Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1857
Peer-review started: April 12, 2014
First decision: May 29, 2014
Revised: June 27, 2014
Accepted: July 29, 2014
Article in press: July 30, 2014
Published online: February 14, 2015
Processing time: 306 Days and 7.4 Hours
AIM: To assess the value of computed tomography (CT) for diagnosis of synchronous colorectal cancers (SCRCs) involving incomplete colonoscopy.
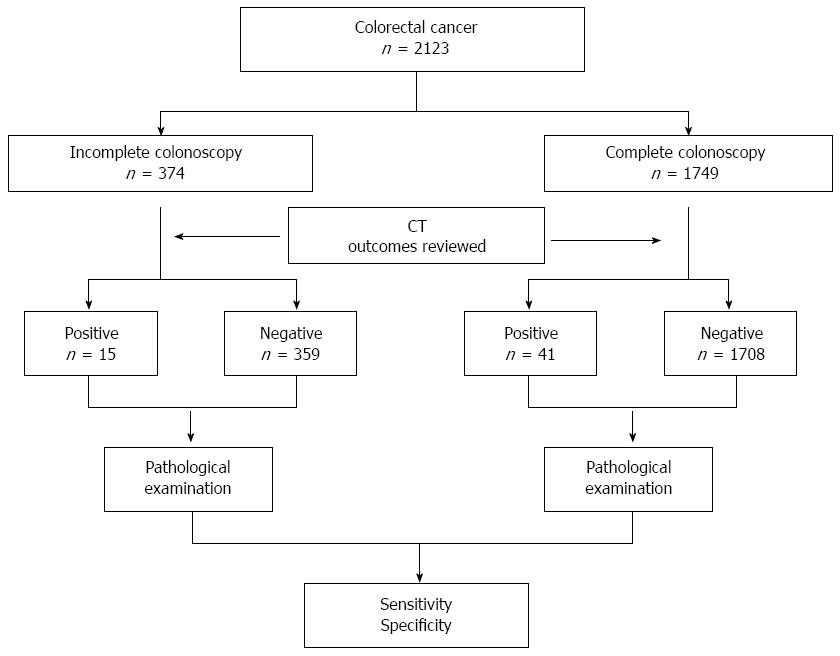
METHODS: A total of 2123 cases of colorectal cancer (CRC) were reviewed and divided into two groups according to whether a complete or incomplete colonoscopy was performed. CT results and final histological findings were compared to calculate the sensitivity and specificity associated with CT for detection of SCRCs following complete vs incomplete colonoscopy. Factors affecting the CT detection were also analyzed.
RESULTS: Three hundred and seventy-four CRC patients underwent incomplete colonoscopy and 1749 received complete colonoscopy. Fifty-six cases of SCRCs were identified by CT, and 36 were missed. In the incomplete colonoscopy group, the sensitivity and specificity of CT were 44.8% and 93.6%, respectively. The positive and negative predictive values were 23.6% and 95.0%, respectively. In contrast, the sensitivity and specificity of CT for the complete colonoscopy group were 68.3% and 97.0%, while the positive and negative predictive values were 22.2% and 98.7%, respectively. In both groups, the mean maximum dimension of the concurrent cancers identified in the CT-negative cases was shorter than in the CT-positive cases (incomplete group: P = 0.02; complete group: P < 0.01) Topographical proximity to synchronous cancers was identified as a risk factor for missed diagnosis (P = 0.03).
CONCLUSION: CT has limited sensitivity in detecting SCRCs in patients receiving incomplete colonoscopy. Patients with risk factors and negative CT results should be closely examined and monitored.
Core tip: This retrospective study aimed to evaluate the diagnostic accuracy of computed tomography (CT) in predicting synchronous colorectal cancers, especially in patients with incomplete colonoscopy. The study suggested that CT remains a feasible option when complete colonoscopy cannot be performed. However, the sensitivity of CT is limited due to several factors such as small tumor size, lumen conditions, and tumor location.
- Citation: Pang EJ, Liu WJ, Peng JY, Chen NW, Deng JH. Prediction of synchronous colorectal cancers by computed tomography in subjects receiving an incomplete colonoscopy: A single-center study. World J Gastroenterol 2015; 21(6): 1857-1864
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1857.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1857
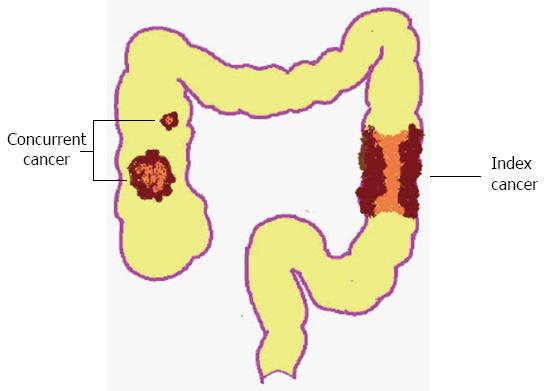
Cases of synchronous colorectal cancers (SCRCs) involve the detection of two or more primary colorectal carcinomas in a single individual upon an initial diagnosis of colorectal cancers (CRC). Currently, the Warren and Gates criteria[1] are used to diagnose SCRCs clinically. These criteria include the following key elements: (1) each tumor must be proved to be malignant; (2) each tumor must be distinct; (3) the probability of one tumor being a metastasis of another must be excluded; and (4) the synchronous lesions must be diagnosed simultaneously or within 6 mo of initial diagnosis[2]. In cases of SCRCs, the most advanced cancer (as determined by TNM staging) is usually defined as the index cancer, while the less-advanced tumors are considered concurrent lesions to the index cancer (Figure 1).
SCRCs are of major clinical importance because a missed diagnosis of concurrent lesions can lead to remnant cancer. The prognosis of SCRCs is typically identical to that of solitary cancer[3,4] as long as any synchronous lesions present are correctly diagnosed prior to surgery. Colonoscopy is ideal for detecting synchronous lesions, except when bowel preparation is inadequate. Moreover, the presence of a scirrhous carcinoma may hinder the passage of an endoscope through the lumen[5]. As a result, incomplete colonoscopy due to these or other factors can result in failure to detect additional lesions. In developing countries, such as China, conventional computed tomography (CT) is typically used to examine the remnant bowel not examined by colonoscopy because CT colonography (CTC) is generally not available. Therefore, the goal of this study was to evaluate the diagnostic accuracy of CT in predicting cases of SCRCs, especially in patients that have undergone incomplete colonoscopy.
Between January 2002 and August 2011, 2186 patients with CRC were treated at the Shanghai Sixth People’s Hospital. Sixty-three patients underwent colonoscopy or CT alone, and these cases were excluded. Cases of SCRCs were defined as patients with more than one primary colorectal adenocarcinoma present at the time of resection, or within 6 mo following resection. For each patient, the most advanced carcinoma according to T staging was identified as the index carcinoma, while the remaining less-advanced cancers were identified as concurrent carcinomas. If carcinomas with the same T stage were present, the lesion with the largest volume was identified as the index carcinoma. The remaining 2123 patients were divided into two groups according to those that underwent incomplete vs complete colonoscopy (Figure 2). The main reasons for incomplete colonoscopy included: colorectal obstruction, elongation of the colon, inadequate bowel preparation, intolerance to the procedure, and diverticular disease.
All patients included underwent preoperative CT using a Lightspeed VCT (GE Medical Systems, United States). Patients were examined in the supine position and received an intravenous injection of contrast medium. Images were obtained using a conventional method with 5-mm thick sections (120 KV, 350 mA). All of the images were retrospectively and independently reviewed by at least two radiologists with knowledge of each patient’s history, clinical presentation, and colonoscopy outcome. However, the radiologists were blinded to any information regarding operative findings or final diagnosis. Final CT interpretations had to be approved by both radiologists. If there were significant discrepancies, the images in question would be submitted to two additional senior radiologists for evaluation in order to obtain a final conclusion.
Detailed pathological characteristics of the concurrent lesions for this cohort were collected by two surgeons (RRS and QCZ), and these characteristics included: maximum lesion dimension, gross appearance, differentiation, and location. The pathological results were taken as the reference standard against which the CT findings were compared. Furthermore, the determination of positive or negative CT findings depended on whether both the index cancer and the concurrent cancers were simultaneously detected.
Overall sensitivity, specificity, positive predictive value, and negative predictive value for CT were calculated using cross-tabulation statistics. Fisher’s exact test or χ2 test was applied to categorical variables. Numerical data (mean maximum dimension and average distance of concurrent lesion to index tumor) are expressed as mean ± SD, and were compared using Student’s t test. A P value < 0.05 was considered statistically significant.
