Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1907
Peer-review started: August 9, 2014
First decision: August 27, 2014
Revised: September 8, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: February 14, 2015
Processing time: 187 Days and 1.1 Hours
AIM: To evaluate the prevalence of double negative (DN) sera and the mechanisms responsible for DN status.
METHODS: Sera of inflammatory bowel disease patients treated with infliximab (IFX) were tested for drug/antibodies to infliximab (ATI) trough levels and the proportion of DN results was compared between a commercially available double antigen ELISA (with labeled IFX as the detection antibody) and an anti-lambda ELISA (with anti-human lambda chain detection antibody). Repeat testing with lower than customary serum dilution (1:10) was performed. Patients with DN status were matched with IFX+/ATI- controls and were followed-up for subsequent development of non-transient ATI to investigate if DN status precedes ATI.
RESULTS: Of 67 sera obtained at time of loss of response, only 6/67 (9%) were DN by anti-lambda ELISA compared to 27/67 (40%) with double antigen ELISA (P < 0.001, Fisher’s Exact test). Of the latter 27 sera, 22% were also DN by anti-lambda ELISA, whereas 44% were actually IFX positive (IFX+ATI-), 30% were ATI positive (IFX-ATI+) and 4% were double positive (IFX+ATI+). Re-testing using a 1:10 dilution converted most DN results into IFX+ and /or ATI+ status. Patients with DN status had shorter survival free of non-transient ATI compared with matched controls (log rank test, P < 0.001). In 9/30 (30%) of these patients, non transient ATI occurred before and after the event at which the DN serum was obtained, supporting the view that a DN result may represent a particular time-point along the two curves of ATI titer rise and infliximab drug level decline.
CONCLUSION: DN status may result from false negative detection of IFX or ATI by double antigen ELISA, suggesting a transitional state of low-level immunogenicity, rather than non-immunological clearance.
Core tip: Among patients who lose response to infliximab (IFX) 10%-60% have low IFX levels in the absence of antibodies to infliximab (ATI) - double negative (DN) status. We explored the prevalence and the mechanisms responsible for DN status. The prevalence of DN sera varied with the assay and dilution used. Patients with DN status had shorter survival free of ATI compared with matched controls (P < 0.001). We believe that DN status may result from false negative detection of IFX or ATI by a conventional ELISA assay, suggesting a transitional state of low-level immunogenicity, rather than non-immunological drug clearance.
- Citation: Ungar B, Anafy A, Yanai H, Ron Y, Yavzori M, Picard O, Fudim E, Loebstein R, Kopylov U, Chowers Y, Dotan I, Eliakim R, Ben-Horin S. Significance of low level infliximab in the absence of anti-infliximab antibodies. World J Gastroenterol 2015; 21(6): 1907-1914
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1907
Infliximab (IFX) is a chimeric mouse - human monoclonal immunoglobulin G1 (IgG1) antibody against tumor necrosis factor α (TNFα). It is effective in inducing and maintaining remission in crohn's disease (CD) and ulcerative colitis (UC)[1-3]. Between 30%-70% of patients who initially respond to IFX subsequently lose their response and experience exacerbation of symptoms, necessitating either dose escalation, switch to another anti-TNF agent, concomitant immunomodulator therapy or surgical intervention[4-6]. Antibodies to infliximab (ATI) develop in approximately 40% of IFX treated patients and correlate with lower IFX trough levels and clinical loss of response (LOR)[7,8]. In 10%-60% of LOR patients, pharmacokinetic tests reveal low IFX trough levels and absence of detectable ATI, designated double negative (DN) status (IFX-/ATI-)[5,9]. Furthermore, several studies, including the SONIC trial, demonstrated that among patients with LOR, the DN status was in fact the more common scenario rather than the expected IFX-/ATI+ status[7,10].
There is a lack of data regarding the mechanisms responsible for the DN status and its consequence. DN status has been attributed to both immune and non-immune clearance of anti-TNF, as well as to technical limitations, such as non-uniform timing of measurement (trough levels are more sensitive than in-between infusions)[5,11]. The uncertainty about the causes and implications of an IFX-/ATI- status makes it hard to establish optimal strategies to prevent and/or manage LOR events in the presence of such a pharmacokinetic situation.
The aims of the present study were to evaluate the frequency and clinical significance of DN status among IFX-treated IBD patients (both in general and at time of LOR) and to investigate the impact of the diagnostic technique on the incidence of this phenomenon.
The study population included IBD patients treated with IFX at the gastroenterology departments of Sheba medical center and the Tel-Aviv Sourasky Medical Center between February 2009 and October 2013, who had available sera stored. All participants provided written informed consent and the ethics committees of the two medical centers approved the study. Pre-infusion sera were obtained and analyzed for trough IFX and ATI levels. Sera of patients whose infusions were delayed for over 2 wk from the scheduled date were excluded.
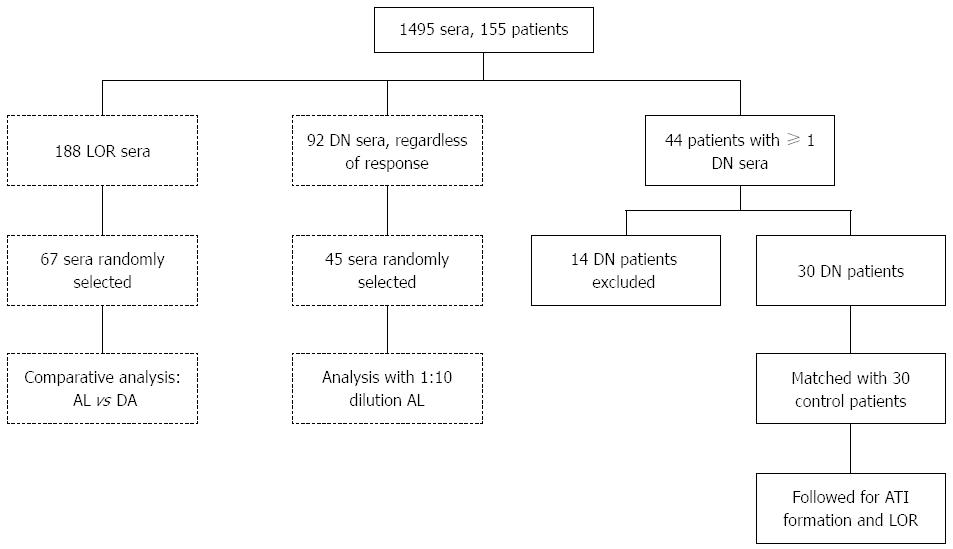
The study consisted of two separate parts: (1) an analytical part, which targeted differences between assays and technical limitations; and (2) a clinical part, aiming to study the natural history of the DN phenomenon (Figure 1). In the analytical part of the study, IFX and ATI trough levels of patients experiencing LOR were evaluated using two different ELISA assays: double antigen and anti-lambda ELISA. Subsequently, the fraction of IgG4 ATI was measured and compared in a sample of patients with discrepant results between the two ELISA assays to investigate if the conflicting results stemmed from a predominant monovalent IgG4 ATI response. Finally, to investigate the analytical accuracy of the anti-lambda ELISA, this assay was repeated in 45 randomly selected DN sera using a serum dilution of 1:10 (rather than the conventional 1:100 dilution). Patients’ sera in this analysis were tested regardless of response status, and sera of healthy volunteers unexposed to IFX served as controls.
The clinical part was a case-control study; cases were patients with IBD who had at least one DN (IFX-ATI-) measurement during routine follow-up (not necessarily at a point of LOR) and controls were IBD patients with positive drug levels without ATI (IFX+ATI-). The starting point of the analysis was defined as the month of the DN event in the cases and the matching month in the controls. The pharmacokinetics at the end of follow-up were correlated to clinical outcome. Cases and controls were matched according to the duration of IFX therapy. Patients with unavailable subsequent measurements were excluded. Antibody formation was defined as positive when a patient tested positive for ATI during follow-up on more than two consecutive time points. Transient antibodies were defined as measurable ATI on up to two consecutive infusions, which disappeared on subsequent infusions without any alteration of therapy[8,12,13]. Permanent, non-transient, ATI comprised the primary end point, while transient ATI were disregarded. Clinical response was defined by an improvement in disease activity indexes, the Harvey-Bradshaw index (HBI) and the simple clinical colitis activity index (SCCAI) for CD and UC patients, respectively, coupled with a treating physician’s decision to continue IFX therapy without alteration. Clinical response was evaluated on the day of IFX infusion. Secondary LOR was defined as increased disease activity (a rise of > 3 points in HBI score or of > 2 points in SCCAI for CD and UC, respectively) after achieving an appropriate induction response[14-16]. When unavailable, clinical response was determined by the documented physician’s global assessment.
IFX levels were measured by a commercially available quantitative ELISA, TNF-α-blocker-monitoring (Immundiagnostik, Bensheim, Germany), following the manufacturer’s instructions. The assay's detection threshold was IFX > 1 μg/mL.
ATI levels were measured by a commercially available qualitative TNF-α-blocker-ADA (antibodies against infliximab, Immundiagnostik, Bensheim, Germany), following the manufacturer’s instructions. The assay’s detection threshold was ATI > 10 AU/mL, which was standardized in our laboratories to 1 AU/mL.
A volume of 100 μL of 1:100 diluted serum was added to pre-plated 750 ng/mL TNFα (Peprotech, Rocky Hill, NJ, United States) and incubated for 90 min. Following washing, horseradish peroxidase (HRP) labeled goat anti-human Fc fragment antibody (MP Biomedicals, Solon, OH, United States) at a concentration of 0.62 μg/mL was added for 60 min and reacted with the tetramethylbenzidine (TMB) substrate. The results were then read on an ELISA reader. Quantification of the measured IFX concentration was done by calibration to a standard curve in which exogenous IFX (Schering Plough, NJ, United States) was added at concentrations between 3 and 200 ng/mL. The assay’s detection threshold was IFX > 0.6 μg/mL.
ATI were determined as previously described[11,17]. Briefly, IFX (0.1 mg/mL) was added to pre-plated TNFα (500 ng/mL) in 100 μL wells of ELISA plates (Nunc, Roskilde, Denmark). After drying, 100 μL of serum (1:100 dilution) was added and incubated for 90 min at room temperature. Plates were then washed and goat anti-human λ chain HRP-labeled antibody (Sertec, Oxford, United Kingdom) was added at a dilution of 2.5 × 104 for 60 min and reacted with the TMB substrate. The results were read by an ELISA reader EL-800 (Biotek Instruments, Winooski, VT, United States) and expressed as mcg/mL-equivalent (mcg/mL-e) after normalization vs results obtained using additions of graded concentrations between 9 and 600 ng/mL of HRP labeled goat anti-human F(ab’)2 fragment antibody (MP Biomedicals). The assay’s detection threshold was ATI > 2.5 μg/mL-eq.
IFX (0.1 mg/mL) was added to pre-plated TNFα (500 ng/mL) in 100 μL wells of ELISA plates (Nunc). After drying, 100 μL of diluted serum (1:100) was added and incubated at room temperature. Plates were then washed and an HRP-labeled monoclonal antibody to human IgG4 (fc-specific, Acris antibodies CN AM20252HR-N) was added and reacted with the TMB substrate. The results were read by an ELISA reader EL-800 (Biotek Instruments) and expressed as mcg/mL. Normalization was obtained using graded concentrations of Human IgG4 Kappa (Millipore CN AG508).
Categorical variables were analyzed by Fisher’s exact test. Kaplan-Meier survival curves were plotted to assess the temporal rate of events and the log rank test was computed for the comparison between survival free durations. Odds ratio and 95%CI were computed for all compared variables. The analysis was performed using MedCalc software (version 12.2.1.0, Mariakerke, Belgium). A two-tailed P < 0.05 was considered statistically significant.
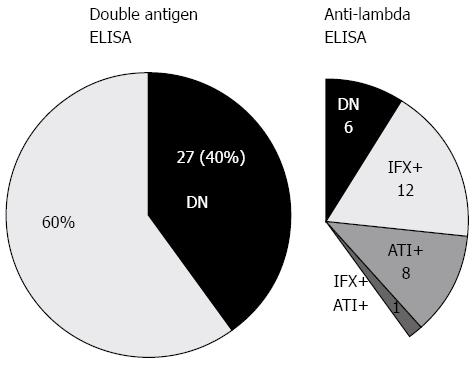
Out of 188 sera obtained from patients with LOR during regular IFX therapy, 67 were randomly selected for comparative analysis using the two techniques (anti-lambda and double antigen ELISA, Figure 1). In this analysis, 27/67 sera (40%) tested IFX-/ATI- with double antigen-ELISA compared to 6/67 (9%) with anti-lambda-ELISA (P < 0.001, Fisher’s exact test). The calculated number needed to test (NNT) for a false-negative DN result by double-antigen ELISA was 3.2. As depicted in Figure 2, when applying anti-lambda-ELISA to the 27 sera that were IFX-/ATI- with the double antigen assay, only 6 (22%) remained DN, while 12 (44%) were actually IFX positive (IFX+ATI-), 8 (30%) were ATI positive (IFX-ATI+) and one serum (4%) was double positive (IFX+ATI+).
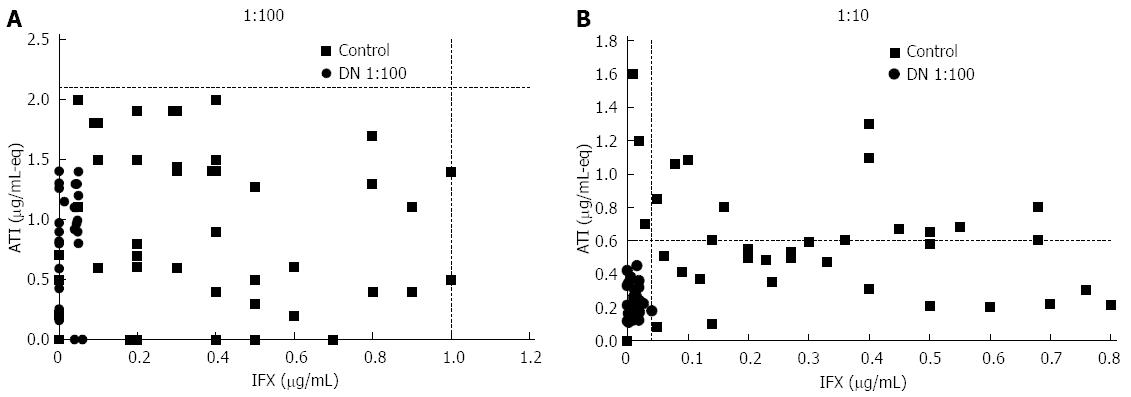
When investigating the occurrence of double negativity regardless of patients’ response status, we found that only 92 of the 1495 sera (6%) analyzed at our center between 2009-2013 were DN by anti-lambda ELISA (Figure 1). To examine whether some of these DN sera represented low-titer ATI or low level IFX, we randomly selected 45 DN sera and re-tested them at a 1:10 dilution to increase analytical sensitivity (compared with standard anti-lambda testing using 1:100 serum dilution). Upon this 1:10 dilution test, 24 (53%) DN sera became IFX positive (IFX+ATI-), 15 (33%) were double positive (IFX+ATI+) and 5 (11%) were ATI positive (IFX-ATI+). Only one serum (2%) retained its double negativity on 1:10 dilution (Figure 3). This transformation into detectable levels on 1:10 dilution was primarily caused by the fact that all 30 sera of healthy controls unexposed to IFX remained DN when tested by 1:10 dilution, but with lower detection cut-off levels.
IgG4 are monovalent antibodies (as opposed to the bivalent IgG1), and are thereby detectable by the anti-lambda ELISA, rather than by the double antigen assay[18]. Therefore, we assumed that a DN status on double antigen ELISA might be a result of non-detection of IgG4 ATI. To test this, we analyzed five sera that were DN by the double antigen ELISA and ATI positive by the anti-lambda ELISA (IFX-ATI-), as well as five sera that were ATI positive on both assays (IFX-ATI+). Contrary to our assumption, IgG4 levels were higher among the double antigen ELISA ATI+ positive sera (Median 6.6, IQR 0.9-7.4 vs median 0.5, IQR 0.07-0.97, P = 0.047, respectively).
To investigate whether DN status is a harbinger of pending immunogenicity, we sought to determine whether patients with DN sera were predisposed to develop ATI compared with patients with measurable IFX (IFX+ATI-). During the study period, 44 out of 155 patients on standard IFX regiment had at least one DN serum sample determined by anti-lambda ELISA (Figure 1). Fourteen of them were excluded from analysis because of missing data (10 were inconsistently followed, two were lost to follow up after the DN event and two received infusions outside our center). Thus, 30 patients (25 CD, 5 UC) were included and matched with 30 controls (27 CD, 3 UC). Median follow up time was 21 ± 25.1 mo vs 20.75 ± 25.5 mo, respectively, P = 0.97. The patients’ demographic and clinical characteristics are presented in Table 1.
| Parameter | Cases | Controls | P value | OR (95%CI) |
| Gender | 0.19 | 2.0 (0.70-5.70) | ||
| Male | 15 (50) | 10 (33.3) | ||
| Female | 15 (50) | 20 (66.6) | ||
| Type of IBD | 0.45 | 0.56 (0.12-2.57) | ||
| CD | 25 (83.3) | 27 (90.0) | ||
| UC | 5 (16.7) | 3 (10.0) | ||
| Duration of IFX therapy1 (mo) | 21 ± 25.1 | 20.75 ± 25.5 | 0.97 | |
| Concomitant therapy | 11 (36.7) | 11 (36.7) | 1.00 | 1.00 (0.35-2.86) |
| Episodic therapy | 6 (20) | 6 (20.0) | 1.00 | 1.00 (0.28-3.54) |
| Median age (yr) | 33 ± 15.2 | 28.5 ± 10.7 | 0.24 | |
| Median disease duration (yr) | 10.5 ± 9 | 9 ± 7.7 | 0.65 | |
| Median age at diagnosis (yr) | 22 ± 12.4 | 20 ± 9.7 | 0.19 | |
| CD - disease location | ||||
| Ileal | 9 (36) | 8 (29.7) | 0.62 | 1.30 (0.41-4.30) |
| Ileo-colonic | 8 (32) | 11 (40.7) | 0.51 | 0.68 (0.22-2.10) |
| Colonic | 8 (32) | 8 (29.6) | 0.85 | 1.10 (0.34-3.63) |
| CD - upper GI involvement | 2 (8) | 2 (7.4) | 0.93 | 1.08 (0.14-8.40) |
| CD - anal/perianal involvement | 13 (52) | 14 (51.8) | 0.99 | 1.00 (0.34-3.00) |
| CD - disease behavior | ||||
| Non stricturing non penetrating | 12 (48) | 12 (44.5) | 0.79 | 1.15 (0.38-3.43) |
| Stricturing | 8 (32) | 10 (37.0) | 0.70 | 0.80 (0.25-2.50) |
| Penetrating | 5 (20) | 5 (18.5) | 0.89 | 1.10 (0.28-4.40) |
| UC - disease location | ||||
| Proctitis | 0 | 0 | ||
| Left-sided colitis | 2 (40) | 1 (33.3) | ||
| Extensive colitis | 3 (60) | 2 (66.7) | ||
| Extra-intestinal manifestations | 15 (50) | 15 (50.0) | 1.00 | 1.00 (0.36-2.75) |
| Smoking | 2 (6.7) | 5 (16.7) | 0.24 | 0.30 (0.06-2.00) |
| Immunomodulator therapy prior to infliximab therapy | 26 (86.7) | 23 (76.7) | 0.32 | 1.97 (0.51-7.63) |
| Adalimumab therapy prior to infliximab therapy | 4 (13.3) | 0 | 0.12 | 10.30 (0.53-201.00) |
| Surgery prior to infliximab therapy | 8 (26.7) | 7 (23.3) | 0.76 | 1.19 (0.37-3.85) |
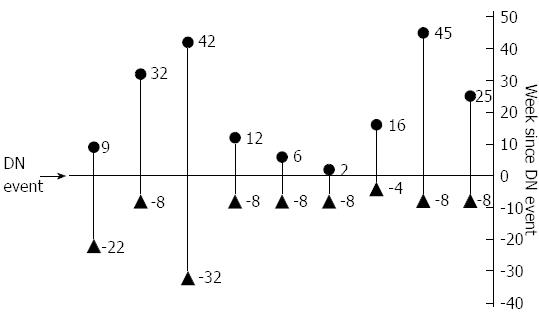
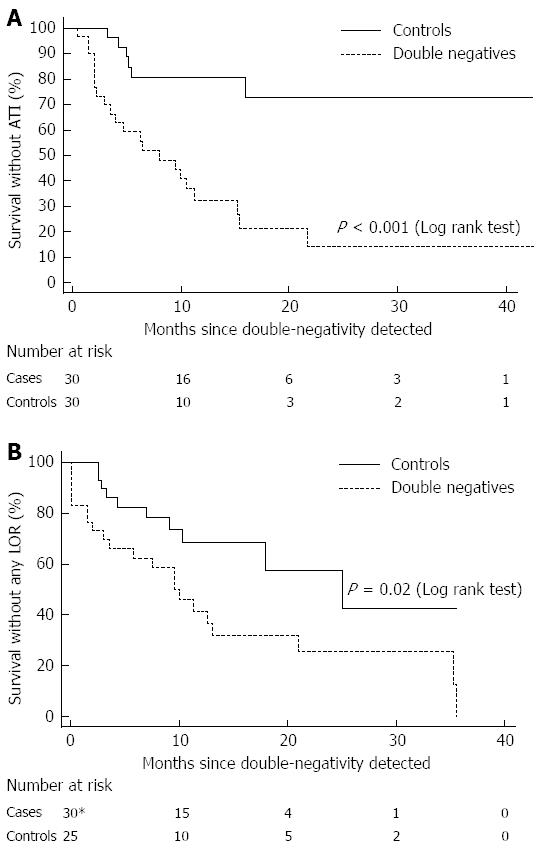
ATI formation was significantly more frequent among the DN group compared with controls (OR = 11, 95%CI: 3.3-36.8, P≤ 0.001). In 9/30 (30%) DN patients who developed non-transient ATI, ATI formation occurred both before and after the event at which the DN serum was obtained (Figure 4), supporting the fact that a DN result may represent a particular time-point along the two curves of ATI titer rise and IFX drug level decline.
To investigate the temporal evolution of immunogenicity in DN patients, Kaplan-Meier analysis was performed. ATI free survival was significantly longer among controls (log rank test, P < 0.001, Figure 5A) than among DN cases. Of note, ATI appearance prior to the DN status was disregarded in this analysis. Nevertheless, when considering ATI existence before the starting point as positive, similar results were obtained (log rank test, P < 0.001). Secondary LOR was also more frequent among DN cases (OR = 4.66 95%CI: 1.57-13.86, P = 0.006) and survival free of secondary LOR was significantly shorter than among controls (P = 0.02, log rank test, Figure 5B).
A substantial portion of IFX treated patients develop low trough levels of IFX in the absence of measurable ATI (IFX-ATI-), i.e., a DN status. Several studies have demonstrated that among patients with LOR, a DN result is more prevalent than antibody positive sera[10,19]. The actual mechanism of LOR remains unclear in most such cases, and the role - if any - of immunogenicity in instigating this phenomenon remains to be determined. In addition to assay limitations and irregular sampling time-points, DN status has been attributed to non-immune clearance of anti-TNF, high tissue inflammatory burden “absorbing” anti-TNF drug and temporal “window phenomenon”, which refers to sampling when all drug-ATI complexes have been cleared[5,11,20].
Studies that employed double antigen ELISA assay reported 20%-40% of the patients as DN, regardless of response status[7,9,21,22]. Vande Casteele et al[12] recently demonstrated a prevalence of only 11% using the homogeneous mobility shift assay (HMSA). Little data exists comparing different methods for IFX and ATI level measurement. Steenholdt et al[23] recently demonstrated a lower detection rate of ATI using double-antigen ELISA than by other essays. In the current study, double negativity was significantly more prevalent when using the double antigen ELISA compared with the anti-lambda ELISA (40% vs 9%, P < 0.001). Furthermore, when applying anti-lambda ELISA to the sera that were IFX-/ATI- by the double antigen assay, only six (22%) remained DN. We assumed that the higher frequency of double negativity using double antigen ELISA stems at least partly from false negative detection of IFX or ATI. As IgG4-ATI levels were not higher among the double antigen ELISA DN sera, double negativity cannot be attributed to the technical inability of double antigen ELISA to detect ATI in patients with a predominance of IgG4-ATI.
Interestingly, only one serum out of 45 examined with 1:100 dilution anti-lambda ELISA retained its double negativity at 1:10 dilution anti-lambda. The other sera became mostly IFX+ATI- or IFX-ATI+. The fact that almost all sera “lost” their DN status at 1:10 dilution implied that at least part of this phenomenon probably arises from low drug and ATI levels close to the detection threshold of the more sensitive anti-lambda assay. These sera may reflect a transitional state of immunological equilibrium between antibody-mediated IFX clearance and endogenous ATI production, rather than genuine non-immunological drug clearance. Notably, in clinical practice, physicians should be aware that some patients may present with DN status at trough merely because of arriving late for a delayed infusion. Such cases were excluded from the present work.
Few studies have addressed the question of subsequent ATI development in patients with DN sera. Hanauer et al[7] demonstrated that only 2.5% of DN patients turned ATI+ at week 76, although IFX infusions were halted at week 46. By contrast, Seow et al[9] showed that 77% of DN UC patients later developed ATI, regardless of response status. In our study, double negativity was also predictive of future non-transient ATI formation. This re-enforced our conclusion that DN status is an immunologically mediated phenomenon, albeit with low titer antibodies close or below the detection level of the assay when employed by certain specifications. As previously demonstrated, transient ATI had little clinical and immunological significance[8,12,13].
There are several limitations to our study. Primarily, the results were obtained with the double antigen ELISA and the anti-lambda ELISA; however, corroborating studies using other assays, such as HMSA, are pertinent. Secondly, because treating physicians were not blinded to the results, one cannot exclude that the DN status of the sera analyzed may have influenced clinical management. However, LOR was defined per clinical indexes and constituted only the secondary outcome. Finally, previous events of positive ATI might influence future ATI formation. To neutralize such past effects, we performed an analysis incorporating former ATI events as if they occurred at time zero, which yielded similar results.
In conclusion, the type of assay employed influences the occurrence of DN status. DN is rarely observed when LOR patients’ sera are analyzed by the sensitive anti-lambda assay, and in many cases it probably results from low-level immunogenicity rather than elusive non-immunogenic mechanisms. Further studies are required to better assess the immunological processes leading to the absence of both drug and ATI, to investigate possible drug clearance pathways and to define appropriate interventions in these patients.
Antibodies to infliximab (ATI) correlate with lower infliximab (IFX) trough levels and loss of response (LOR). However, 10%-60% of LOR patients have low IFX levels in the absence of ATI, which are designated double negative (DN) status.
The clinical and immunological significance of the DN status is currently unknown.
Only 9% of sera obtained at time of LOR were DN by anti-lambda ELISA compared with 40% with double antigen ELISA (P < 0.001, Fisher’s Exact test). Re-testing with 1:10 dilution converted most of the DN results into IFX+ and /or ATI+ status. Patients with DN status had shorter survival free of non-transient ATI compared with matched controls (log rank test, P < 0.001).
The type of assay employed influences the occurrence of DN status. It is rarely observed when LOR patients’ sera are analyzed by the sensitive anti-lambda assay, and in many cases it probably results from low-level immunogenicity rather than non-immunogenic mechanisms. Further studies are required to better assess the immunological processes leading to the absence of both drug and ATI, to investigate possible drug clearance pathways and to define appropriate interventions in these patients.
DN status: A serum sample that is negative for both infliximab and ATI (IFX-ATI-). Double antigen ELISA: A commercially available ELISA assay for the detection of ATI, which incorporates infliximab as the detection antibody. Anti-lambda ELISA assay: An in-house developed ELISA assay for the detection of ATI, which incorporates an anti-human λ chain antibody as the detection antibody. LOR: Loss of clinical response to infliximab therapy.
This manuscript evaluates the prevalence and clinical significance of DN status among infliximab-treated inflammatory bowel disease patients. The article is well written and is suitable to be published.
P- Reviewer: De Silva AP, Trifan A S- Editor: Ma YJ L- Editor: Stewart GJ E- Editor: Ma S
| 1. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 2. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1553] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 3. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 4. | Kopylov U, Mantzaris GJ, Katsanos KH, Reenaers C, Ellul P, Rahier JF, Israeli E, Lakatos PL, Fiorino G, Cesarini M. The efficacy of shortening the dosing interval to once every six weeks in Crohn’s patients losing response to maintenance dose of infliximab. Aliment Pharmacol Ther. 2011;33:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, Olson A, Bao W, Rutgeerts P. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 448] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Ungar B, Chowers Y, Yavzori M, Picard O, Fudim E, Har-Noy O, Kopylov U, Eliakim R, Ben-Horin S; ABIRISK consortium. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2374] [Article Influence: 158.3] [Reference Citation Analysis (1)] |
| 11. | Kopylov U, Mazor Y, Yavzori M, Fudim E, Katz L, Coscas D, Picard O, Chowers Y, Eliakim R, Ben-Horin S. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis. 2012;18:1628-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Vande Casteele N, Gils A, Singh S, Ohrmund L, Hauenstein S, Rutgeerts P, Vermeire S. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 13. | Steenholdt C, Al-khalaf M, Brynskov J, Bendtzen K, Thomsen OØ, Ainsworth MA. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2188] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 16. | Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 17. | Ben-Horin S, Yavzori M, Katz L, Kopylov U, Picard O, Fudim E, Coscas D, Bar-Meir S, Goldstein I, Chowers Y. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut. 2011;60:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Afif W, Loftus EV, Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 20. | Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079-187; quiz 1079-187;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 483] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 22. | Pariente B, Pineton de Chambrun G, Krzysiek R, Desroches M, Louis G, De Cassan C, Baudry C, Gornet JM, Desreumaux P, Emilie D. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:1199-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Steenholdt C, Ainsworth MA, Tovey M, Klausen TW, Thomsen OO, Brynskov J, Bendtzen K. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit. 2013;35:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |