with immobilized anti-CD3 antibody and incubated for one week. Afterwards, the lymphocyte suspension was transferred to a gas permeable bag for two more weeks. The resulting activated lymphocytes were harvested, passed through a 100 μm filter membrane and resuspended in 100 mL normal saline containing 1% human serum albumin for intravenous and/or intrapleural infusion.
Fasting venous blood samples (2-3 mL) were drawn in EDTA-Na2 anticoagulant tubes, during the morning, from gastric cancer patients before EAAL generation. After red blood cell hemolysis, blood samples were mixed with 10 μL fluorescence labeled antibodies, including CD3-FIT, CD8-APC, CD4-PerCP-Cy5.5, CD27-PerCP-Cy5.5, CD28-PE, CD25-PE, CD127-Alexa Fluor® 647, CD16-PE, CD56-APC and their isotype negative controls (BD Biosciences, United States), respectively, and incubated in the dark at room temperature for 20 min. Samples were analyzed by flow cytometry on a BD Accuri C6 (BD Biosciences, United States). A total of 5 × 105 EAALs were washed twice with PBS, resuspended in 100 μL PBS, and labeled with 10 μL fluorescence antibodies as described above. After two more washes, EAAL samples were resuspended in 1 mL PBS for flow cytometry.
Cell survival was calculated by estimating the number of live cells. A cell suspension was stained with typan blue solution, and undyed cells were regarded as live. Cell survival rate was calculated as undyed cells/total cells × 100%.
The proliferation of the cells was estimated as proliferation multiplicity. Isolated PBMCs from the patients were appropriately diluted and cultured. Before and after culture, a cell suspension was counted using a counting chamber under a microscope. The total cell number was calculated by the cell concentration multiplied by the volume, and the cell proliferation multiplicity was calculated as the ratio of cell numbers before and after culture.
According to the safety requirement of an important cellular immunotherapy product, PROVENGE® (Dendreon Corporation, Settle, WA, United States), which was approved by United States Food and Drug Administration in April, 2010, and China’s pharmacopeia, the sterility test for bacteria and fungi was performed during the culturing of EAALs. Upon the day when the EAALs were harvested and ready to be delivered back the cancer patients, Gram staining, sterility and bacterial endotoxin tests were performed. Negative for sterility test and endotoxin concentration < 2.5 EU/mL in EAAL cells were regarded as the criteria for EAAL treatment.
The treatment regimen and the retrospective case-control study were both approved by the Medical Ethics Committee of PLA General Hospital, China. All of the included patients signed written informed consent. The patients enrolled in the present study were admitted to our hospital from October 2006 to December 2009. The inclusion criteria for the EAAL treatment group were as follows; the patients received EAAL therapy according to the cell therapy records accessed through the China PLA General Hospital electronic medical reviewing system, and the patients were histologically or cytologically diagnosed with gastric cancer and had a life expectancy > 12 wk with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0-2. Patients with an ECOG score > 2, an incomplete medical history, or who were lost to follow-up were excluded. All patients agreed to their treatment regimen and signed informed consent. Forty-two of these patients had undergone adoptive EAAL cellular immunotherapy and constituted the EAAL group. Clinical data including age, gender, clinical stage, chemotherapeutic regimens, and hospitalization, surgical, radiotherapy, and survival data were collected along with EAAL therapy details and side effects by medical record review. In parallel, 42 patients were selected for the control group from the China PLA General Hospital electronic medical reviewing system as histologically confirmed gastric cancer patients who were admitted to the same hospital in the same first admission month as the EAAL patients, and had experienced surgery, chemotherapy or radiotherapy. Patients with a history of cell therapy and ECOG > 2 were excluded. The control candidates were grouped and numbered according to their clinical cancer stages, and were randomly selected to match the number of the patients as EAAL patients using the Statistical Package for Social Science (SPSS) 17.0 (SPSS Inc., Chicago, IL, United States). Clinical data were collected in the same fashion and those with incomplete medical history or lost to follow-up were substituted.
Patients were followed and the relationship between treatment and overall survival (OS), from the diagnosis (nearly identical to the start of chemotherapy because the patients underwent the treatment soon after the diagnosis) until death or last follow-up, of gastric cancer patients was retrospectively analyzed in the EAAL and control groups. In addition, the safety of EAAL immunotherapy was evaluated.
Statistical analyses were performed using SPSS 17.0. Summary statistics were given for patient characteristics and treatment administration. Frequencies were reported as number and percentage. Phenotypes of lymphocyte cells in peripheral blood and harvested EAALs were expressed as means ± SD, and comparisons between groups were made by self-paired t tests. Comparisons of basic clinical characteristics between the immunotherapy and control groups were carried out by the Pearson χ2 test. OS was analyzed by the Kaplan-Meier method and the differences in the distributions were compared by the log-rank test. Factors that might affect patients’ OS were analyzed by the COX multivariate regression method. Subgroup analysis was used to analyze the OS in different subgroups of patients who had received EAAL immunotherapy. A P value < 0.05 was considered statistically significant.
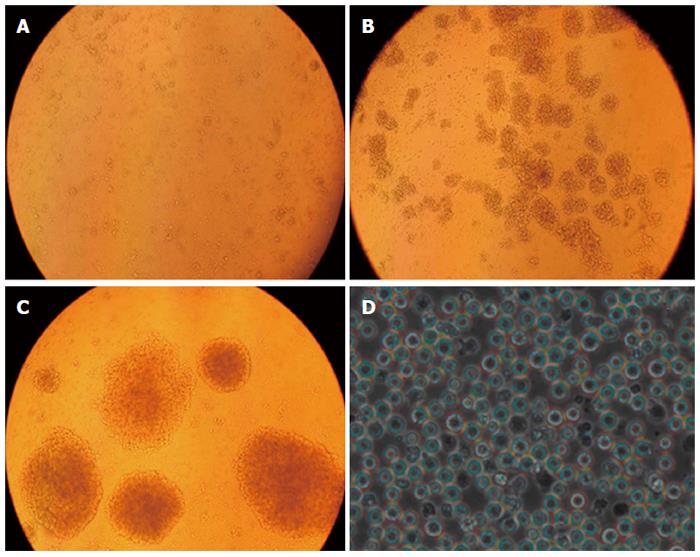
A representative example of T cell proliferation from a patient in the EAAL cohort is shown in Figure 1. Initially, cells were attached to sidewalls of the culture flask, gradually becoming larger and round, and forming colonies. On about day 14, adherent cells and colonies fell off, forming a cell suspension (Figure 1).
After 13.55 ± 1.25 d of culture, the total cell number went from about 7.65 × 106± 1.52 × 106 to 8.76 × 109± 1.82 × 109, and the proliferation multiplicity obtained by calculating the difference in cell number before and after culture was 1156.57 ± 167.88. The survival rate of effector cells was 97.57% ± 0.94% (Table 1).
| Property | Value |
| Proliferation time (d) | 13.55 ± 1.25 |
| Number of cells before proliferation (106/L) | 7.65 ± 1.52 |
| Number of cells after proliferation (109/L) | 8.76 ± 1.82 |
| Proliferation multiplicity | 1156.57 ± 167.88 |
| Survival rate of cells | 97.57% ± 0.94% |
After in vitro culture and proliferation, the percentages of CD3+, CD3+CD8+, CD8+CD27+, CD8+CD28+, and CD3+CD16+/CD56+ cells increased remarkably (P < 0.05), while those of CD3+CD4+, CD4+CD25+, CD3-CD16+/CD56+ (NK cells) were overtly decreased (P < 0.05); no significant change was observed in CD4+CD25+CD127- cells (P = 0.448, Table 2).
| Phenotype | Before (%) | After (%) | P valuea |
| CD3+ | 67.39 ± 7.55 | 96.71 ± 3.85 | < 0.001 |
| CD3+CD4+ | 33.22 ± 11.57 | 10.17 ± 8.83 | < 0.001 |
| CD3+CD8+ | 27.85 ± 8.51 | 79.33 ± 13.58 | < 0.001 |
| CD8+CD28+ | 13.19 ± 6.08 | 51.01 ± 15.34 | < 0.001 |
| CD3-CD16+/CD56+ | 28.12 ± 12.10 | 5.56 ± 6.48 | < 0.001 |
| CD3+CD16+/CD56+ | 9.19 ± 6.42 | 12.73 ± 7.65 | 0.019 |
| CD45RA+ | 60.84 ± 8.26 | 9.74 ± 5.87 | < 0.001 |
| CD45RO+ | 46.53 ± 10.01 | 94.32 ± 4.57 | < 0.001 |
| CD4+CD25+ | 5.61 ± 4.19 | 3.17 ± 2.15 | 0.002 |
| CD4+CD25+CD127- | 1.00 ± 0.46 | 0.94 ± 1.50 | 0.448 |
| CD29+ | 62.15 ± 8.77 | 93.04 ± 3.98 | < 0.001 |
| CD4+CD29+ | 20.11 ± 5.97 | 12.36 ± 7.55 | < 0.001 |
Eighty-four patients were enrolled in the study (aged 40-85 years). Among the 58 screened patients who had undergone EAAL therapy from October 2009 to December 2012, 42 were included in the EAAL group. The cellular immunotherapy ranged from 2-24 treatments, total immunotherapy times were 242, and median immunotherapy times were 5. Based on clinical stage at the beginning of the study the EAAL group was further subdivided: 10 patients with stage I and II disease formed EAAL group 1; 12 individuals with stage IIIa and IIIb disease constituted EAAL group 2; 20 patients with stage IIIc and IV disease were included in EAAL group 3. Control subgroups with corresponding number of patients were obtained after random selection from 246 patients fulfilling the inclusion criteria for the control group.
The EAAL group was composed of 34 males and 8 females, while 33 males and 9 females constituted the control group. The patient age in the EAAL and control groups was 57.54 ± 11.93 and 58.98 ± 11.17 years, respectively, indicating that this parameter was similar in both groups (P = 0.740). Patients were further subdivided into < 60 and ≥ 60 years.
According to the number of chemotherapy cycles patients were divided into subgroups with ≤ 6 and > 6 cycles. Finally, surgery and radiotherapy status of patients allowed the formation of Yes and No subgroups. Detailed basic clinical characteristics of the enrolled patients are summarized in Table 3 and statistical analysis showed that there were no significant differences between the two groups in these parameters (P > 0.05).
| Characteristic | Patients treated with EAALs (n) | Patients treated without EAALs (n) | P value |
| Patient number | 42 | 42 | |
| Age (yr) | |||
| < 60 | 24 | 22 | 0.661 |
| ≥ 60 | 18 | 20 | |
| Sex | |||
| Male | 34 | 33 | 0.786 |
| Female | 8 | 9 | |
| Stage | |||
| I and II | 10 | 9 | 0.909 |
| IIIa and IIIb | 12 | 11 | |
| IIIc and IV | 20 | 22 | |
| Surgery | |||
| Yes | 33 | 37 | 0.242 |
| No | 9 | 5 | |
| Radiotherapy | |||
| Yes | 8 | 4 | 0.212 |
| No | 34 | 38 | |
| Chemotherapy | |||
| ≤ 6 cycles | 19 | 24 | 0.275 |
| > 6 cycles | 23 | 18 |
Patients in the EAAL group received a total 288 cycles of chemotherapeutic regimens with a median of 7 cycles per patient, while 2 patients received no chemotherapy. Seven patients received post-surgery adjuvant chemotherapy alone and EAAL treatment afterwards; 13 individuals used first-line chemotherapeutic regimens in combination with EAAL treatment and 20 patients received second-line or combined chemotherapeutic regimens. EAAL cell therapy was administered alone in 2 patients because they were radically resected stage I patients, with post-surgery adjuvant chemotherapy in 13 patients, with first-line chemotherapeutic regimens in 16 patients, with second-line or combined chemotherapeutic regimens in 16 patients, and with both first-line and second-line regimens in 7 patients.
Patients in the control group received a total 264 cycles of chemotherapeutic regimens with a median of 6 cycles per patient, while 3 patients received no chemotherapy. Six patients were administered post-surgery adjuvant chemotherapy alone; 16 individuals used first-line chemotherapeutic regimens, and 17 patients were treated with second-line or combined chemotherapeutic regimens.
Chemotherapeutic regimens utilized in the study population included mFLOFOX6 (oxaliplatin, fluorouracil, leukovorin), mDCF (taxotere, cisplatin, fluorouracil), DF (taxotere, fluorouracil), mECF (epirubicin, cisplatin, fluorouracil), XELOX (oxaliplatin, capecitabine), SOX (oxaliplatin, tegafur/gimeracil/oteracil capsule), FOLFIRI (CPT-11, fluorouracil, leukovorin), capecitabine alone, and tegafur/gimeracil/oteracil capsule alone.
Patients were further subdivided according to the number of chemotherapy cycles received (≤ 6 and > 6 cycles), their status of surgery, and radiotherapy (negative or positive). Clinical characteristics are detailed in Table 3 and no statistical significance was found between the two groups (P > 0.05 for all).
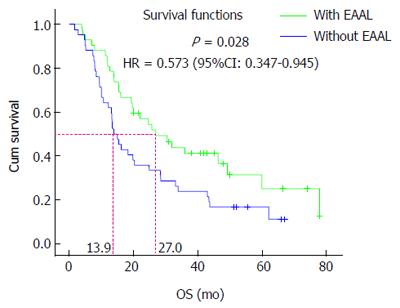
At the last follow-up on December 31, 2012, 28 (28/42, 66.7%) patients had died in the EAAL immunotherapy group, indicating a median OS of 27.0 mo. In the control group, 36 (36/42, 85.71%) patients had died and the median OS was 13.9 mo. All deaths were associated with tumor progression. Further analysis showed that OS time in the EAAL group was significantly higher than that obtained for the control group (P = 0.028, HR = 0.573, 95%CI: 0.347-0.945, Figure 2). The 1- to 5- year survival rates were 80.95%, 54.35%, 41.10%, 36.54% and 25.05%, respectively, in the EAAL group, and 61.90%, 33.33%, 16.67%, 11.11% and 11.11%, respectively, in controls. These data suggest a slightly better 1- to 5-year patient survival in the immunotherapy group compared with the control group, although the differences were not statistically significant (P > 0.05 for all, Table 4).
| Group | n | 1 yr survival rate (%)(95%CI) | 2-yr survival rate (%)(95%CI) | 3-yr survival rate (%)(95%CI) | 4-yr survival rate (%)(95%CI) | 5-yr survival rate (%)(95%CI) |
| Immunotherapy group | 42 | 80.95 | 54.35 | 41.1 | 36.54 | 25.05 |
| (69.08-92.83) | (39.16-69.54) | (25.79-56.41) | (20.52-52.55) | (7.78-42.33) | ||
| Control group | 42 | 61.9 | 33.33 | 16.67 | 11.11 | 11.11 |
| (47.22-76.59) | (19.08-47.59) | (5.40-27.94) | (0-22.75) | (0-22.75) | ||
| P value | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
Results of subgroup analyses are displayed in Table 5. For the subgroup composed of clinical stage IIIb and IV patients, the median OS was longer in the EAAL group than in the controls (14.0 mo vs 10.0 mo, P = 0.03). In addition, for patients who underwent surgery, the median OS was longer in EAAL treated individuals than the control group (36.0 mo vs 15.4 mo, P = 0.045). However, other subgroups showed no significant differences in median OS between the EAAL and control groups (P > 0.05).
| Subgroup | Median OS (mo) | RR (95%CI) | P value | |
| Immunotherapy group | Control group | |||
| Age (yr) | ||||
| < 60 | 25.8 | 13.4 | 0.65 (0.333-1.272) | 0.209 |
| ≥ 60 | 46.5 | 13.9 | 0.502 (0.233-1.079) | 0.078 |
| Gender | ||||
| Male | 20.0 | 15.0 | 0.439 (0.146-1.32) | 0.143 |
| Female | 30.5 | 13.9 | 0.625 (0.355-1.099) | 0.103 |
| Clinical stage | ||||
| I and II | 60.0 | 62.2 | 1.212 (0.293-5.013) | 0.791 |
| IIIa and IIIb | 49.3 | 28.4 | 0.358 (0.128-1.003) | 0.051 |
| IIIc and IV | 14.0 | 10.0 | 0.484 (0.251-0.933) | 0.03a |
| Surgery | ||||
| Yes | 36.0 | 15.4 | 0.561 (0.319-0.986) | 0.045a |
| No | 19.7 | 9.4 | 0.265 (0.069-1.015) | 0.053 |
| Radiotherapy | ||||
| Yes | 60.0 | 43.5 | 0.382 (0.094-1.55) | 0.178 |
| No | 22.0 | 13.4 | 0.624 (0.364-1.069) | 0.086 |
| Chemotherapy cycles | ||||
| ≤ 6 cycles | 27.0 | 10.0 | 0.544 (0.255-1.159) | 0.115 |
| > 6 cycles | 30.5 | 18.4 | 0.554 (0.28-1.093) | 0.089 |
COX multivariate regression analysis showed that gender, age, cancer stage, surgery, radiotherapy, chemotherapy, and EAAL immunotherapy were independent risk factors for OS in gastric cancer patients (Table 6).
| Factor | Wald | P value | HR | 95%CI |
| Gender | 2.527 | 0.112 | 1.723 | 0.881-3.369 |
| Age | 1.799 | 0.180 | 0.659 | 0.359-1.212 |
| Clinical stage | 41.852 | < 0.001 | ||
| Clinical stage (1) | 37.267 | < 0.001a | 0.050 | 0.019-0.131 |
| Clinical stage (2) | 22.155 | < 0.001a | 0.172 | 0.083-0.358 |
| Surgery | 0.474 | 0.491 | 0.777 | 0.378-1.595 |
| Chemotherapy cycles | 0.069 | 0.793 | 1.083 | 0.597-1.966 |
| Radiotherapy | 0.581 | 0.446 | 1.386 | 0.599-3.209 |
| Application of EAALs | 7.819 | 0.005a | 2.249 | 1.274-3.969 |
Fifty grade 1 or 2 and self-limiting adverse events developed in 242 EAAL transfers (Table 7). No patient showed pulmonary or renal symptoms, sign of infection, hepatic function deterioration, or autoimmune disorder. There was no treatment-related death recorded.
Tumor cells adopt diverse mechanisms to escape tumor-specific immunity in the neoplastic process. The pathological interactions between cancer cells and host immune cells create an immunosuppressive network, not only in the tumor microenvironment, but also systemically[25,26]. Transfusion of an adequate quantity of lymphocytes, which are capable of recognizing and lysing tumor cells, is the basis for successful adoptive cell therapy[27,28]. Previous reports have suggested that T-cells from non-tumor-bearing hosts can boost anti-tumor immunity to break the morbid equilibrium formed between tumor cells and the host[29,30]. Indeed, cell transfer therapy for cancer has been recognized as the fourth anticancer modality after operation, chemotherapy, and radiotherapy[31]. However, the use of several immune cell types has been hampered by serious drawbacks including the poor efficacy and/or the complexity of cell propagation[17-19]. Interestingly, these shortcomings can be overcome through infusion of a large number of EAALs, as demonstrated in HCC[23]. An additional advantage of EAALs is that their use presents no risk of violating medical ethics since the effector cells originate from the patient’s PBMCs.
Herein, we assessed a variety of molecular markers to further characterize EAAL phenotypes. We found that CD3+ and CD3+CD8+ T lymphocytes represented more than 95% and 80% of total EAALs, respectively, while the proportions of CD3+CD4+ and CD3−CD16+/CD56+ NK cells were relatively low. CD8+CD27+ and CD8+CD28+ cytotoxic T lymphocytes (CTLs) and CD3+CD16+/CD56+ T lymphocytes are essential effector cells, which play an important role in anti-tumor immunity[32-34]. Therefore, the high contents described above for these cell types in EAALs may result in increased anti-tumor immunity.
The expression of regulatory T cell (Treg) specific transcription factors such as Foxp3[35,36] was not assessed in this study. However, the rather low percentage (0.80% ± 1.59%) of CD4+CD25+CD127- T cells, which were considered CD4+CD25+Foxp3+ cells[37], implied the extremely low proportion of Treg cells in EAALs. These findings indicate that EAAL would not suppress immunity in patients.
As a result, although not all lymphocytes are tumor-specific, the high expression of CD3, CD8, CD27, CD28, CD56 and CD16 in EAALs implies that a large number of EAALs have the potential to exert or improve anti-tumor effects.
In order to further characterize the clinical effect of EAALs, we adopted a case-control study to retrospectively analyze whether EAALs could prolong the OS of gastric cancer patients. We demonstrated that the baseline clinical features were similar and comparable in the EAAL and control groups. Interestingly, the Kaplan-Meier survival analysis showed that median OS time was significantly longer in EAAL treated individuals than in the control group (P = 0.028, HR = 0.573, 95%CI: 0.347-0.945), indicating a 42.7% decrease in the risk of death in gastric cancer patients after treatment with EAALs. In addition, EAAL immunotherapy seemed to improve 1- to 5-year survival rates in gastric cancer patients, although the differences did not reach statistical significance.
We demonstrated by COX multivariate regression analysis that clinical stage and EAAL immunotherapy were independent risk factors for OS in gastric cancer patients. The hazard ratios for clinical stages I and II were 0.050 (95%CI: 0.019-0.131) and 0.172 (95%CI: 0.083-0.358), respectively, suggesting that stage I and II patients might live longer than patients with more advanced stage disease. These data corroborated previous reports and clinical observations[38,39]. The hazard ratio for application of EAAL immunotherapy was 2.249 (1.274-3.969), which suggested that patients receiving EAAL immunotherapy could live 2.249 times longer than those in the control group.
In the subgroup analysis, the OS in clinical stages IIIc and IV patients who had received surgery could be prolonged by EAAL immunotherapy (P < 0.05). The OS was also improved by EAAL immunotherapy in other subgroups, but the difference was not significant (P > 0.05). These findings indicate that advanced or locally advanced gastric cancer patients might benefit more from EAAL immunotherapy.
As for safety of EAAL immunotherapy, the most common adverse reactions were fever (10.74%) and chill (5.37%). Other adverse reactions included headache, nausea, itching, rash, tachycardia and diarrhea, with low incidence rates of no more than 3%. Importantly, all the adverse reactions were of grade 1 or 2 and self-limiting, suggesting a good safety profile for EAAL immunotherapy.
In conclusion, the in vitro induction and proliferation method described in this study was easy and highly efficient, with good repeatability and biological safety. Our data suggest that EAAL immunotherapy might prolong the OS in gastric cancer patients. Meanwhile, a good level of safety was obtained for EAAL cellular immunotherapy with only mild adverse reactions. However, this study was a retrospective observational study. Prospective cohort clinical studies with larger sample sizes are required for confirmation of these findings.
We wish to thank Tong-Jun Lin for his guidance in the writing of this manuscript. Bo-Tao Guo is acknowledged for performing preliminary calculations in this study. We are also grateful to the medical personnel of Department of Oncology Medicine at the Chinese PLA General Hospital, where the patients reported herein were treated.
Adoptive cellular immunotherapy is a process by which the patient’s own peripheral immune cells are collected and their numbers are increased by growing them in vitro. This then allows the cells to be delivered back to the patient’s blood stream and results in increased anti-tumor immunity. Expanded activated autologous lymphocytes (EAALs) have been found to be particularly successful in this therapy in hepatocellular carcinoma.
Many different in vitro proliferated effector cells have been tested for use as adaptive immunotherapy. These include lymphokine-activated killer cells, anti-CD3 induced activated killer cells, activated natural killer cells, dendritic cells, tumor-infiltrating lymphocytes, and cytokine-induced killer cells. The current research hotspot is to find the most effective method for this immunotherapy.
Previously EAALs have shown promise as the most successful adaptive immunotherapy cell type in improving cancer patient outcomes. This is the first investigation into their use in gastric cancer patients.
The study results suggest that EAAL immunotherapy may improve overall survival of gastric cancer patients.
Effector cells are immune cells that become active in order to defend the body in an immune response. EAALs are the patient’s white blood cells (lymphocytes), which have been activated and grown outside the body in a culture dish.
The manuscript entitled: “Prolonged survival in gastric cancer patients after adoptive immunotherapy” is an interesting study. The novelty of the immunotherapy should be acknowledged.
P- Reviewer: Coccolini F, Formica V, Mohammadi M, Sadik R S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 657] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 2. | Ballen KK, Colvin G, Dey BR, Porter D, Westervelt P, Spitzer TR, Quesenberry PJ. Cellular immune therapy for refractory cancers: novel therapeutic strategies. Exp Hematol. 2005;33:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Papa MZ, Mulé JJ, Rosenberg SA. Antitumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo: successful immunotherapy of established pulmonary metastases from weakly immunogenic and nonimmunogenic murine tumors of three district histological types. Cancer Res. 1986;46:4973-4978. [PubMed] |
| 4. | Schoof DD, Gramolini BA, Davidson DL, Massaro AF, Wilson RE, Eberlein TJ. Adoptive immunotherapy of human cancer using low-dose recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988;48:5007-5010. [PubMed] |
| 5. | Yun YS, Hargrove ME, Ting CC. In vivo antitumor activity of anti-CD3-induced activated killer cells. Cancer Res. 1989;49:4770-4774. [PubMed] |
| 6. | Herberman RB. Cancer immunotherapy with natural killer cells. Semin Oncol. 2002;29:27-30. [PubMed] |
| 7. | Klingemann H, Boissel L. Targeted cellular therapy with natural killer cells. Horm Metab Res. 2008;40:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Guo H, Qian X. Clinical applications of adoptive natural killer cell immunotherapy for cancer: current status and future prospects. Onkologie. 2010;33:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901-3909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Hart DN, Hill GR. Dendritic cell immunotherapy for cancer: application to low-grade lymphoma and multiple myeloma. Immunol Cell Biol. 1999;77:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother. 2004;53:275-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | Nguyen LT, Yen PH, Nie J, Liadis N, Ghazarian D, Al-Habeeb A, Easson A, Leong W, Lipa J, McCready D. Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs). PLoS One. 2010;5:e13940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Olioso P, Giancola R, Di Riti M, Contento A, Accorsi P, Iacone A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol. 2009;27:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997-4002. [PubMed] |
| 16. | Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer. 2011;2:363-368. [PubMed] |
| 17. | Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622-632. [PubMed] |
| 18. | Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O’Connell M, Khan A, Vlamis V, Vogelzang NJ, Bajorin DF. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824-832. [PubMed] |
| 19. | Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159-1166. [PubMed] |
| 20. | Linn YC, Hui KM. Cytokine-induced killer cells: NK-like T cells with cytotolytic specificity against leukemia. Leuk Lymphoma. 2003;44:1457-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Sekine T, Shiraiwa H, Yamazaki T, Tobisu K, Kakizoe T. A feasible method for expansion of peripheral blood lymphocytes by culture with immobilized anti-CD3 monoclonal antibody and interleukin-2 for use in adoptive immunotherapy of cancer patients. Biomed Pharmacother. 1993;47:73-78. [PubMed] |
| 23. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 24. | Tsoukas CD, Landgraf B, Bentin J, Valentine M, Lotz M, Vaughan JH, Carson DA. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J Immunol. 1985;135:1719-1723. [PubMed] |
| 25. | Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1564] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 26. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3521] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 27. | Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281-355. [PubMed] |
| 28. | June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 402] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 29. | Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 421] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 30. | Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 513] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 31. | Qian X, Wang X, Jin H. Cell transfer therapy for cancer: past, present, and future. J Immunol Res. 2014;2014:525913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Raitakari M, Brown RD, Sze D, Yuen E, Barrow L, Nelson M, Pope B, Esdale W, Gibson J, Joshua DE. T-cell expansions in patients with multiple myeloma have a phenotype of cytotoxic T cells. Br J Haematol. 2000;110:203-209. [PubMed] |
| 33. | Fenton RG, Turcovski-Corrales SM, Taub DD. Induction of melanoma antigen-specific cytotoxic T lymphocytes in vitro by stimulation with B7-expressing human melanoma cell lines. J Immunother. 1998;21:95-108. [PubMed] |
| 34. | Schmidt-Wolf IG, Lefterova P, Johnston V, Huhn D, Blume KG, Negrin RS. Propagation of large numbers of T cells with natural killer cell markers. Br J Haematol. 1994;87:453-458. [PubMed] |
| 35. | Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606-612. [PubMed] |
| 36. | Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Hartigan-O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Sun Z, Wang ZN, Zhu Z, Xu YY, Xu Y, Huang BJ, Zhu GL, Xu HM. Evaluation of the seventh edition of American Joint Committee on Cancer TNM staging system for gastric cancer: results from a Chinese monoinstitutional study. Ann Surg Oncol. 2012;19:1918-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |