Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2614
Peer-review started: September 13, 2014
First decision: October 14, 2014
Revised: October 24, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: March 7, 2015
Processing time: 178 Days and 4.3 Hours
AIM: To investigate perfusion change in contrast-enhanced ultrasonography (CEUS) to evaluate liver fibrosis based on biliary obstruction using an animal model.
METHODS: New Zealand white rabbits (3-4 kg) underwent bile duct ligation to form a biliary obstruction model. We performed liver CEUS and laboratory tests on the day before the operation (day 0) and every 7 postoperative days until the rabbits were sacrificed. After CEUS, signal intensity of liver parenchyma with a time-intensity curve was analyzed. Perfusion parameters were automatically calculated from region-of-interests, including peak signal intensity, mean transit time, area under the curve and time to peak. Histological grades of liver fibrosis were assessed according to the Metavir score system immediately after sacrifice. Generalized estimating equations were used to analyze the association between liver fibrosis grades and perfusion parameters for statistical analysis. The perfusion parameters were measured on the last day and the difference between day 0 and the last day were evaluated.
RESULTS: From the nine rabbits, histological grades of liver fibrosis were grade 1 in one rabbit, grade 2 and 3 in three rabbits each, and grade 4 in two rabbits. Among the four CEUS parameters, only the peak signal intensity measured on the last day demonstrated a significant association with liver fibrosis grades (OR = 1.392, 95%CI: 1.114-1.741, P = 0.004). The difference in peak signal intensity between day 0 and the last day also demonstrated an association with liver fibrosis (OR = 1.191, 95%CI: 0.999-1.419, P = 0.051). The other parameters tested, including mean transit time, area under the curve, and time to peak, showed no significant correlation with liver fibrosis grades.
CONCLUSION: This animal study demonstrates that CEUS can be used to evaluate liver fibrosis from biliary obstruction using peak signal intensity as a parameter.
Core tip: Contrast-enhanced ultrasonography (CEUS) was proposed for the evaluation of liver fibrosis. However, no previous studies have evaluated the utility of CEUS for liver fibrosis caused by biliary obstruction, which can be the major cause of liver fibrosis in children. Therefore, we investigated the feasibility of CEUS to evaluate liver fibrosis from biliary obstruction by quantitatively assessing perfusion changes in an animal model with bile duct ligation. We also demonstrated that the parameter of peak signal intensity from CEUS could be useful to evaluate liver fibrosis from biliary obstruction.
- Citation: Shin HJ, Chang EY, Lee HS, Hong JH, Park G, Kim HG, Kim MJ, Lee MJ. Contrast-enhanced ultrasonography for the evaluation of liver fibrosis after biliary obstruction. World J Gastroenterol 2015; 21(9): 2614-2621
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2614
Contrast-enhanced ultrasonography (CEUS) is an examination based on blood pool tracer visualization using ultrasound contrast agents during real-time ultrasonography (US) imaging. CEUS assessment can be made by canceling and separating of linear ultrasound signals from tissues and by utilizing non-linear signals from microbubbles[1]. Although visualization of the microvasculature is limited in conventional US, computed tomography (CT) and magnetic resonance imaging (MRI), it becomes possible through CEUS. Moreover, CEUS can quantitatively measure blood flow and perfusion using contrast kinetic curves obtained from quantification software[2]. This method had high diagnostic performance and clinical utility for detecting and characterizing focal liver lesions[3,4]. Moreover, the European Federation of Societies for Ultrasound in Medicine and Biology documented guidelines for utilizing CEUS, not only for liver assessment, but also for other organs, especially for diagnosing focal lesions and monitoring treatment response[1,5].
Previous studies also demonstrated the role of CEUS for evaluating liver fibrosis because local and hemodynamic changes accompanied fibrotic liver changes[6]. The severity of cirrhosis can be evaluated with enhanced signal intensity of the liver parenchyma on CEUS[7,8]. However, the utility of CEUS in liver fibrosis from biliary obstruction has not been evaluated.
Liver fibrosis occurs in adults, but can also occur in children; however, the causes of liver fibrosis are different in children compared with adults. The major indication for liver transplantation in children is biliary atresia, which causes biliary cirrhosis[9], despite the fact that the pathophysiology of biliary atresia is not well known. Hartley et al[10] commented that biliary atresia might be a final common phenotype for several pathogenic processes. Additionally, liver fibrosis progresses rapidly before and even after performing a hepatoportoenterostomy for bile drainage[11]. Therefore, early and accurate evaluation of liver fibrosis in pediatric patients with biliary atresia is important. Liver fibrosis evaluation with CEUS can be particularly useful in pediatric patients because of the absence of radiation exposure and because it is easy to perform without sedation. However, to our knowledge, no previous studies have evaluated the utility of CEUS for liver fibrosis caused by biliary obstruction.
Therefore, the aim of this study was to investigate the feasibility of CEUS to evaluate liver fibrosis from biliary obstruction by quantitatively assessing perfusion change using an animal model. We performed serial follow-up before and after operation of biliary obstruction and examined US features and changes in perfusion parameter patterns from CEUS that correlated with liver fibrosis induced by biliary obstruction.
This study protocol was approved by our institutional animal experimental committee and performed according to local animal care guidelines. Nine male New Zealand white rabbits that weighed 3-4 kg were included. Under general anesthesia, the common bile ducts were ligated with suture material by one pediatric surgeon (E.Y.C.). The rabbits were maintained on a standard diet after the operation.
Laboratory tests were also performed before and after the operation, including measurement of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels. The upper normal limits of the laboratory tests were 36 IU/L for AST, 72 IU/L for ALT, and 1.0 mmol/L for total bilirubin. Preoperative tests were performed just before starting the operation. Postoperative tests were performed at the postoperative day 3, day 7, every 7 d, and the last was performed prior to sacrifice. The time of sacrifice was agreed upon by the veterinarian (G.P.) and radiologists according to the rabbits’ condition.
Abdominal US was performed while the rabbits were under sedation on the day before the operation (day 0) and every 7 postoperative days until the rabbits were sacrificed. Additional examination was performed just before the sacrifice, if possible.
The imaging study, including CEUS, was performed using an ultrasound unit (iU22, Philips Medical Systems, Best, The Netherlands) equipped with a L12-5 linear transducer (5-12 MHz). All US evaluations were performed by one of two board-certified radiologists (M.J.L. and H.J.S.). Before the CEUS study, intrahepatic bile duct dilatation from successful bile duct ligation was confirmed on US. For CEUS imaging, we captured the images of relatively homogeneous liver parenchyma without shadowing from the bowel or ribs. Lipid-based ultrasound contrast agent (SonoVue, Bracco, Milan, Italy) dissolved with a 5-mL physiologic saline solution was used. Then 0.4 mL of mixed contrast agent was administered as a bolus into the ear vein of the rabbit, followed by a 2-mL saline flush. The images were recorded continuously for three minutes from the time of contrast injection, and these procedures were repeated twice. The time interval between the two CEUS examinations was more than five minutes.
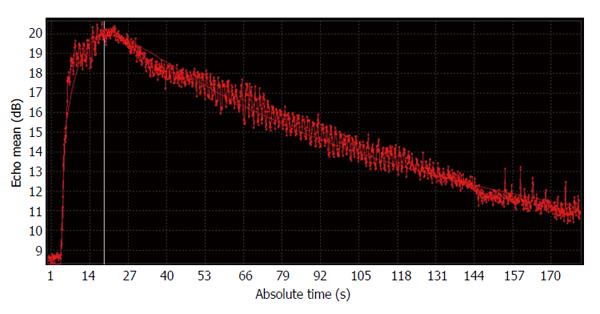
The cine clips were downloaded in the DICOM format for offline processing (QLAB software, Philips Medical Systems, Best, The Netherlands) with a bolus kinetic model for acquiring the perfusion parameters from the CEUS examinations. We drew a region-of-interest (ROI) in the liver parenchyma and avoided hepatic vessels or dilated bile ducts. The software automatically presented absolute time to echo mean (dB) curve with a curve fitting method (Figure 1). It also automatically calculated perfusion parameters of the ROI, including peak signal intensity (PSI, dB), mean transit time (MTT, s), area under the curve (AUC, dB s) and time to peak (TTP, s). The values were obtained three times from the two examinations by the same radiologists with a blind-check of parameters in the same rabbits. From the three values, mean values were obtained as representative values.
Immediately after sacrifice, liver specimens were fixed in 10% formalin, embedded in paraffin, cut into 3-mm-thick sections, and stained with Masson’s trichrome. Histological grades of liver fibrosis were assessed according to the Metavir score system[12,13]. The fibrosis score was evaluated from this semiquantitative classification system as follows: 0 = no fibrosis, 1 = portal fibrosis without septa, 2 = few septal fibrosis, 3 = numerous septal fibrosis without cirrhosis, and 4 = cirrhosis[12].
Histopathologic results were used as a standard reference. Generalized estimating equations were used to analyze the association between liver fibrosis grades and perfusion parameters. The US examination parameters measured on the last day and the difference between day 0 (before operation) and the last day were evaluated. Odds ratios (ORs) with 95%CI were calculated for all parameters. Statistically significant results were assumed when the P value was less than 0.05 in all cases. Analysis was performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, United States).
From the nine rabbits, the CEUS and laboratory tests were performed until postoperative day 7 in two rabbits, day 10 in one rabbit, day 14 in three rabbits, day 18 in one rabbit, and day 21 in two rabbits. Histological grades of liver fibrosis were grade 1 in one rabbit, grade 2 and 3 in three rabbits each, and grade 4 in two rabbits. The follow-up duration in each fibrosis grade was 18 d in fibrosis grade 1, 10-14 d in grade 2, 7-14 d in grade 3, and 21 d in grade 4.
The laboratory test results are summarized in Table 1. The AST, ALT, and total bilirubin levels were within normal range in the initial preoperative study and all the values were markedly increased on postoperative day 7. The levels of AST and ALT decreased during follow-up. However, total bilirubin levels were persistently elevated during follow-up (mean: 5.4-5.7 mmol/L).
| Preoperative day (day 0)(n = 9) | Postoperative day 7(n = 9) | Postoperative day 14(n = 6) | Postoperative day 21(n = 2) | |
| Aspartate aminotransferase (IU/L) | 24.6 ± 14.0 | 94.3 ± 55.1 | 46.7 ± 16.9 | 55.5 ± 2.1 |
| Alanine aminotransferase (IU/L) | 64.8 ± 28.8 | 217.8 ± 94.6 | 49.7 ± 10.2 | 94 ± 12.7 |
| Total bilirubin (mmol/L) | 0.2 ± 0.1 | 5.4 ± 3.1 | 5.7 ± 3.5 | 5.5 ± 1.5 |
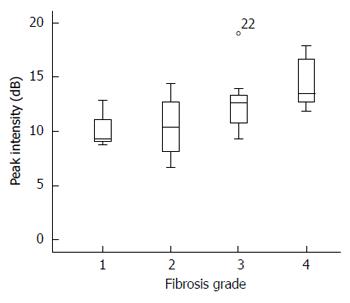
Before the CEUS study, intrahepatic bile duct dilatation was detected in all rabbits. On CEUS, the time-intensity curve was obtained as a typical pattern after bolus injection, showing an early and rapid rising slope and a gradual decreasing slope after reaching PSI (Figure 1). The perfusion analysis results obtained from this curve are summarized in Table 2. Among the four perfusion parameters, only PSI measured on the last day demonstrated a significant association with liver fibrosis grades (OR = 1.392, 95%CI: 1.114-1.741, P = 0.004) (Figure 2). The difference between the mean value on the last day and that of day 0 (before operation) also indicated that PSI was associated liver fibrosis (OR = 1.191, 95%CI: 0.999-1.419, P = 0.051). The other parameters including MTT, AUC, and TTP showed no significant correlation with liver fibrosis grades.
| Fibrosis grades | Grade 1(n = 1) | Grade 2(n = 3) | Grade 3(n = 3) | Grade 4(n = 2) | Odds ratio(95%CI) | P value1 |
| Mean value on the last day | ||||||
| Peak signal intensity (dB) | 10.28 | 10.53 ± 1.53 | 12.64 ± 0.29 | 14.35 ± 0.33 | 1.392 (1.114-1.741) | 0.0042 |
| Mean transit time (s) | 63.27 | 49.15 ± 6.7 | 45.40 ± 5.91 | 45.78 ± 0.97 | 0.979 (0.946-1.013) | 0.215 |
| Area under the curve (dB s) | 1160.84 | 945.08 ± 196.93 | 1146.56 ± 39.96 | 1406.26 ± 3.36 | 1.001 (1.000-1.003) | 0.113 |
| Time to peak (s) | 23.18 | 20.35 ± 3.26 | 21.85 ± 3.37 | 27.33 ± 3.39 | 1.052 (0.959-1.154) | 0.284 |
| Mean value difference on the last day and mean value on day 0 | ||||||
| Peak signal intensity (dB) | 1.63 | -0.95 ± 1.1 | 0.15 ± 1.38 | 5.2 ± 0.84 | 1.191 (0.999-1.419) | 0.051 |
| Mean transit time (s) | 2.78 | -4.64 ± 7.67 | -14.24 ± 13.21 | 4.67 ± 0.57 | 1.001 (0.977-1.026) | 0.946 |
| Area under the curve (dB s) | 196.26 | -272.71 ± 154.65 | -259.65 ± 267.88 | 631.21 ± 92.03 | 1.001 (1.000-1.002) | 0.160 |
| Time to peak (s) | 1.6 | 1.76 ± 4.96 | -0.12 ± 3.52 | 10.42 ± 2.13 | 1.053 (0.969-1.143) | 0.222 |
Liver cirrhosis is characterized by morphologic and hemodynamic changes of the general liver. The morphologic changes include coarse liver parenchymal echogenicity, liver surface nodularity, left lobe hypertrophy, enlargement of the hilar periportal space, and secondary changes from portal hypertension, such as splenomegaly and ascites[13,14]. Hemodynamic change is due to global change in liver perfusion that results from sequential changes of arterialized capillaries, decreased portal flow and increased arterial flow that compensates for the other changes[14-16]. Various imaging modalities have been used to evaluate liver fibrosis to detect morphologic or hemodynamic changes.
Even though liver biopsy is a gold standard for diagnosing liver fibrosis, it is limited because of its invasive nature and sampling errors[17,18]. Abdominal US is commonly performed in clinical practice because it can evaluate morphologic and hemodynamic changes using grayscale and Doppler studies[13]. Previous studies demonstrated that the accuracy for detecting liver cirrhosis using US was 82%-88%[14,19]. However, this evaluation has limited accuracy for early liver fibrosis detection. CT also can be used to evaluate liver fibrosis and a recent study demonstrated that early fibrotic change could be detected using perfusion CT[20]. However, CT is associated with radiation hazards and insufficient spatial resolution in small infants, and is not advantageous because it uses iodinated contrast agents[14]. MRI can also be used to evaluate liver fibrosis and is better than CT. However, MRI is limited because of the non-linear relationship between tracer concentration and acquired signal intensity[14]. Additionally the cost, need for sedation, and longer examination time are also important drawbacks of MRI in pediatric patients[13]. A Fibroscan showed good correlation with liver fibrosis detection[17]; however, this method lacks anatomical information. The acoustic radiation force impulse imaging and shear wave elastography allow liver fibrosis assessment, but standard cutoff values between normal and pathologic conditions have not yet been identified and the recent report demonstrated that the obtained values differed according to the frequency of transducers and acquisition depths[21]. Therefore, CEUS can be applied as an optimal tool to assess liver fibrosis because it includes grayscale images from US and even subtle perfusion changes can be quantitatively detected from contrast administration, without radiation exposure or need for sedation in infants.
Several clinical studies used CEUS for adults with chronic viral liver disease[15,22-26]. Ridolfi et al[22] showed that PSI was higher in cirrhosis patients, as in our study, and TTP and MTT were shorter in cirrhotic patients compared with the control group. In the other previous studies, liver transit time was widely used for assessing liver fibrosis and cirrhosis[15,23,24,26]. The liver transit time was significantly shortened in advanced liver fibrosis by arteriovenous shunting and arterialized capillaries[14,16,26].
Two CEUS studies that assessed liver fibrosis evaluation were performed in an animal model and had different results[6,27]. In these studies, liver fibrosis was induced by administering carbon tetrachloride (CCl4) via intraperitoneal routes. Zhang et al[27] applied CEUS in rabbits and showed that PSI was decreased with the development of liver fibrosis, which was contrary to our results. In their study, hepatic artery to vein transmission time was significantly shorter in the advanced fibrosis group than the other groups. They suggested that decreased PSI resulted from decreased sinusoid volume caused by fibrosis and explained that decreased transit time through the liver resulted from increased intrahepatic shunts, similar to the explanation given in a human study. Ying et al[6] used CEUS in rats and also demonstrated that PSI was significantly decreased. However, TTP was significantly increased in the advanced fibrosis group compared with the early fibrosis group. They explained that delayed TTP was obtained from decreased hepatic sinusoidal volume derived from fibrotic change, which could lead to reduced liver blood flow velocity, overall decreased portal vein volume, and a longer perfusion time for liver parenchyma.
In the literature reviewed, PSI and TTP showed different results for evaluating advanced liver fibrosis. The two main explanations for hepatic perfusion change were decreased sinusoidal volume by fibrotic change and increased intrahepatic shunt flow causing a considerable amount of blood bypass. However, these pathogeneses could vary in our study. Chronic viral hepatitis in adults and intraperitoneal CCl4 injection in animals are different conditions compared with the bile duct ligation used in our study. The former conditions mainly induce hepatotoxicity, while bile duct ligation can cause cholestasis, recurrent cholangitis, and biliary cirrhosis. Previous animal studies revealed that liver fibrosis from hepatitis resulted from the activated hepatic stellate cells and portal myofibroblasts which lead to progressive portal fibrosis, periportal fibrosis and development of regenerative nodules[6,27,28]. However, liver fibrosis from obstructive cholestasis is different from this pattern. Obstructive cholestasis leads to bile duct proliferation and hyperplasia of bile duct epithelium which aggravates the generation of profibrogenic cytokine transforming growth factor β to form a scar[29,30]. Moreover, we used rabbits that had body weights of 3-4 kg, which was similar to full-term neonates, to evaluate liver fibrosis from biliary atresia. Using rats as an animal model has limitations because of their small liver volume. This is because it is more complicated to avoid small liver vessels for quantitative CEUS analysis.
In our study, laboratory tests showed that AST and ALT levels were markedly increased on postoperative day 7 and decreased during follow-up. However, these levels did not reach the normal range. Even though inflammatory changes from bile duct obstruction decreased gradually during the follow-up, it persisted in addition to liver fibrosis. This is similar to clinical conditions in biliary atresia. In children with biliary atresia, the intrahepatic inflammatory processes can continue for at least six months after Kasai portoenterostomy, which might cause progressive fibrosis and cirrhosis[10,31]. Moreover, the progression of fibrosis is more likely when cholangitis is recurrent[32]. Lee et al[29] demonstrated that biliary atresia was characterized by portal tract inflammation and biliary cirrhosis could develop in late stages. They showed that patients with biliary atresia had significantly larger hepatic artery, representing hypertrophic and hyperplastic changes that resulted from liver fibrosis. A recent review of hepatic fibrosis and cirrhosis explained that before an overall decrease in hepatic perfusion as a late change, hepatic arterial flow increases as a compensatory response resulting from arterialization of capillaries and reduced portal venous flow[14]. The increased PSI in our study could result from the increased compensatory hepatic arterial flow that occurs before advanced liver cirrhosis. However, we could not evaluate the diameter or Doppler flow pattern of the hepatic artery from rabbits due to small size and large variation of rabbit hepatic arteries detected during the operation. Moreover, bile duct ligation does not have the same pathophysiology as biliary atresia. Further studies are needed to validate the utility of perfusion analysis using CEUS to evaluate liver fibrosis in children with biliary atresia.
Our study had several limitations. First, the number of subjects was small. Only one rabbit was included in fibrosis grade 1. This could lower the statistical power of our study and could be a key reason why other parameters, including MTT, AUC and TTP, were not significantly correlated with fibrosis grade, in contrast to other studies[6,13,22,25]. However, our study demonstrated the feasibility of CEUS to evaluate liver fibrosis from biliary obstruction using an animal model. Though the contrast agent for CEUS has not been approved yet for pediatric patients, this study demonstrated the usefulness of CEUS for assessing liver fibrosis in children with biliary atresia. Second, we did not evaluate intra- and inter-observer variability while performing CEUS and achieving perfusion values from ROI. However, we tried to reduce variability by evaluating CEUS in the most homogeneous hepatic parenchyma for 3 min with two contrast injections. The values from the ROI were obtained three times to reduce intra-observer variability. Third, different pharmacokinetics, according to the contrast agent, could affect the results of perfusion parameters[14]. Moreover, a post-processing procedure is required to obtain perfusion parameters using different software without standardization. Therefore, additional studies with a large sample size and including patients with biliary atresia are necessary to establish the potential of CEUS for evaluating liver fibrosis from biliary obstruction.
In conclusion, this animal study demonstrates that CEUS can be used to evaluate liver fibrosis from biliary obstruction using PSI. Further studies are needed to validate the clinical utility of CEUS in the evaluation of liver fibrosis in children with biliary atresia.
The authors thank Mr. Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea, for his help with the figure.
Contrast-enhanced ultrasonography (CEUS) is an examination based on blood pool tracer visualization using ultrasound contrast agents during real-time ultrasonography (US) imaging. CEUS was proposed for the evaluation of liver fibrosis and previous studies demonstrated the role of CEUS for evaluating liver fibrosis for detecting local and hemodynamic changes accompanied by fibrotic liver changes. However, no previous studies have evaluated the utility of CEUS for liver fibrosis caused by biliary obstruction, which can be the major cause of liver fibrosis in children.
Liver cirrhosis is characterized by morphologic and hemodynamic changes of the general liver. Hemodynamic change is due to global change in liver perfusion that results from sequential changes of arterialized capillaries, decreased portal flow and increased arterial flow that compensates for the other changes. To assess liver fibrosis, the current research hotspot is the utilization of CEUS in this area because it includes grayscale images from US and even subtle perfusion changes can be quantitatively detected from contrast administration, without radiation exposure or need for sedation in infants.
Two previous CEUS studies performed liver fibrosis evaluation in an animal model and had different results. In these studies, liver fibrosis was induced by administering carbon tetrachloride via intraperitoneal routes to make hepatotoxicity. However, to make a liver fibrosis model specifically induced by obstructive cholestasis, we performed bile duct ligation in rabbits that had body weights of 3-4 kg, which was similar to full-term neonates, to evaluate liver fibrosis from biliary atresia. We performed liver CEUS in preoperative and postoperative days sequentially and obtained perfusion parameters quantitatively. The parameters were compared with final histopathologic fibrosis grade according to the Metavir score system immediately after sacrifice.
The study results suggest that the peak signal intensity measured on the last day demonstrated a significant association with liver fibrosis grades and the difference in peak signal intensity between the day before the operation and the last day also showed an association with liver fibrosis. It implies that CEUS can be useful to evaluate liver fibrosis induced from biliary obstruction using peak signal intensity as a parameter.
CEUS is a new examination method using real time US to visualize the administered ultrasound contrast agent which travels the blood stream in the form of microbubbles. Therefore, CEUS can quantitatively measure blood flow and perfusion changes using contrast kinetic curves derived from quantification software.
This is an interesting research study which evaluated the utility of recently developed CEUS to demonstrate the relationship between perfusion parameters and liver fibrosis grades. This study also correlated the histological fibrosis grades with vascular change of the liver using an animal model focused on liver fibrosis from bile duct obstruction.
P- Reviewer: Mennigen R S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 680] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 2. | Tranquart F, Mercier L, Frinking P, Gaud E, Arditi M. Perfusion quantification in contrast-enhanced ultrasound (CEUS)--ready for research projects and routine clinical use. Ultraschall Med. 2012;33 Suppl 1:S31-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Quaia E, De Paoli L, Angileri R, Cabibbo B, Cova MA. Indeterminate solid hepatic lesions identified on non-diagnostic contrast-enhanced computed tomography: assessment of the additional diagnostic value of contrast-enhanced ultrasound in the non-cirrhotic liver. Eur J Radiol. 2014;83:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cui XW, Ignee A, Jedrzejczyk M, Dietrich CF. Dynamic Vascular Pattern (DVP), a quantification tool for contrast enhanced ultrasound. Z Gastroenterol. 2013;51:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 499] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 6. | Ying M, Leung G, Lau TY, Tipoe GL, Lee ES, Yuen QW, Huang YP, Zheng YP. Evaluation of liver fibrosis by investigation of hepatic parenchymal perfusion using contrast-enhanced ultrasound: an animal study. J Clin Ultrasound. 2012;40:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Giuseppetti GM, Argalia G, Abbattista T. Liver cirrhosis: evaluation of haemodynamic changes using an ultrasound contrast agent. Eur J Radiol. 2004;51:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Fujita Y, Watanabe M, Sasao K, Wakui N, Shinohara M, Ishii K, Sumino Y. Investigation of liver parenchymal flow using contrast-enhanced ultrasound in patients with alcoholic liver disease. Alcohol Clin Exp Res. 2004;28:169S-173S. [PubMed] |
| 9. | Cocciolillo S, Parruti G, Marzio L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J Hepatol. 2014;6:496-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 638] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 11. | Tomita H, Masugi Y, Hoshino K, Fuchimoto Y, Fujino A, Shimojima N, Ebinuma H, Saito H, Sakamoto M, Kuroda T. Long-term native liver fibrosis in biliary atresia: development of a novel scoring system using histology and standard liver tests. J Hepatol. 2014;60:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 13. | Liu GJ, Ji Q, Moriyasu F, Xie XY, Wang W, Wong LH, Lin MX, Lu MD. Value of contrast-enhanced ultrasound using perflubutane microbubbles for diagnosing liver fibrosis and cirrhosis in rats. Ultrasound Med Biol. 2013;39:2158-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Aubé C. Imaging modalities for the diagnosis of hepatic fibrosis and cirrhosis. Clin Res Hepatol Gastroenterol. 2015;39:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Staub F, Tournoux-Facon C, Roumy J, Chaigneau C, Morichaut-Beauchant M, Levillain P, Prevost C, Aubé C, Lebigot J, Oberti F. Liver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoring. Eur Radiol. 2009;19:1991-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ridolfi F, Abbattista T, Marini F, Vedovelli A, Quagliarini P, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis. 2007;39:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Shin NY, Kim MJ, Lee MJ, Han SJ, Koh H, Namgung R, Park YN. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med. 2014;33:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] |
| 19. | Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472-478. [PubMed] |
| 20. | Ronot M, Asselah T, Paradis V, Michoux N, Dorvillius M, Baron G, Marcellin P, Van Beers BE, Vilgrain V. Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion CT. Radiology. 2010;256:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Chang S, Kim MJ, Kim J, Lee MJ. Variability of shear wave velocity using different frequencies in acoustic radiation force impulse (ARFI) elastography: a phantom and normal liver study. Ultraschall Med. 2013;34:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Ridolfi F, Abbattista T, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound evaluation of hepatic microvascular changes in liver diseases. World J Gastroenterol. 2012;18:5225-5230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 23. | Cobbold JF, Patel D, Fitzpatrick JA, Patel N, Crossey MM, Abdalla MS, Goldin RD, Vennart W, Thomas HC, Taylor-Robinson SD. Accuracy and reliability of microbubble ultrasound measurements for the non-invasive assessment of hepatic fibrosis in chronic hepatitis C. Hepatol Res. 2012;42:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Tang A, Kim TK, Heathcote J, Guindi M, Jang HJ, Karshafian R, Burns PN, Wilson SR. Does hepatic vein transit time performed with contrast-enhanced ultrasound predict the severity of hepatic fibrosis? Ultrasound Med Biol. 2011;37:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Orlacchio A, Bolacchi F, Petrella MC, Pastorelli D, Bazzocchi G, Angelico M, Simonetti G. Liver contrast enhanced ultrasound perfusion imaging in the evaluation of chronic hepatitis C fibrosis: preliminary results. Ultrasound Med Biol. 2011;37:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Blomley MJ, Lim AK, Harvey CJ, Patel N, Eckersley RJ, Basilico R, Heckemann R, Urbank A, Cosgrove DO, Taylor-Robinson SD. Liver microbubble transit time compared with histology and Child-Pugh score in diffuse liver disease: a cross sectional study. Gut. 2003;52:1188-1193. [PubMed] |
| 27. | Zhang L, Duan YY, Yin JK, Cui JH, Zhang Y, Cao TS. Grey scale enhancement by a new self-made contrast agent in early cirrhotic stage of rabbit liver. BMC Gastroenterol. 2007;7:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Lefkowitch JH. Liver biopsy assessment in chronic hepatitis. Arch Med Res. 2007;38:634-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Lee MS, Kim MJ, Lee MJ, Yoon CS, Han SJ, Oh JT, Park YN. Biliary atresia: color doppler US findings in neonates and infants. Radiology. 2009;252:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol. 1998;153:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Narayanaswamy B, Gonde C, Tredger JM, Hussain M, Vergani D, Davenport M. Serial circulating markers of inflammation in biliary atresia--evolution of the post-operative inflammatory process. Hepatology. 2007;46:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Wu ET, Chen HL, Ni YH, Lee PI, Hsu HY, Lai HS, Chang MH. Bacterial cholangitis in patients with biliary atresia: impact on short-term outcome. Pediatr Surg Int. 2001;17:390-395. [PubMed] |