A total of 374 CRC patients underwent incomplete colonoscopy and four cases of SCRCs were missed due to poor bowel preparation. A total of 1749 patients received complete endoscopy and no case of SCRCs was missed. Cancer specimens were obtained from all 2123 patients either by surgery (n = 1916) or colonoscopy (n = 207). Using CT, 56 cases of SCRCs were identified, and 36 cases were not. Regarding the missed cases, 30 patients had SCRCs detected during surgery, while the remaining six were identified during postoperative colonoscopy that was performed up to 6 mo after surgery. There were no patients with local recurrence in the anastomosis. In addition, all of the cases of SCRCs in this cohort only had one concurrent lesion detected, and none of the patients had three or more CRCs simultaneously identified. There were no significant differences in patient sex, age, gross tumor appearance, cancer differentiation, location, or the presence of colonic obstruction for the incomplete and complete colonoscopy groups (Table 1).
| Concurrent lesions | Incomplete colonoscopy | P value | Complete colonoscopy | P value | ||
| CT+ (n = 13) | CT- (n = 16) | CT+ (n = 43) | CT- (n = 20) | |||
| Sex (M/F) | 8/5 | 11/5 | 26/17 | 16/4 | ||
| Median age (yr) | 63 | 69 | 66 | 63 | ||
| Mean maximum dimension (mm) | 38 ± 11 | 24 ± 5 | 0.02 | 37 ± 13 | 25 ± 9 | < 0.01 |
| Gross appearance | 0.20 | 0.18 | ||||
| Protruding | 9 | 7 | 23 | 10 | ||
| Ulcerated | 2 | 5 | 9 | 8 | ||
| Sclerotic | 2 | 4 | 11 | 2 | ||
| Differentiation | 0.17 | 0.06 | ||||
| Well | 3 | 4 | 9 | 8 | ||
| Moderate | 5 | 7 | 22 | 4 | ||
| Poor | 5 | 5 | 12 | 8 | ||
| Location | 0.71 | 0.41 | ||||
| Ascending colon | 5 | 4 | 14 | 6 | ||
| Transverse colon | 2 | 2 | 6 | 4 | ||
| Descending colon | 4 | 5 | 13 | 6 | ||
| Sigmoid colon | 2 | 3 | 8 | 1 | ||
| Rectum | 0 | 2 | 2 | 3 | ||
| Colonic obstruction | 0.46 | - | ||||
| Yes | 6 | 10 | 0 | 0 | ||
| No | 7 | 6 | 43 | 20 | ||
| Average distance to index tumor (cm) | 36.77 ± 6.86 | 19.88 ± 4.16 | 0.04 | 36.65 ± 3.55 | 24.00 ± 5.11 | 0.05 |
| Located in the same bowl segments with index tumor | 0.03 | 0.03 | ||||
| Yes | 3 | 11 | 13 | 12 | ||
| No | 10 | 5 | 30 | 8 | ||
The mean maximum dimension of the concurrent cancers identified in the CT-negative cases was shorter than that of the CT-positive cases, and this was independent of whether a complete or incomplete colonoscopy was performed (Table 1). Regarding the missed lesions for the two groups, the largest diameters were 65 mm and 45 mm for the incomplete and complete colonoscopy groups, respectively. Moreover, the smallest cancers visualized by CT for the two groups had mean maximum dimensions of 25 mm and 20 mm, respectively, and in both groups they presented as local thickening of the colon.
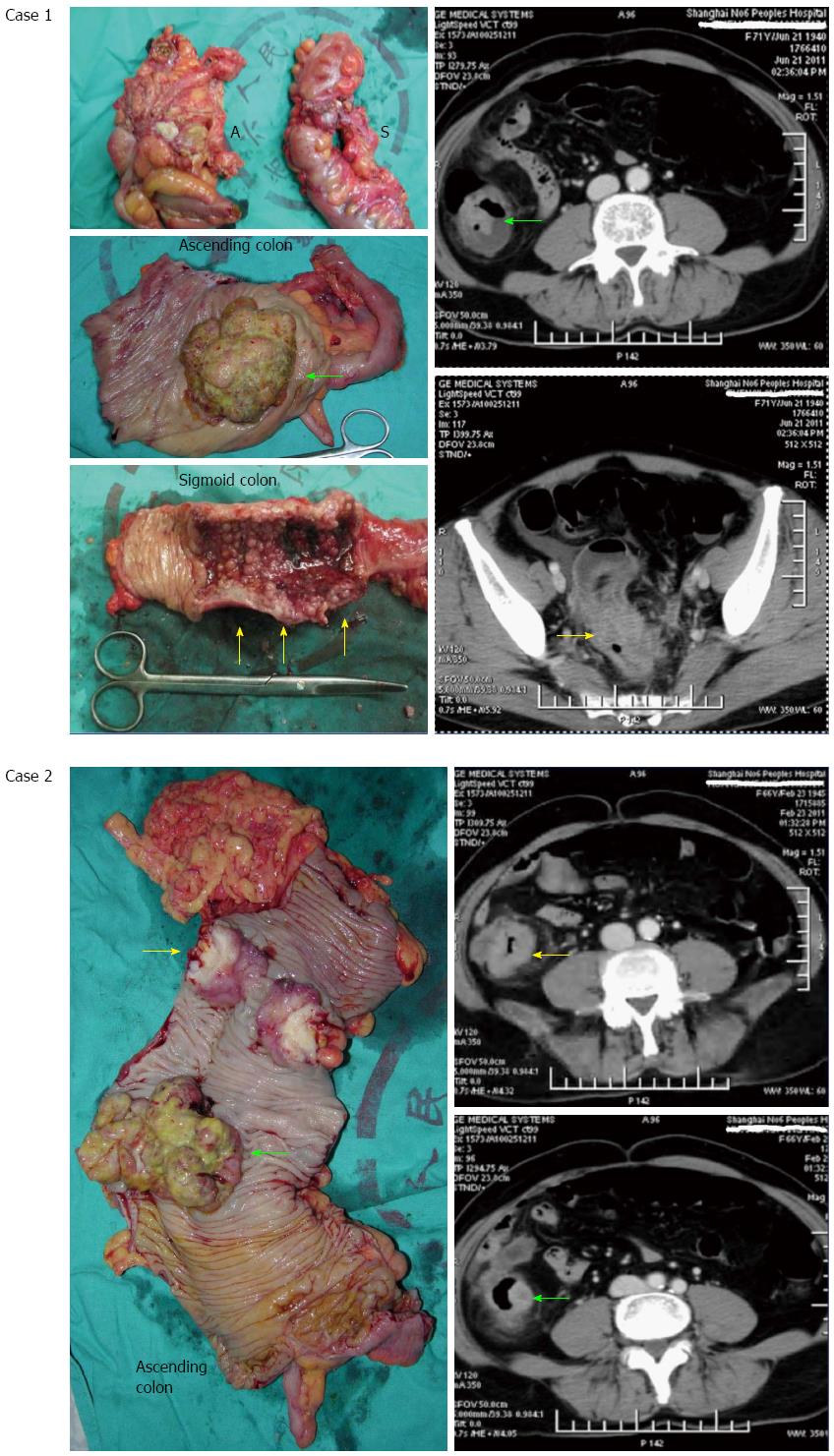
Concurrent cancers were located in the same bowel segment as the index cancers in 14 patients in the incomplete group and 25 in the complete group. Furthermore, > 60% of these concurrent cancers were missed by CT (Figure 3), with 68.8% (11/16) being in the incomplete colonoscopy group and 60.0% (12/20) in the complete colonoscopy group (Table 1). In contrast, 23.1% and 30.2% of cases, respectively, involved the index and concurrent cancers in different bowel segments (Table 1). Furthermore, the mean distance from index cancer to concurrent cancers was 36.77 cm (CT positive) vs 19.88 cm (CT negative) in the incomplete group and 36.65 cm (CT positive) vs 24.00 cm (CT negative) in the complete group. Therefore, topographical adjacency between index and concurrent cancers is one of the risk factors for missing diagnosis of SCRCs. The anatomical distribution of the index and concurrent cancers detected are listed in Table 2.
| Index lesion | Concurrent lesions | ||||
| Ascending colon | Transverse colon | Descending colon | Sigmoid colon | Rectum | |
| Incomplete group | |||||
| Ascending colon | 6 | 1 | 3 | 1 | 1 |
| Transverse colon | 0 | 2 | 1 | 0 | 0 |
| Descending colon | 2 | 1 | 4 | 1 | 0 |
| Sigmoid colon | 1 | 0 | 1 | 2 | 1 |
| Rectum | 0 | 0 | 0 | 1 | 0 |
| Total | 9 | 4 | 9 | 5 | 2 |
| Complete group | |||||
| Ascending colon | 10 | 1 | 4 | 3 | 1 |
| Transverse colon | 3 | 3 | 2 | 0 | 1 |
| Descending colon | 2 | 7 | 1 | 2 | |
| Sigmoid colon | 0 | 2 | 1 | 4 | 0 |
| Rectum | 2 | 2 | 5 | 1 | 1 |
| Total | 20 | 10 | 19 | 9 | 5 |
A histological examination revealed 92 cases of SCRCs in this cohort, with 29 in the incomplete group and 63 in the complete group. The corresponding sensitivity and specificity of CT for the incomplete group was 44.8% and 93.6%, respectively (Table 3), while the positive and negative predictive values were 23.6% and 95.0%, respectively. In contrast, these values for the complete colonoscopy group were 68.3%, 97.0%, 22.2% and 98.7%, respectively.
| CT findings | Histological findings | Sensitivity (%) | Specificity (%) | ||
| Positive | Negative | Total | |||
| Incomplete group | 44.8 (26.5, 64.3) | 93.6 (90.5, 96.0) | |||
| Positive | 13 | 22 | 35 | ||
| Negative | 16 | 323 | 339 | ||
| Complete group | 68.3 (55.3, 79.4) | 97.0 (96.0, 97.8) | |||
| Positive | 43 | 51 | 94 | ||
| Negative | 20 | 1635 | 1655 | ||
| Total | 92 | 2031 | 2123 | ||
The prevalence of SCRCs has been reported to range from 3% to 6%[3,6,7]. In the present study, the prevalence was 4.3% for 92 cases of SCRCs recorded within 10 years in a single-center database. Although the prevalence of SCRCs is not high, missed diagnosis can lead to errors in treatment and poor prognosis. Currently, colonoscopy is regarded as the best method for the direct detection of SCRCs. However, for cases that involve elongation of the colon[8], poor bowel preparation, diverticulitis, or bowel obstruction[9], colonoscopy may not be able to visualize entire segments of the colon. In a recent study, CTC was shown to be a valuable technique for preoperative colonic visualization of patients who had undergone incomplete colonoscopy[10,11]. Furthermore, positron emission tomography (PET)/CT has also been recommended as a supplementary technique to increase the accuracy of CTC[12]. However, the use of CTC is not widespread, mainly due to the lack of accepted guidelines for analysis of the results, and limited numbers of professionals with this experience[13]. In addition, most patients in developing countries cannot afford to undergo PET. Therefore, CT is a more accessible method, and the aim of the present study was to evaluate the predictive value of CT for cases of SCRCs, especially when colonoscopy was incomplete.
The overall sensitivity of CT in the present study was 44.8% for cases involving incomplete colonoscopy and 68.8% for complete colonoscopy. Both of these percentages are lower than those reported for solitary cancers detected by CT (range: 72%-84%)[14,15]. Moreover, the sensitivity of CT for colorectal neoplasms depends on the condition of the lumen and the size of the lesion. Correspondingly, sensitivity of CT can reach as high as 95% when the lumen is distended with air, yet can be as low as 68% when the colon is collapsed[15]. In the present series, > 50% of the incomplete colonoscopy cases were due to poor bowel preparation as a result of obstruction. In addition, concurrent lesions that were smaller in size were more likely to be neglected by CT in both the incomplete and complete colonoscopy groups. For example, the average maximum dimension in the CT-positive group was about 38 mm, and it was about 25 mm in the negative group. In most cases, smaller concurrent lesions also initially presented as local thickening on CT, and in the incomplete group were often obscured by remnant fecal material caused by poor bowel preparation. However, recent studies[16-18] have demonstrated that 7%-11% of cases involving colonic wall thickening detected by CT were predicted to have an underlying carcinoma, and colonoscopy was proposed. Taken together, these results indicate that missed diagnosis occurs in a certain proportion of SCRCs when complete colonoscopy is not additionally performed.
Another important factor associated with false-negative findings on CT in the present cohort was proximity of index and concurrent cancers within the same bowel segment. Previously, the right and left colon have been reported to be frequent sites of SCRCs[4]. Moreover, the topographical proximity of carcinomas on CT may lead to misdiagnosis of SCRCs as a solitary cancer (Figure 3). For example, two individual cancers in the same bowel segment could be misinterpreted as an extension of a single cancer by CT. Generally, the direction of CRC infiltration is not along the long axis of the bowel, but rather is circumferential. Therefore, when an abnormal length of colon involvement by cancer presents on CT, SCRCs should be considered. For these suspected cases, careful palpation should be performed at the beginning of an operation because the resection range of bowel segments depends on the location of the cancers. Furthermore, if the SCRCs can be detected initially, then an extended bowel segment procedure can be scheduled in advance. Alternatively, if the concurrent cancer is detected only after the resection of the index cancer, removal of an additional bowel segment may be needed. Although supplementary resection is not complex, it is inconsistent with the en bloc principle of radical cancer resection.
There were some limitations associated with the present study. First, this was a retrospective single-center study. Therefore, differences in experience and professionalism among the radiologists of this center may have influenced the detection accuracy of SCRCs by CT. Second, the CT instrument used in this study was older and may have had a lower detection rate for SCRCs. Third, the operator-dependent changes should be taken into consideration, which may also have influenced the imaging results. Therefore, the results of the present study need to be verified in a multicenter prospective investigation with a large number of cases that can be analyzed using up-to-date CT instruments.
In conclusion, the present study provides evidence regarding the capacity for CT to diagnose SCRCs, especially in patients that have undergone incomplete colonoscopy. The limited detection rate of CT that was observed may have been due to several factors such as small tumor size, lumen conditions, and tumor location. However, CTC and PET/CT are not readily available at all institutions, especially in developing countries, therefore, CT remains a feasible option when complete colonoscopy cannot be performed. Accordingly, it is important to identify the factors that can lead to false-negative results. Furthermore, both careful exploration during intraoperative colonoscopy[19,20] and close monitoring during the follow-up period are key to obtaining accurate diagnosis and treatment of SCRCs.
We thank Qing-Chao Zhu and Rong-Rong Shen for their assistance in reviewing the endoscopic and radiological results.
Synchronous colorectal cancers (SCRCs) refer to two or more primary colorectal cancers (CRCs) in a single individual with a prevalence ranging from 3% to 6%. SCRCs are clinically important because neglect of lesions leads to remnant cancer. Colonoscopy is ideal for detecting synchronous lesions but some factors may prevent the colonoscope from visualizing whole bowel segments. Conventional computed tomography (CT) is usually applied in these cases to examine the remnant bowel not accessible to colonoscopy.
CT colonography (CTC) is also known as virtual colonoscopy. With advancements made in scanning technology and three-dimensional (3D) postprocessing modalities, this method has been used to detect colorectal cancers, especially small tumors. CTC has been shown to be a valuable technique for preoperative colonic visualization of patients who have undergone incomplete colonoscopy. However, good bowel preparation is necessary for accurate diagnosis.
Currently, colonoscopy is regarded as the best method for the direct detection of SCRCs. However, for cases that involve elongation of the colon, poor bowel preparation, diverticulitis, or bowel obstruction, colonoscopy may not be able to visualize entire segments of the colon. CTC or positron emission tomography (PET) was shown to be valuable for preoperative detection of lesions in patients undergoing incomplete colonoscopy. PET and CTC in most patients in developing countries are not widespread, mainly due to limited numbers of well-trained professionals, and high expense. Therefore, CT is a more accessible method, and the present study was the first to evaluate the predictive value of CT for cases of SCRCs, especially when colonoscopy was incomplete.
The study suggested that CT remains a feasible option when complete colonoscopy cannot be performed. However, the sensitivity is limited when the tumor is small or index cancer is adjacent to concurrent cancers.
SCRCs refer to two or more primary CRCs consistent with the following key elements: (1) each tumor must be proved to be malignant; (2) each tumor must be distinct; (3) the probability of one tumor being a metastasis of another must be excluded; and (4) the synchronous lesions must be diagnosed simultaneously or within 6 mo of initial diagnosis.
Pang et al evaluated the diagnostic accuracy of CT in patients with SCRCs. It was a well-designed study, and the paper is well written. It addresses an interesting clinical area.
P- Reviewer: Chapel A, Miheller P, Zhi QM S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Warren S, Gates O. Carcinoma of ceruminous gland. Am J Pathol. 1941;17:821-826.3. [PubMed] |
| 2. | Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10:2136-2139. [PubMed] |
| 3. | Latournerie M, Jooste V, Cottet V, Lepage C, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous colorectal cancers. Br J Surg. 2008;95:1528-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Chen HS, Sheen-Chen SM. Synchronous and “early” metachronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum. 2000;43:1093-1099. [PubMed] |
| 5. | Neri E, Turini F, Cerri F, Faggioni L, Vagli P, Naldini G, Bartolozzi C. Comparison of CT colonography vs. conventional colonoscopy in mapping the segmental location of colon cancer before surgery. Abdom Imaging. 2010;35:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lam AK, Carmichael R, Gertraud Buettner P, Gopalan V, Ho YH, Siu S. Clinicopathological significance of synchronous carcinoma in colorectal cancer. Am J Surg. 2011;202:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Nosho K, Kure S, Irahara N, Shima K, Baba Y, Spiegelman D, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S. A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology. 2009;137:1609-20.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Hanson ME, Pickhardt PJ, Kim DH, Pfau PR. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol. 2007;189:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Morini S, Zullo A, Hassan C, Lorenzetti R, Campo SM. Endoscopic management of failed colonoscopy in clinical practice: to change endoscopist, instrument, or both? Int J Colorectal Dis. 2011;26:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | McArthur DR, Mehrzad H, Patel R, Dadds J, Pallan A, Karandikar SS, Roy-Choudhury S. CT colonography for synchronous colorectal lesions in patients with colorectal cancer: initial experience. Eur Radiol. 2010;20:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Liedenbaum MH, de Vries AH, van Rijn AF, Dekker HM, Willemssen FE, van Leerdam ME, van Marrewijk CJ, Fockens P, Bipat S, Bossuyt PM. CT colonography with limited bowel preparation for the detection of colorectal neoplasia in an FOBT positive screening population. Abdom Imaging. 2010;35:661-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kinner S, Antoch G, Bockisch A, Veit-Haibach P. Whole-body PET/CT-colonography: a possible new concept for colorectal cancer staging. Abdom Imaging. 2007;32:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Rockey DC. Computed tomographic colonography: ready for prime time? Gastroenterol Clin North Am. 2010;39:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ozel B, Pickhardt PJ, Kim DH, Schumacher C, Bhargava N, Winter TC. Accuracy of routine nontargeted CT without colonography technique for the detection of large colorectal polyps and cancer. Dis Colon Rectum. 2010;53:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Balthazar EJ, Megibow AJ, Hulnick D, Naidich DP. Carcinoma of the colon: detection and preoperative staging by CT. AJR Am J Roentgenol. 1988;150:301-306. [PubMed] |
| 16. | Patel P, Widjaja D, Blum S, Glandt M, Akella J, Chilimuri S, Balar B. Significance of bowel wall thickening on computed tomography scan: higher risk of pathology among African Americans compared to Hispanics. J Natl Med Assoc. 2009;101:345-348. [PubMed] |
| 17. | Wolff JH, Rubin A, Potter JD, Lattimore W, Resnick MB, Murphy BL, Moss SF. Clinical significance of colonoscopic findings associated with colonic thickening on computed tomography: is colonoscopy warranted when thickening is detected? J Clin Gastroenterol. 2008;42:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Moraitis D, Singh P, Jayadevan R, Cayten CG. Colonic wall thickening on computed tomography scan and clinical correlation. Does it suggest the presence of an underlying neoplasia? Am Surg. 2006;72:269-271. [PubMed] |
| 19. | Kaibara N, Kimura O, Nishidoi H, Miyano Y, Koga S. Intraoperative colonoscopy for the diagnosis of multiple cancers of the large intestine. Jpn J Surg. 1982;12:117-121. [PubMed] |
| 20. | Kuramoto S, Ihara O, Sakai S, Tsuchiya T, Oohara T. Intraoperative colonoscopy in the detection of nonpalpable colonic lesions--how to identify the affected bowel segment. Surg Endosc. 1988;2:76-80. [PubMed] |