Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1814
Peer-review started: May 24, 2014
First decision: July 15, 2014
Revised: October 20, 2014
Accepted: December 19, 2014
Article in press: December 22, 2014
Published online: February 14, 2015
Processing time: 263 Days and 6.7 Hours
AIM: To investigate the mechanisms by which Csk-binding protein (CBP) inhibits tumor progression in esophageal carcinoma.
METHODS: A CBP overexpressing esophageal carcinoma cell line (TE-1) was established. The growth, invasion, and migration of CBP-TE-1 cells, as well as the expression of Src were then determined and compared with those in normal TE-1 cells.
RESULTS: The expression of Src was decreased by the overexpression of CBP in TE-1 cells. The growth, invasion, and migration of TE-1 cells were decreased by the overexpression of CBP.
CONCLUSION: This study indicates that CBP may decrease the metastasis of esophageal carcinoma by inhibiting the activation of Src. CBP may be a potential tumor suppressor and targeting the CBP gene may be an alternative strategy for the development of therapies for esophageal carcinoma.
Core tip: Csk-binding protein (CBP) is a ubiquitously expressed transmembrane protein and functions as a suppressor of Src-mediated tumor progression by promoting the inactivation of Src. Here, we established a CBP overexpressing esophageal carcinoma cell line (TE-1) and found that the overexpression of CBP significantly decreased the proliferation, invasion, and migration of TE-1 cells, accompanied by decreased activation of Src. These results indicate that CBP may decrease the metastasis of esophageal carcinoma by inhibiting the activation of Src. Targeting the CBP gene may be an alternative strategy for the development of therapies for esophageal carcinoma.
- Citation: Zhou D, Dong P, Li YM, Guo FC, Zhang AP, Song RZ, Zhang YM, Li ZY, Yuan D, Yang C. Overexpression of Csk-binding protein decreases growth, invasion, and migration of esophageal carcinoma cells by controlling Src activation. World J Gastroenterol 2015; 21(6): 1814-1820
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1814.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1814
Csk-binding protein (CBP), a ubiquitously expressed transmembrane protein, functions as a suppressor of Src-mediated tumor progression by promoting the inactivation of Src[1,2]. CBP is widely reported to act as a scaffold in the Csk-mediated negative regulation of Src family kinases (SFKs)[3,4]. First, CBP is phosphorylated by SFKs, then it associates with C-terminal Src kinase (Csk) through a specific site (Tyr-317 in humans) and brings it into proximity with membrane-associated SFKs. After that, Csk phosphorylates the C-terminal negative regulatory tyrosine residue of SFKs, which suppresses their activation[1]. SFKs are membrane-associated non-receptor protein tyrosine kinases that play pivotal roles in regulating various cellular processes including proliferation, differentiation, adhesion, migration, and survival[5]. Thus, CBP plays the opposite role in various Csk-mediated cellular processes and might be a significant target for Csk-mediated tumors.
Recent studies have shown that CBP is expressed at low levels in various human cancer cells[6-8], suggesting that CBP may be an important suppressor in the progression of various human cancers. We have previously reported that the expression of CBP is markedly down-regulated in esophageal carcinoma[9]; however, the mechanisms by which the down-regulation of CBP affects the progression of esophageal carcinoma remain unknown. Therefore, we established an esophageal carcinoma cell line stably overexpressing CBP (TE-1). We found that overexpression of CBP decreased the growth, invasion, and migration of esophageal carcinoma cells.
The human esophageal carcinoma cell line TE-1 was provided by the Cell Bank of the Chinese Academy of Sciences, Shanghai, China. Cells were cultured in RPM1640 medium supplemented with 10% fetal bovine serum.
A lentiviral-delivered CBP vector was constructed and prepared by Shanghai qcbio Science & Technologies Co., Ltd. (Shanghai, China), as described by Lois et al[10]. Briefly, primers were designed according to the CBP sequence (Genbank Accession Number NC_000008.10). The primer sequences were: CBP-F, 5’-GGAATTCCCTGCCATGGGGCCCGCG-3’; CBP-R, 5’-GGAATTCGAGCCTGGT AATATCTCTGCCT-3’. The target gene was obtained by polymerase chain reaction and was inserted into the pUC57 vector. Subsequently, both pLenO-DCE and pUC57-CBP were digested by EcoRI and NotI. After ligation, the pLenO-DCE-CBP vector was constructed. After sequencing, the pLenO-DCE-CBP vector was transfected into 293T cells and the lentiviral-delivered CBP vector was prepared.
Briefly, 1 × 106 TE-1 cells were seeded in each well of a 6-well plate in 500 μL of complete medium at 37 °C in a 5% CO2 incubator for 24 h, and then transduced by lentiviral vectors at a multiplicity of infection of 10:1[11]. Transduction was carried out in the presence of polybrene (8 μg/mL). After washing three times with PBS, 1 mL of RPMI1640 was added in each well. Cells were seeded at 37 °C in a 5% CO2 incubator for 48 h. Fluorescence microscopy was used to observe the transduction. G418 (400 μg/mL) was used for screening. Transduced cells were passaged and seeded for further experiments. Also, the pLenO-DCE-(-) vector was transduced into TE-1 cell as a blank transfection control.
Cells (5 × 103) were seeded in a 96-well plate (BD Biosciences, United States) and harvested for the MTT assay at different time points from days 1-6. Cell samples were incubated with 20 μL of MTT (5 mg/mL; Sigma, United States) for 6 h. Following the removal of the MTT solution, formazan crystals were dissolved in 150 μL of dimethyl sulfoxide (DMSO, Sigma, United States). The absorption of the solution was measured at 570 nm[12].
Invasion chambers coated with Matrigel were purchased from BD Biosciences. Assays were conducted as described by Seton-Rogers et al[13]. Briefly, cells (1 × 105) were added to the top chambers (in 300 μL of RPMI1640) of 24-well Transwell plates (BD Biosciences; 8 μm pore size). After 24 h, the top (non-migrated) cells were removed, and the bottom (migrated) cells were fixed with 70% methanol and stained with trypan blue to visualize nuclei. The number of migrating cells in five fields was counted at 100× magnification, and the mean for each chamber was determined with ImageJ (version 1.38, National Institutes of Health). Experiments were repeated a minimum of three times.
Scratch assay was used for the detection of cell migration as described by Gough et al[14]. Briefly, cells (1 × 106) were seeded in a 6-well plate (BD Biosciences, United States) until cells reached 100% confluence. A p200 pipette tip was then used to create a scratch of the cell monolayer. After washing the plate once with PBS and replacing with the new medium, the cells were incubated at 37 °C in a 5% CO2 incubator. After 24 h, the number of cells that had migrated into the scratch was calculated at 100× magnification.
Cells were lysed on ice in RIPA buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 2 mmol/L sodium fluoride, 2 mmol/L Na3VO42, 1 mmol/L EDTA, and 1 mmol/L EGTA). Total protein extracts were analyzed by Western blot, as described previously[15]. Proteins (20 μg) were separated by SDS-PAGE (Invitrogen) and transferred to PVDF membranes. The membranes were blotted for 1 h with 5% milk. Membranes were incubated with a primary antibody (1:500 dilution) against CBP or Src (Santa Cruz Biotechnology, Inc., United States) at 4 °C overnight. After incubation with a horseradish peroxidase-conjugated secondary antibody (1:1000 dilution) for 3 h at 37 °C, signals were detected by ECL chemiluminescence for 5 min. The films were analyzed by densitometry with image software.
Data are expressed as mean ± SE and were statistically evaluated by one-way ANOVA followed by a Newman-Keuls test. P < 0.05 was considered statistically significant.
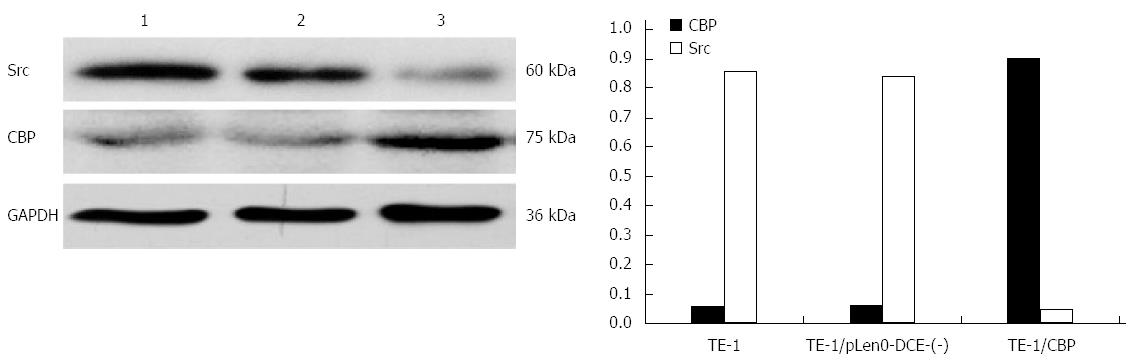
To check the overexpression of CBP in our stably transfected cells and to determine if overexpression of CBP leads to decreased expression of Src, we assessed the expression of Src in our TE-1 esophageal carcinoma cells that had been transduced with lentiviral constructs to overexpress CBP. As illustrated in Figure 1, our stably transduced cells overexpressed CBP, and CBP overexpression significantly decreased the protein levels of Src (P < 0.05) (Figure 1), supporting the previous finding that CBP down-regulates the activity of Src[1,2].
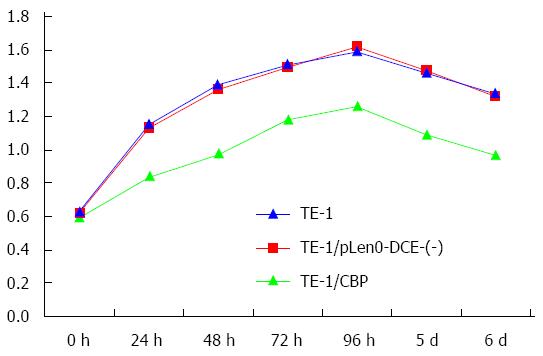
We assessed the cell growth of TE-1, pLenO-DCE-(-) vector transfected TE-1 (TE-1/pLenO-DCE) and CBP overexpressing TE-1 (TE-1/CBP) cells. As illustrated in Figure 2, compared with TE-1 and TE-1/pLenO-DCE cells, the cell growth of TE-1/CBP cells decreased significantly (P < 0.05) (Figure 2).
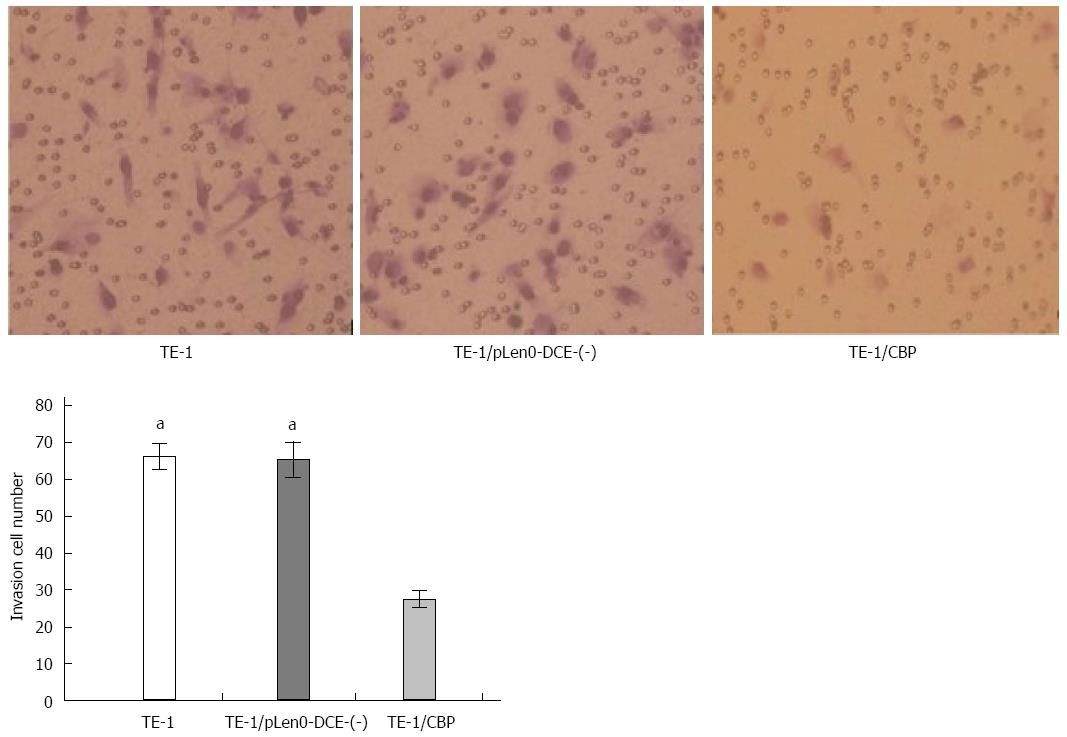
As illustrated in Figure 3, Transwell analysis showed that the numbers of TE-1 cells (62.2 ± 3.6) and TE-1/pLenO-DCE cells (65.4 ± 4.8) passing through the Matrigel were markedly higher than that of TE-1/CBP cells (27.6 ± 2.2) (P < 0.05) (Figure 3).
