Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2419
Peer-review started: July 15, 2014
First decision: August 15, 2014
Revised: September 28, 2014
Accepted: November 19, 2014
Article in press: November 19, 2014
Published online: February 28, 2015
Processing time: 228 Days and 23.1 Hours
AIM: To determine the upper cut-off values of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in a Northern Chinese population.
METHODS: A total of 3769 subjects in Jilin Province Northeast China were stratified to determine the potential factors affecting serum ALT and AST levels. The upper cut-off values of serum ALT and AST in these subjects were determined using receiver operating characteristic analysis and their sensitivity and specificity were evaluated.
RESULTS: Stratification analysis revealed that serum ALT and AST levels were associated with gender, alcohol consumption, serum cholesterol and triglyceride levels, and body mass index. The upper cut-off values of serum ALT and AST were 22.15 U/L and 25.35 U/L for healthy men and 22.40 U/L and 24.25 U/L for healthy women, respectively. The new cut-off values had a higher sensitivity, but a slightly lower specificity than the current standards.
CONCLUSION: Our results indicate that the new upper cut-off values of serum ALT and AST are markedly lower than current standards and may be valuable for the evaluation of liver function.
Core tip: This study examined the potential factors affecting serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in a Chinese population and determined the upper cut-off values of serum ALT and AST in these subjects. Serum ALT and AST levels were found to be associated with gender, alcohol consumption, serum cholesterol and triglyceride levels, and body mass index. The upper cut-off values of serum ALT and AST were 22.15 U/L and 25.35 U/L for healthy men and 22.40 U/L and 24.25 U/L for healthy women, respectively, which are markedly lower than current standards.
- Citation: Zhang P, Wang CY, Li YX, Pan Y, Niu JQ, He SM. Determination of the upper cut-off values of serum alanine aminotransferase and aspartate aminotransferase in Chinese. World J Gastroenterol 2015; 21(8): 2419-2424
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2419.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2419
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are two biomarkers frequently used for the assessment of hepatic diseases. ALT is predominantly expressed by hepatocytes, while AST is mainly expressed by both myocardial cells and hepatocytes. An increase in the levels of serum ALT and AST usually reflects various degrees of hepatic injury. As a result, changes in the levels of serum ALT and AST are useful serological markers for the diagnosis of hepatic diseases and for the evaluation of therapeutic efficacy and adverse hepatic effects of drug treatment[1]. Therefore, the application of an optimal upper cut-off value for serum ALT and AST levels is of particular significance due to their high clinical relevance.
The levels of serum ALT and AST can be affected by age, gender, alcohol consumption, body mass index (BMI), fasting blood glucose, strenuous physical exercise, dietary and living habits, nutrition, metabolic status, and drug treatment[2-5]. During the past few decades, social, economic and people’s health conditions in China have notably changed. However, since their introduction in the 1950s, the old upper cut-off values for the levels of serum ALT (40 U/L) and AST (50 U/L) have been used in China without substantial modification[1,6-8]. Due to limited knowledge, the upper cut-off values for the levels of serum ALT and AST were established without consideration of various risk factors. Whether the current upper cut-off values can accurately reflect healthy liver function in the Chinese population is questionable. Indeed, these upper cut-off values have recently been challenged in other countries[3,9,10]. Previous studies have found that some patients with chronic hepatitis B (CHB) or chronic hepatitis C (CHC) have persistently normal levels of serum ALT, but many of them display severe liver inflammation and fibrosis, as evidenced by histological examination of biopsied specimens[10,11]. Apparently, the upper cut-off values for the levels of serum ALT and AST may underestimate the prevalence of chronic liver diseases. In addition, hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are highly prevalent in China, which can progress to CHB and CHC, respectively, or even to liver cirrhosis and hepatocellular carcinoma (HCC). Therefore, it is critical to re-evaluate the upper cut-off values for the levels of serum ALT and AST in Chinese for the early and accurate diagnosis of liver diseases.
In the present study, we evaluated the upper cut-off values of the levels of serum ALT and AST in a Northeast Chinese population and identified potential risk factors that affected the values of serum ALT and AST levels in the diagnosis of liver diseases.
Data were collected from an epidemiological study of chronic diseases in Jilin, China from September 2007 to October 2007. The questionnaire-based study was supervised and assisted by the National Bureau of Statistics of China. This study questionnaire was approved by the Ethics Committee of the First Hospital of Jilin University. A total of 6043 subjects from local rural and urban areas were included in the study, of whom 3769 (1798 men and 1971 women) received physical examinations (including height, body weight, waist circumference, and blood pressure), B-mode ultrasonography, and blood tests (liver function tests, routine blood tests, and anti-hepatitis C virus antibodies (HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb). The study population had an average age of 45.0 ± 19.0 years and consisted predominantly of Han Chinese (n = 3749, 99.5%). The exclusion criteria included: subjects with hepatic steatosis; liver cirrhosis; liver enlargement; a history of HBV or HCV infection; current administration of medicine and a history of ALT and/or AST levels > 60 U/L; triglyceride (TG) ≥ 1.7 mmol/L; cholesterol ≥ 6.0 mmol/L; low density lipoprotein (LDL) ≥ 4.3 mmol/L; high density lipoprotein (HDL) < 1.04 mmol/L in men or < 1.3 mmol/L in women; BMI ≥ 23; alcohol consumption > 40 g/mo; daily cigarette consumption > 30. According to the assay results, we excluded 1632 individuals with factors affecting serum ALT and AST levels. The upper cut-off values of ALT and AST were evaluated in the remaining 2137 individuals.
Individuals were fasted overnight and their blood samples were collected, followed by centrifugation to prepare serum samples. The levels of fasting blood glucose, serum ALT and AST levels were measured using specific kits, according to the manufacturers’ instructions (Kehua Bio-Engineering, Shanghai, China). The levels of serum anti-hepatitis C virus antibodies, HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb were determined by ELISA using assay kits from Abbott (Illinois, United States). Blood cell counts were performed using an automatic biochemical analyzer (7600-010, Hitachi, Tokyo, Japan).
Statistical analysis was performed with SPSS 17.0 for Windows. All data were tested for their distribution. Normally distributed data are presented as mean ± standard derivation (SD), and non-normally distributed data are presented as median ± interquartile range (IQR). The association between the levels of serum ALT and AST levels and factors, including gender, age, alcohol intake, exercise, smoking, cholesterol, TG, LDL, blood glucose, and BMI, was analyzed using the Kruskal-Wallis test for categorical data and linear regression analysis for continuous data. The upper cut-off values of serum ALT and AST levels were determined using the ROC curve. The old and new upper cut-off values of ALT and AST levels in this population were analyzed using the χ2 test. A P value less than 0.05 was considered statistically significant.
In this study, abnormal rates of serum ALT and AST levels in males were 16% and 3.3%, respectively. The abnormal rates in females were 5.9% and 1.7%, respectively; the rates of ALT and AST abnormalities in drinkers (alcohol consumption > 40 g/mo) were 15.3% and 3.4%, respectively. The abnormal rates in non-drinkers were 8.2% and 1.9%, respectively; for HBV positive subjects were 25.3% and 11.8%, respectively; for HBV negative subjects were 10.1% and 2.0%, respectively; for HCV positive subjects were 22.5% and 17.5% respectively; for HCV negative subjects were 10.7% and 2.3%, respectively. As shown in Table 1, serum cholesterol, TG, LDL, BMI, alcohol consumption, smoking, gender, and hepatic diseases were associated with the serum levels of ALT and AST in this population. Furthermore, HDL levels were positively correlated with serum AST levels, and serum cholesterol, LDL and TG levels were positively correlated with serum ALT levels in this population. In addition, a higher level of serum HDL was the strongest factor associated with abnormal levels of serum AST, followed by higher levels of serum cholesterol, and LDL. Moreover, regular alcohol consumption was strongly associated with abnormal levels of serum ALT and AST in this population.