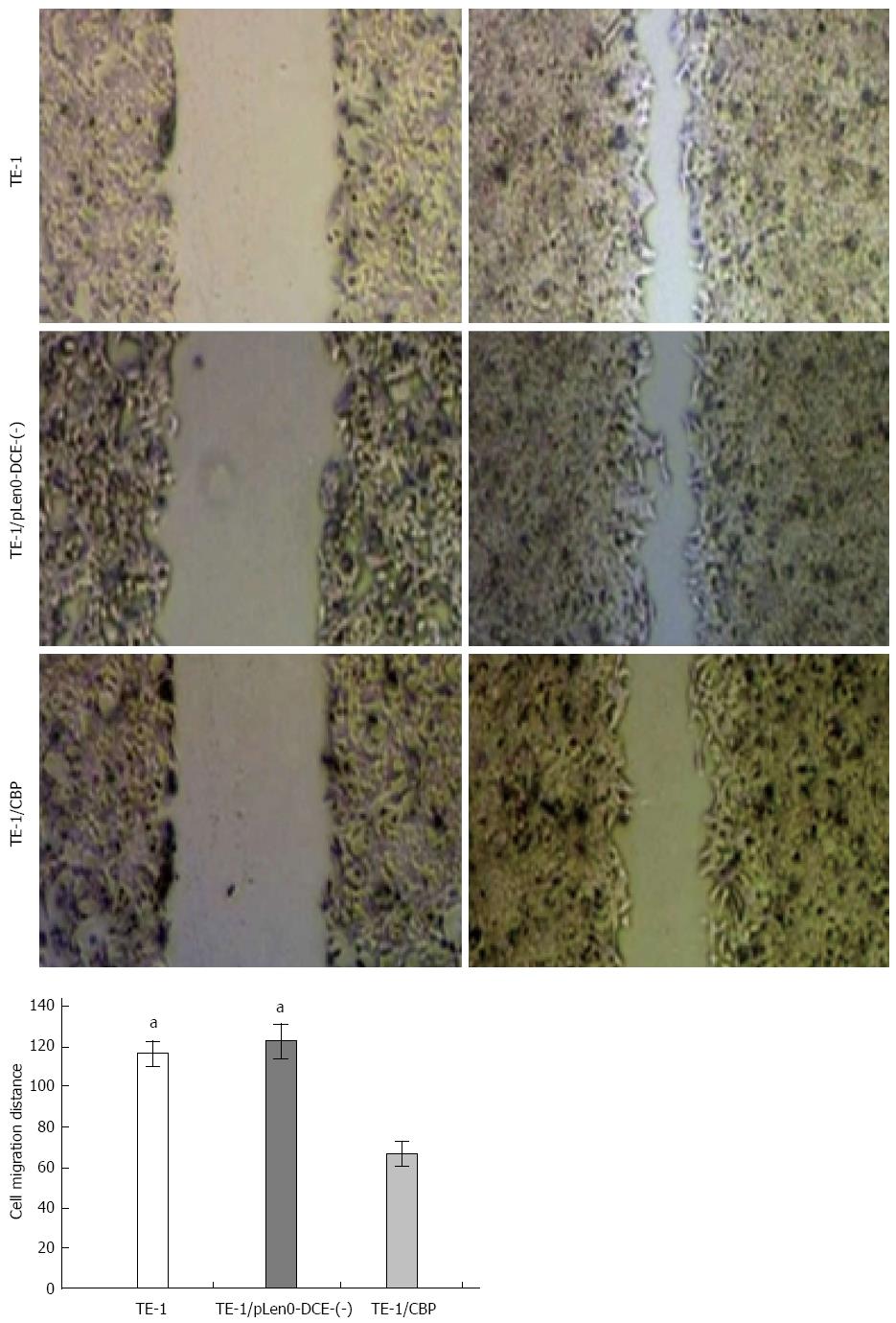
As illustrated in Figure 4, scratch analysis showed that the distance of TE-1 cells (116.2 ± 6.4 μm) and TE-1/pLenO-DCE cells (122.4 ± 8.8 μm) migrating into the scratch was markedly greater than that of TE-1/CBP cells (66.6 ± 6.2 μm) (P < 0.05) (Figure 3).
CBP is weakly expressed in many types of human cancers, including non-small cell lung cancer[7], esophageal cancer[9], and head and neck squamous cell cancer[16], suggesting that CBP may play an important suppressive role in the progression, development, and invasion of cancers. Recently, we reported that the expression of CBP is down-regulated in esophageal carcinoma[9], but the functions of CBP in esophageal carcinoma remain undetermined. To investigate the role of CBP in esophageal carcinoma, we examined the alteration of cell growth, invasion and migration of esophageal carcinoma cells after overexpressing CBP.
We up-regulated the expression of CBP by transfection of TE-1 esophageal carcinoma cells with CBP constructs. Our data demonstrate that CBP overexpression resulted in a decrease in the growth, invasion, and migration of TE-1 cells. These findings indicate that CBP plays an important role in the suppression of tumor progression. Since tumor progression inhibition is a prerequisite for efficient tumor therapy[17,18], CBP might be a promising target for tumor therapy. Our data also indicate that controlling the activation of the Src pathway may be one of the underlying mechanisms by which CBP up-regulates the growth, invasion and migration of esophageal carcinoma cells.
Src signaling, a prominent cancer cell growth-promoting and invasion-promoting pathway[19,20], can be activated by receptor tyrosine kinases and integrins to promote the growth, migration, and invasion of tumor cells. Although many studies have demonstrated the role of CBP in promoting the inactivation of Src signaling[1,2], we sought to investigate whether the up-regulation of CBP is able to decrease the growth, invasion, and migration of esophageal carcinoma cells through the inactivation of Src signaling. We found that CBP overexpression decreased the activity of Src in TE-1 esophageal carcinoma cells, and reduced esophageal carcinoma cell growth, invasion, and migration. Thus, we propose that CBP up-regulation decreases esophageal carcinoma progression, at least partially, through the inactivation of Src signaling. Further studies are needed to determine the molecular mechanism(s) by which CBP regulates Src signaling.
In summary, we demonstrated that CBP inhibits the growth, invasion, and migration of esophageal carcinoma cells by inactivating Src signaling, suggesting that CBP plays an important role in the inhibition of tumor progression and development of esophageal carcinoma. CBP as an effective target aiming to modulate Src signaling may be a reasonable approach to treating esophageal carcinoma.
Csk-binding protein (CBP) is a ubiquitously expressed transmembrane protein and functions as a suppressor of Src-mediated tumor progression by promoting the inactivation of Src. The authors previously reported that expression of CBP is markedly down-regulated in esophageal carcinoma; however, the mechanisms by which the down-regulation of CBP affects the progression of esophageal carcinoma remain unknown.
Previous studies have suggested that CBP has an opposing role in various Csk-mediated cellular processes and might be a significant target in Csk-mediated tumors. CBP is weakly expressed in many types of human cancers, including non-small cell lung cancer, esophageal cancer, and head and neck squamous cell cancer, suggesting that CBP may play an important suppressive role in the progression, development, and invasion of cancers.
The authors have reported that the expression of CBP is down-regulated in esophageal carcinoma, but the functions of CBP in esophageal carcinoma remain to be determined. To investigate the role of CBP in esophageal carcinoma, the authors examined the alterations in the growth, invasion, and migration of esophageal carcinoma cells overexpressing CBP. The data demonstrate that CBP overexpression resulted in a decrease in the growth, invasion, and migration of TE-1 cells. These findings indicate that CBP plays an important role in suppressing tumor progression.
This study indicates that CBP plays an important role in the inhibition of tumor progression and the development of esophageal carcinoma. CBP as an effective target aiming to modulate Src signaling may be a reasonable approach to treating esophageal carcinoma.
This study indicates that CBP overexpression resulted in decreased growth, invasion, and migration of TE-1 cells. These findings indicate that CBP plays an important role in suppressing tumor progression. These findings are interesting, and overall the writing is good.
P- Reviewer: Fujiwara N S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Ma S
| 1. | Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 423] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Jiang LQ, Feng X, Zhou W, Knyazev PG, Ullrich A, Chen Z. Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation. Oncogene. 2006;25:5495-5506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Matsuoka H, Nada S, Okada M. Mechanism of Csk-mediated down-regulation of Src family tyrosine kinases in epidermal growth factor signaling. J Biol Chem. 2004;279:5975-5983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Place AT, Chen Z, Bakhshi FR, Liu G, O’Bryan JP, Minshall RD. Cooperative role of caveolin-1 and C-terminal Src kinase binding protein in C-terminal Src kinase-mediated negative regulation of c-Src. Mol Pharmacol. 2011;80:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906-7909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 725] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 6. | Oneyama C, Hikita T, Enya K, Dobenecker MW, Saito K, Nada S, Tarakhovsky A, Okada M. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol Cell. 2008;30:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Kanou T, Oneyama C, Kawahara K, Okimura A, Ohta M, Ikeda N, Shintani Y, Okumura M, Okada M. The transmembrane adaptor Cbp/PAG1 controls the malignant potential of human non-small cell lung cancers that have c-src upregulation. Mol Cancer Res. 2011;9:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Suzuki K, Oneyama C, Kimura H, Tajima S, Okada M. Down-regulation of the tumor suppressor C-terminal Src kinase (Csk)-binding protein (Cbp)/PAG1 is mediated by epigenetic histone modifications via the mitogen-activated protein kinase (MAPK)/phosphatidylinositol 3-kinase (PI3K) pathway. J Biol Chem. 2011;286:15698-15706. [PubMed] |
| 9. | Zhou D, Zhang AP, Liu T, Li ZY, Yang YZ, Song RZ. [Expression of Csk-binding protein in esophageal carcinoma and its possible implications]. Nanfang Yike Daxue Xuebao. 2011;31:1781-1783. [PubMed] |
| 10. | Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1585] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 11. | Lee JS, Hmama Z, Mui A, Reiner NE. Stable gene silencing in human monocytic cell lines using lentiviral-delivered small interference RNA. Silencing of the p110alpha isoform of phosphoinositide 3-kinase reveals differential regulation of adherence induced by 1alpha,25-dihydroxycholecalciferol and bacterial lipopolysaccharide. J Biol Chem. 2004;279:9379-9388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Song KS, Li G, Kim JS, Jing K, Kim TD, Kim JP, Seo SB, Yoo JK, Park HD, Hwang BD. Protein-bound polysaccharide from Phellinus linteus inhibits tumor growth, invasion, and angiogenesis and alters Wnt/β-catenin in SW480 human colon cancer cells. BMC Cancer. 2011;11:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, Brugge JS. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA. 2004;101:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Gough W, Hulkower KI, Lynch R, McGlynn P, Uhlik M, Yan L, Lee JA. A quantitative, facile, and high-throughput image-based cell migration method is a robust alternative to the scratch assay. J Biomol Screen. 2011;16:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3’-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310-3319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, Freilino M, Nozawa H, Thomas SM, Gooding WE, Siegfried JM. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284-4291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010;51:523-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 467] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 19. | Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Michels S, Trautmann M, Sievers E, Kindler D, Huss S, Renner M, Friedrichs N, Kirfel J, Steiner S, Endl E. SRC signaling is crucial in the growth of synovial sarcoma cells. Cancer Res. 2013;73:2518-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 758] [Reference Citation Analysis (0)] |