| Factors | ALT | AST | ||||||||
| Normal n = 3361 | Abnormal n = 408 | OR (95%CI) | Regression coefficients orχ2value | P value | Normal n = 3677 | Abnormal n = 92 | OR (95%CI) | Regression coefficients orχ2value | P value | |
| Gender | ||||||||||
| Male | 1506 (44.8) | 292 (71.6) | 1.0 | 342.1254 | < 0.0001 | 1739 (47.3) | 59 (64.1) | 1.0 | 192.1106 | < 0.0001 |
| Female | 1855 (55.2) | 116 (28.4) | 0.32 (0.26- 0.40) | 1938 (52.7) | 33 (35.9) | 0.50 (0.33-0.77) | ||||
| Age1 | 46 ± 19 | 43 ± 16 | 0.98 (0.98-0.99) | -0.09070 | 0.0017 | 45 ± 19 | 45 ± 16 | 1.00 (0.98-1.02) | 0.03095 | 0.0891 |
| Drinking | ||||||||||
| No | 2220 (66.1) | 198 (48.5) | 1.0 | 2371 (64.5) | 47 (51.1) | 1.0 | ||||
| Previous | 53 (1.6) | 13 (3.2) | 2.75 (1.47-5.13) | 182.001 | < 0.0001 | 65 (1.8) | 1 (1.1) | 0.78 (0.11-5.71) | 120.0163 | < 0.0001 |
| Current | 1088 (32.4) | 197 (48.3) | 2.03 (1.65-2.51) | 1241 (33.8) | 44 (47.8) | 1.79 (1.18-2.71) | ||||
| Smoking | ||||||||||
| No | 2120 (63.1) | 238 (58.3) | 1.0 | 29.8714 | < 0.0001 | 2295 (62.4) | 63 (68.5) | 1.0 | 9.9796 | < 0.0001 |
| Smoker | 1241 (36.9) | 170 (41.7) | 1.22 (0.99-1.50) | 1382 (37.6) | 29 (31.5) | 0.76 (0.49-1.19) | ||||
| HBV | ||||||||||
| Negative | 3234 (96.2) | 365 (89.5) | 1.0 | 55.4066 | < 0.0001 | 3527 (95.9) | 72 (78.3) | 1.0 | 62.2821 | < 0.0001 |
| Positive | 127 (3.8) | 43 (10.5) | 3.00 (2.09-4.31) | 150 (4.1) | 20 (21.7) | 6.53 (3.88-11.01) | ||||
| HCV | ||||||||||
| Negative | 3330 (99.1) | 399 (97.8) | 1.0 | 10.4863 | 0.0012 | 3644 (99.1) | 85 (92.4) | 1.0 | 13.3629 | 0.0003 |
| Positive | 31 (0.9) | 9 (2.2) | 2.42 (1.15-5.13) | 33 (0.9) | 7 (7.6) | 9.10 (3.91-21.14) | ||||
| BMI | 22.2 ± 3.91 | 23.67 ± 4.47 | 1.16 (1.13-1.20) | 1.17256 | < 0.0001 | 22.49 ± 3.70 | 22.81 ± 4.33 | 1.07 (1.00-1.14) | 0.25393 | 0.0008 |
| Cholesterol1 | 4.23 ± 1.2 | 4.57 ± 1.22 | 1.42 (1.28-1.56) | 3.01080 | < 0.0001 | 4.26 ± 1.20 | 4.50 ± 1.42 | 1.42 (1.19-1.70) | 1.37468 | < 0.0001 |
| LDL1 | 2.90 ± 0.90 | 3.20 ± 0.90 | 1.37 (1.23-1.52) | 2.56544 | < 0.0001 | 3.00 ± 0.90 | 3.10 ± 1.10 | 1.23 (1.05-1.45) | 1.16700 | < 0.0001 |
| HDL1 | 1.30 ± 0.40 | 1.30 ± 0.45 | 1.06 (0.90-1.25) | -0.36483 | 0.5918 | 1.30 ± 0.40 | 1.40 ± 0.60 | 1.24 (1.03- 1.50) | 1.47788 | 0.0005 |
| Triglyceride1 | 1.17 ± 0.97 | 1.76 ± 1.66 | 1.24 (1.17-1.30) | 2.03651 | < 0.0001 | 1.21 ± 1.05 | 1.51 ± 1.63 | 1.14 (1.07-1.22) | 0.93710 | < 0.0001 |
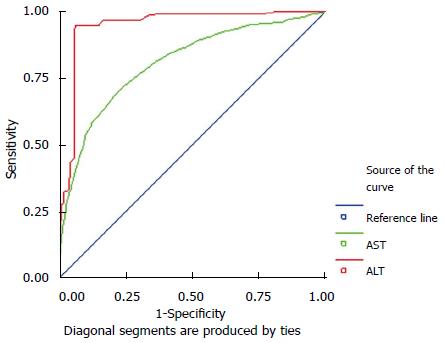
Upper normal limits for the levels of serum ALT and AST in men: The areas under the receiver operating characteristic (ROC) curve of ALT and AST were 0.94 and 0.80, respectively (Figure 1, Table 2), which were statistically different from A = 0.5 (ALT: P < 0.05; AST: P < 0.05). The upper normal limit for the level of serum ALT was 22.15 U/L with a sensitivity of 0.89 and a specificity of 0.86. The upper normal limit for the level of serum AST was 25.35 U/L with a sensitivity of 0.85 and a specificity of 0.81 (Table 3).
| Standard | Sensitivity (%) | Specificity (%) | P | |
| ALT | Old | 42.23 | 94.52 | < 0.01 |
| New | 88.76 | 84.55 | < 0.01 | |
| AST | Old | 40.21 | 95.53 | < 0.01 |
| New | 84.32 | 82.15 | < 0.01 |
| Gender | Area | SE | P | Sensitivity | Specificity | Upper normal limit | |
| Men | ALT | 0.94 | 0.01 | < 0.05 | 0.89 | 0.86 | 22.15 |
| AST | 0.80 | 0.01 | < 0.05 | 0.85 | 0.81 | 25.35 | |
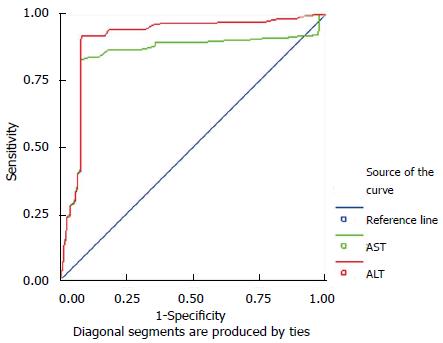
| Women | ALT | 0.90 | 0.006 | < 0.05 | 0.90 | 0.82 | 22.40 |
| AST | 0.84 | 0.008 | < 0.05 | 0.85 | 0.80 | 24.25 |
Comparison of the new cut-off values with the current standards for the levels of serum ALT and AST in men: The new cut-off values for the levels of serum ALT and AST had slightly lower specificity, but much higher sensitivity than that of current standards (Table 2).
Upper normal limits for the levels of serum ALT and AST in women: The areas under the ROC curve of the levels of serum ALT and AST in women were 0.90 and 0.84, respectively (Figure 2), which were statistically different from A = 0.5 (ALT: P < 0.05; AST: P < 0.05). The upper normal limit of the level of serum ALT was 22.4 U/L with a sensitivity of 0.90 and a specificity of 0.82. The upper normal limit of the level of serum AST was 24.25 U/L with a sensitivity of 0.85 and a specificity of 0.80 (Table 3).
Comparison of the new cut-off values with the current standards for the levels of serum ALT and AST in women: The new cut-off values for the levels of serum ALT and AST had slightly lower specificity, but much higher sensitivity than that of current standards (Table 4).
| Standard | Sensitivity (%) | Specificity (%) | P | |
| ALT | Old | 58.12 | 96.52 | < 0.01 |
| New | 92.45 | 85.25 | < 0.01 | |
| AST | Old | 49.15 | 92.75 | < 0.01 |
| New | 84.62 | 80.34 | < 0.01 |
The levels of serum ALT and AST are the most commonly used serological markers for the evaluation of hepatic diseases. However, a number of recent studies have challenged the currently used cut-off values for the levels of serum ALT and AST due to the presence of normal or near normal levels of serum ALT and AST in patients with chronic liver diseases[3,9,10,12]. The goal of CHB treatment is to reduce viral replication, subsequently decrease liver inflammation, fibrosis, and the risk of developing cirrhosis and HCC. Treatment guidelines for initiating treatment are based on the presence of active liver inflammation or elevated serum levels of liver transaminases[13,14]. According to the current upper normal limits for the levels of serum ALT, although 40% of HBeAg-positive patients had persistently normal serum ALT levels they had significant liver fibrosis on liver biopsy[15]. In another study, 35% of adult (> 35 years) HBeAg-positive patients had normal levels of serum ALT, but they already had advanced liver fibrosis[16]. Therefore, disease activity in patients during the immune tolerance phase may be erroneously judged by assessing HBV DNA and serum ALT levels alone[15,17]. In a retrospective study of 192 CHB patients, it was found that 37% of patients with significant hepatic fibrosis and inflammation had persistently normal levels of serum ALT and these patients with higher than normal levels of serum ALT (> 25 U/L) had been recommended for a liver biopsy[18]. A large prospective study of 142055 subjects in South Korea showed that the mortality rate of patients with ALT levels close to the normal upper limit (30-39 IU/L) was higher than those with lower ALT levels (20-29 IU/L)[9]. This finding suggests that the normal upper limit of the levels of serum ALT should be reduced by 25%. Establishment of new criteria for determination of the upper normal limits for serum ALT and AST levels may increase the sensitivity for the evaluation of chronic hepatitis, and help evaluate disease progression. It will also help patients receive anti-viral therapy at an early stage, reduce the needs for unnecessary liver biopsy, decrease diagnostic risks and costs, and increase patients’ tolerance.
Similarly, a recent Israeli study also showed that the levels of serum ALT in healthy subjects were lower than those commonly used[4]. In addition, an Iranian study assessing the upper normal limit of the levels of serum ALT in 1928 randomly recruited subjects found that individuals with normal body weight and without diabetes had significantly lower levels of serum ALT than those in the whole study population, suggesting a new cut-off value for the level of serum ALT to truly distinguish healthy subjects from those with abnormal liver function[6]. Likewise, another study in Brazil recommended that the normal upper limit of the level of serum ALT should be reduced to half the present standard for hepatitis C patients in need of chronic hemodialysis[19].
In agreement with the previous findings, our study of 2137 healthy subjects found that the upper normal limits of the levels of serum ALT and AST were markedly lower than the current standards after adjusting for potential confounders.
Further analysis revealed that the specificities of the cut-off values for the levels of serum ALT and AST were slightly lower than those of the current standards, but the sensitivities of the new cut-off values were significantly higher than those of the current standards. Therefore, the new cut-off values were better than the current standards.
Our study also found that abnormal levels of serum ALT and AST commonly occurred in men. We stratified the healthy subjects according to gender and found that the upper normal limits of the levels of serum ALT and AST were 22.4 U/L and 24.25 U/L for women and 22.15 U/L and 25.35 U/L for men, respectively. The marked discrepancies between the currently used upper normal limits of the levels of serum ALT and AST and the new values we described may be explained by the fact that many “healthy” individuals in the initial ALT studies may have had abnormal liver function, such as alcohol-related liver disease or nonalcoholic fatty liver disease[20]. Our findings support the notion that high levels of plasma TGs and cholesterol, high BMI, and regular alcohol consumption were associated with increased levels of serum ALT and that the former three are associated with the development of non-alcoholic liver diseases.
We found that the levels of serum TGs were correlated with the levels of serum ALT and AST. These findings were consistent with previous studies showing that higher levels of serum ALT and AST are associated with the development of metabolic syndrome[2,4,5,12,21]. For example, in a recent Chinese study, which evaluated the relationship between the levels of serum ALT and metabolic syndrome (MS) in patients with nonalcoholic fatty liver disease, a strong association between serum ALT levels and MS was identified and the cluster of MS components was suggested to be the predictor for ALT elevations[21].
However, we recognize that our study had limitations. First, this study did not consider the potential effects of occupation and environment on the reference values for the levels of serum ALT and AST. Second, it is possible that some subjects with liver diseases were not identified using B-mode ultrasonography on physical examination. Moreover, information regarding family and medical histories of individual subjects was simply obtained from the questionnaires, and accordingly, might not be accurate.
In summary, our data showed that the upper normal limits for the levels of serum ALT and AST were significantly lower than the currently used values. Further studies are necessary for the establishment of new reference ranges of the levels of serum ALT and AST for the evaluation of liver function in the clinic.
In addition, given the strong gender effect on the levels of serum ALT and AST, we suggest that new upper normal limits of ALT and AST need to be determined for both men and women. In our future study, we will increase the sample size and include cohorts from different geographical areas, to ensure that our results are more reliable and will provide important evidence for the prevention and management of liver disorders.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are two biomarkers frequently used for the assessment of hepatic diseases. Changes in the levels of serum ALT and AST are useful serological markers for the diagnosis of hepatic diseases and for the evaluation of therapeutic efficacy and adverse hepatic effects of drug treatment. Due to limited knowledge, the upper cut-off values for the levels of serum ALT and AST were established without consideration of various risk factors. Whether the current upper cut-off values can accurately reflect healthy liver function in the Chinese population is questionable.
The upper cut-off values for the levels of serum ALT and AST may underestimate the prevalence of chronic liver diseases. In addition, hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are highly prevalent in China, which can progress to chronic hepatitis B and C, respectively, or even to liver cirrhosis and hepatocellular carcinoma. It is critical to re-evaluate the upper cut-off values for the levels of serum ALT and AST in Chinese for the early and accurate diagnosis of liver diseases.
The results indicate that the new upper cut-off values of serum ALT and AST are markedly lower than current standards and may be valuable for the evaluation of liver function.
Present data showed that the upper normal limits for the levels of serum ALT and AST were significantly lower than the currently used values. In addition, given the strong gender effect on the levels of serum ALT and AST, we suggest that new upper normal limits of ALT and AST need to be determined for both men and women.
ALT, a transaminase enzyme, is found in plasma and in various bodily tissues, but is most commonly associated with the liver. AST is a pyridoxal phosphate-dependent transaminase enzyme found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells. Serum ALT and AST are commonly measured clinically as markers of liver health.
This is a good retrospective study in which the authors determined the upper normal limits for the levels of serum ALT and AST in Chinese population and suggest that the upper normal limits for the levels of serum ALT and AST were dramatically lower than the currently used values.
P- Reviewer: Chiang TA, Jin B, Morales-Gonzalez JA S- Editor: Qi Y L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 693] [Article Influence: 27.7] [Reference Citation Analysis (4)] |
| 2. | Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. J Gastroenterol Hepatol. 2007;22:1482-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Jamali R, Pourshams A, Amini S, Deyhim MR, Rezvan H, Malekzadeh R. The upper normal limit of serum alanine aminotransferase in Golestan Province, northeast Iran. Arch Iran Med. 2008;11:602-607. [PubMed] |
| 4. | Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, Noff D, Zelber-Sagie S, Sheinberg B, Oren RHalpern Z. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006;26:445-450. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Mohamadnejad M, Pourshams A, Malekzadeh R, Mohamadkhani A, Rajabiani A, Asgari AA, Alimohamadi SM, Razjooyan H, Mamar-Abadi M. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J Gastroenterol. 2003;9:2322-2324. [PubMed] |
| 6. | Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med. 2002;137:49-51. [PubMed] |
| 7. | Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch Intern Med. 1991;151:260-265. [PubMed] |
| 8. | Siest G, Schiele F, Galteau MM, Panek E, Steinmetz J, Fagnani F, Gueguen R. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem. 1975;21:1077-1087. [PubMed] |
| 9. | Gruis M. Beyond maternity: postpartum concerns of mothers. MCN Am J Matern Child Nurs. 1977;2:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [PubMed] |
| 11. | Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 815] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, Sansonetti N, Opolon P. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology. 1998;27:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2400] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 14. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2169] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 15. | Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, Bose S. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 16. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol. 2009;7:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Hardy JA, Davies DC. Alzheimer’s disease. Br J Hosp Med. 1988;39:372-33, 372-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Lopes EP, Gouveia EC, Albuquerque AC, Sette LH, Mello LA, Moreira RC, Coelho MR. Determination of the cut-off value of serum alanine aminotransferase in patients undergoing hemodialysis, to identify biochemical activity in patients with hepatitis C viremia. J Clin Virol. 2006;35:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Hoda K, Nguyen MH. Hronic hepatitis B virus infection in patients with “Normal” ALT levels. Cur Hep Rep. 2007;6:24-29. |
| 21. | Chen ZW, Chen LY, Dai HL, Chen JH, Fang LZ. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. J Zhejiang Univ Sci B. 2008;9:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |