Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1804
Peer-review started: July 26, 2014
First decision: August 27, 2014
Revised: September 19, 2014
Accepted: October 21, 2014
Article in press: October 21, 2014
Published online: February 14, 2015
Processing time: 201 Days and 6.7 Hours
AIM: To investigate the role of the overexpression of B7-H3 in apoptosis in colorectal cancer cell lines and the underlying molecular mechanisms.
METHODS: SW620 cells that highly overexpressed B7-H3 (SW620-B7-H3-EGFP) and HCT8 cells stably transfected with B7-H3 shRNA (HCT8-shB7-H3) were previously constructed in our laboratory. Cells transfected with pIRES2-EGFP were used as negative controls (SW620-NC and HCT8-NC). Real-time PCR and western blotting analysis were used to detect the mRNA and protein expressions of the apoptosis regulator proteins Bcl-2, Bcl-xl and Bax. A cell proliferation assay was used to evaluate the survival rate and drug sensitivity of the cells. The effect of drug resistance was detected by a cell cycle assay. Active caspase-3 western blotting was used to reflect the anti-apoptotic ability of cells. Western blotting was also performed to determine the expression of proteins associated with the Jak2-STAT3 signaling pathway and the apoptosis regulator proteins after the treatment with AG490, a Jak2 specific inhibitor, in B7-H3 overexpressing cells. The data were analyzed by GraphPad Prism 6 using a non-paired t-test.
RESULTS: Whether by overexpression in SW620 cells or downregulation in HCT8, B7-H3 significantly affected the expression of anti- and pro-apoptotic proteins, at both the transcriptional and translational levels, compared with the negative control (P < 0.05). A cell proliferation assay revealed that B7-H3 overexpression increased the drug resistance of cells and resulted in a higher survival rate (P < 0.05). In addition, the results of cell cycle and active caspase-3 western blotting proved that B7-H3 overexpression inhibited apoptosis in colorectal cancer cell lines (P < 0.05). B7-H3 overexpression improved Jak2 and STAT3 phosphorylation and, in turn, increased the expression of the downstream anti-apoptotic proteins B-cell CLL/lymphoma 2 (Bcl-2) and Bcl-xl, based on western blotting (P < 0.05). After treating B7-H3 overexpressing cells with the Jak2-specific inhibitor AG490, the phosphorylation of Jak2 and STAT3, and the expression of Bcl-2 and Bcl-xl, decreased accordingly (P < 0.05). This finding suggested that the Jak2-STAT3 pathway is involved in the mechanism mediating the anti-apoptotic ability of B7-H3.
CONCLUSION: The overexpression of B7-H3 induces resistance to apoptosis in colorectal cancer cell lines by upregulating the Jak2-STAT3 signaling pathway, potentially providing new approaches to the treatment of colorectal cancer.
Core tip: The expression of B7-H3 has been positively correlated with poor prognosis in colorectal cancer. Previous studies revealed the relationship between B7-H3 and tumor invasion and metastasis. In the present study, the role of B7-H3 in apoptosis in colorectal cancer was investigated. Our results showed that overexpression of B7-H3 induced resistance to apoptosis in colorectal cancer cell lines by upregulating the Jak2-STAT3 signaling pathway. These results provide a new vision for designing therapeutics targeting B7-H3 and its associated signaling pathways in the treatment of colorectal cancer.
- Citation: Zhang T, Jiang B, Zou ST, Liu F, Hua D. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol 2015; 21(6): 1804-1813
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1804.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1804
B7-H3, first identified in 2001, is a member of the human B7 family of proteins, sharing 20%-27% amino acid identity with other B7 family members. As an important co-stimulatory molecule, B7-H3 promotes the proliferation of T cells and induces interferon (IFN)-γ production in the presence of T cell receptor signaling[1]. However, B7-H3 also acts as a T cell co-inhibitor. Most of the published data support the notion that B7-H3 inhibits T cell activation. Both mouse and human B7-H3 inhibit CD4 T cell activation and the production of effector cytokines such as IFN-γ and interleukin (IL)-4[2]. However, the function of B7-H3 in natural immunity and cancer immunity remains unclear.
The expression of B7-H3 in human tumor cells is positively correlated with the degree of disease malignancy, and B7-H3 participates in the process of tumor cell immune escape[3]. B7-H3 is highly expressed in many types of solid tumors, such as prostate cancer[4], pancreatic cancer[5], breast cancer[6], and gastric cancer[7]. Previous studies demonstrated a relationship between the expression of B7-H3 and poor prognosis in cancer patients[5,8,9]. The expression of B7-H3 is also closely related to colorectal cancer (CRC)[10]. Expression of B7-H3 not only has a negative relationship with prognosis in CRC[11] and the number of T cells in the tumor microenvironment[12] but also is positively correlated with invasion[13] and metastasis[14] in CRC.
The relationship between B7-H3 and the outcome of CRC cannot simply be explained by the regulation of B7-H3 in the immune system. The mechanism of abnormal B7-H3 expression in CRC and its role in the changes of tumor biological behavior need to be determined. Apoptosis, the process of programmed cell death, is an important field in tumor study[15]. However, few published papers have studied the relationship between B7-H3 and apoptosis, particularly in CRC. Therefore, we focused on the function of B7-H3 in apoptosis in CRC cells to discover the signal transduction pathway involved.
Anti-human B7-H3, Bcl-2, Bcl-xl, Jak2, pJak2Tyr1007/1008, STAT3, pSTAT3Tyr705, and active caspase-3 antibodies were purchased from Abcam (Cambridge, MA, United States). An antibody against Bcl-2-associated X protein (Bax) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, United States). The horseradish peroxidase conjugated secondary anti-mouse and anti-rabbit antibodies and the GAPDH antibody were from Beyotime (Nantong, China). Tryphostins AG490 was from Sigma-Aldrich (St. Louis, MO, United States). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratory (Kumamoto, Japan).
Two human CRC cell lines, SW620 and HCT8, exhibited different expression levels of B7-H3. We constructed SW620 cells that expressed high level of B7-H3 (SW620-B7-H3-EGFP), and HCT8 cells stably transfected with B7-H3 shRNA (HCT8-shB7-H3). Cells transfected with pIRES2-EGFP were used as negative controls (SW620-NC and HCT8-NC). All cells were cultured in Dulbecco’s high glucose modified eagles medium (DMEM) (HyClone GE Healthcare Life Sciences, South Logan, UT, United States) supplemented with 10% fetal bovine serum at 37 °C in a humidified atmosphere with 5% CO2. AG490, a Jak2 protein tyrosine kinase inhibitor, was dissolved in DMSO at a final concentration of 100 μmol/L. Clinical chemotherapeutics Oxaliplatin (L-OHP) and 5-fluorouracil (5-Fu) were used to detect the anti-apoptotic ability of cancer cells.
Total RNA was isolated from 1.5 × 106 cells using TRIzol, following the manufacturer’s instructions and quantified by a NanoDrop 2000 (Thermo Scientific, Waltham, MA, United States). Total RNA was treated with RNase-free DNase to remove residual genomic DNA. The first strand cDNA was synthesized from 1 μg RNA using an oligo-dT primer and AMV reverse transcriptase.
The expression levels of B7-H3, Bcl-2, Bcl-xl and Bax were analyzed relative to the level of the β-actin gene transcript using a Prism 7300 real-time polymerase chain reaction (PCR) instrument (Applied Biosystems Inc., Foster City, CA, United States). First-strand cDNA was amplified in a 20 μL PCR reaction mixture: 10 μL 2 × SYBR green PCR master mix, 0.4 μL 50 × ROX, 0.4 μL of each specific primer sets, and ddH2O added to 20 μL. The sequences of primers were as follows: β-actin 5’- AGCGAGCATCCCCCAAAGTT-3’ (sense), 5’- GGGCACGAAGGCTCATCATT-3’ (antisense); B7-H3 5'-AGCACTGTGGTTCTGCCTCACA-3' (sense), 5'-CACCAGCTGTTTGGTATCTGTCAG-3' (antisense); Bcl-2 5’-CTGCACCTGACGCCCTTCACC-3’ (sense), 5’-CACATGACCCCACCGAACTCAAAGA-3’ (antisense); Bcl-xl 5’-GATCCCCATGGCAGCAGTAAAGCAAG-3’ (sense), 5’-CCCCATCCCGGAAGAGTTCATTCACT-3’ (antisense); Bax 5’-TCAACTGGGGCCGGGTTGTC-3’ (sense), 5’-CCTGGTCTTGGATCCAGCC-3’ (antisense). The PCR cycling consisted of 40 cycles of amplification of the cDNA with annealing at 60 °C.
Western blotting was performed on whole-cell extracts prepared by lysing 1 × 106 cells in RIPA lysis buffer containing phosphatase inhibitor, protease inhibitor and 100 mmol/L PMSF (KeyGEN BioTECH, China) for 20 min on ice. The proteins were separated by 10% SDS-PAGE, except for active caspase-3 (15%), and then transferred onto a PVDF membrane (Merck Millipore, Germany). The membranes were blocked with 5% nonfat dry milk for 1 h at room temperature, and then incubated with the indicated antibodies at a concentration of 1:1000, except for Bax (1:100), at 4 °C overnight, followed by incubation with secondary antibody for 1 h at room temperature. The immunoreactive bands were visualized using Beyo ECL Plus (Beyotime, China).
Cells were seeded in 96-well plates at 5 × 103-8 × 103 cells/well and cultured overnight in DMEM. The next day, the medium was replaced with DMEM containing the different drugs with a two-fold concentration gradient. After 48 h, cell proliferation was quantified by a CCK-8 assay to calculate the inhibition rate. The assays were repeated at least three times.
To analyze the effect of drugs on the different phases of the cell cycle, cells were incubated with different drug concentrations for 48 h. Cells harvested from each sample were then fixed with cold 70% ethanol at 4 °C overnight. The cells then were incubated with RNase A at 37 °C for 30 min and stained for 30 min in propidium iodide staining solution in the dark. Cell cycle analyses were performed with a FACScantoII flow cytometer and ModFit LT software (Verity Software House, ME, United States). For apoptosis analyses, we referred to the data of the sub-G1 peak.
Differences in mean values between groups were analyzed by a non-paired t-test. At least three independent experiments were performed for all the studies. Differences were considered to be statistically significant when P values were < 0.05. All of the data were analyzed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, United States).
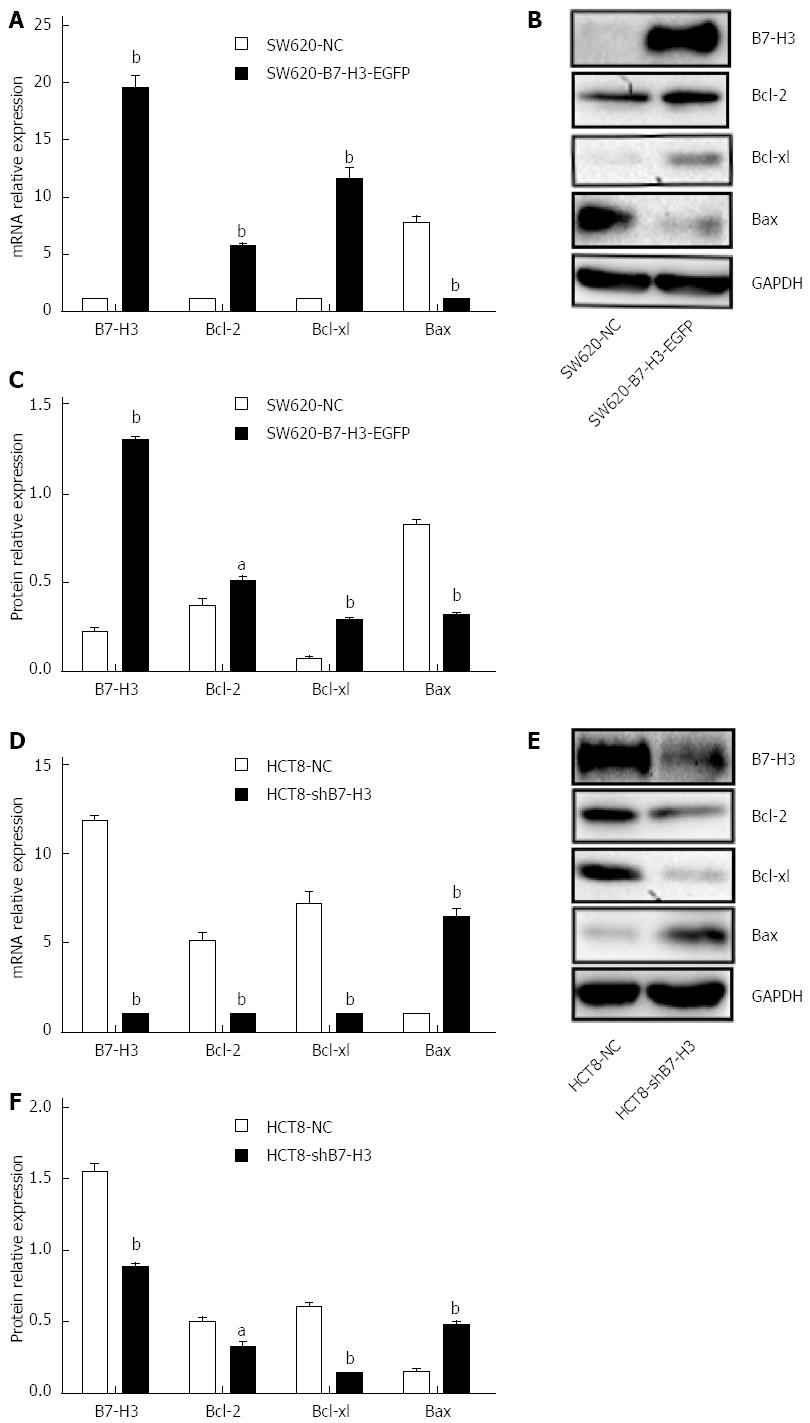
To investigate the relationship between B7-H3 and apoptosis in CRC cell lines, we performed western blotting with cell extracts from SW620-NC, SW620-B7-H3-EGFP, HCT8-NC and HCT8-shB7-H3 to demonstrate the expression of the apoptosis regulator proteins of the Bcl-2 family, including the anti-apoptotic proteins Bcl-2 and Bcl-xl and the pro-apoptotic protein Bax (Figure 1). Both B7-H3 overexpression in SW620 cells and downregulation in HCT8 cells affected the expression of anti- and pro-apoptotic proteins, at both the transcriptional and translational levels. In SW620-B7-H3-EGFP, the anti-apoptotic proteins Bcl-2 and Bcl-xl showed increased expression compared with SW620-NC (P < 0.05), while expression of the pro-apoptotic protein Bax decreased (P < 0.05). We observed a similar phenomenon in HCT8 cells (P < 0.05). The expressions of B7-H3 and the anti-apoptotic proteins were positively correlated in CRC cell lines. This suggested that the overexpression of B7-H3 might increase the resistance to apoptosis in tumor cells.
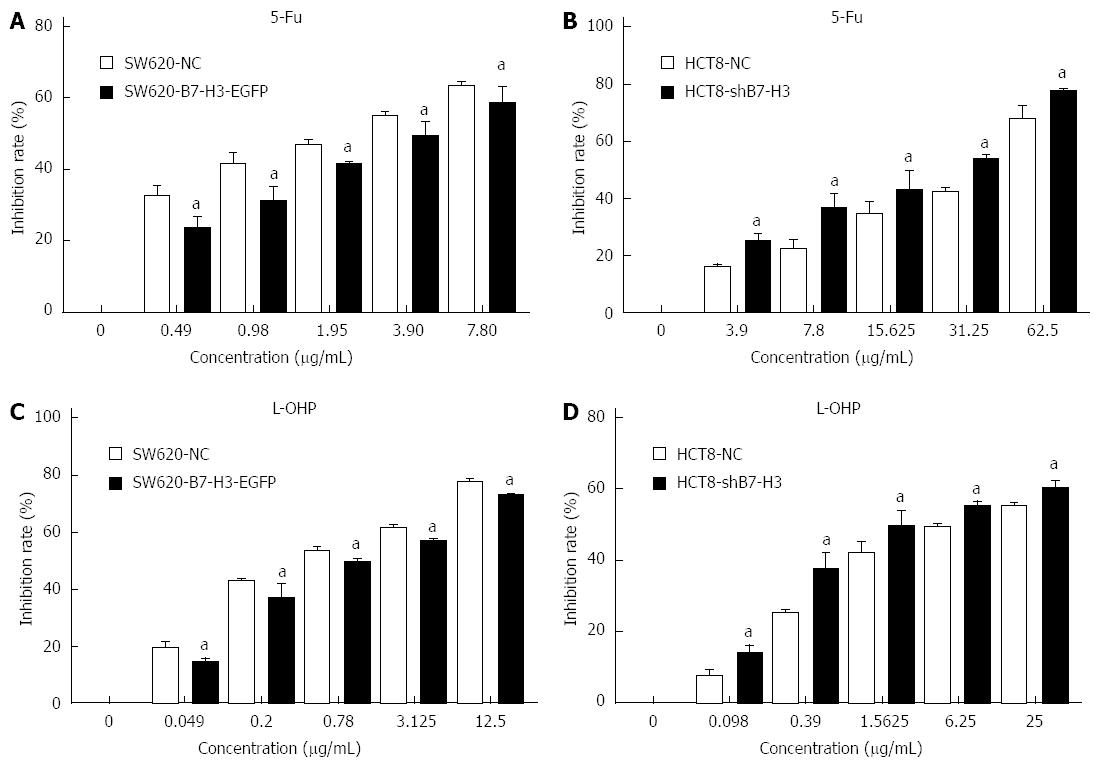
To investigate whether B7-H3 altered the survival of CRC cells after chemotherapeutic treatment, we used a cell proliferation assay to detect the inhibition rate of SW620-NC, SW620-B7-H3-EGFP, HCT8-NC and HCT8-shB7-H3 treated with different concentrations of L-OHP and 5-Fu for 48 h (Figure 2). After treatment with L-OHP or 5-Fu at any concentration, the inhibition rate of SW620-B7-H3-EGFP was less than that of SW620-NC (P < 0.05). The HCT8 cells showed similar results (P < 0.05). Therefore, we hypothesized that overexpression of B7-H3 increased the cells’ resistance to drugs, resulting in a higher survival rate in the cells that overexpressed B7-H3.
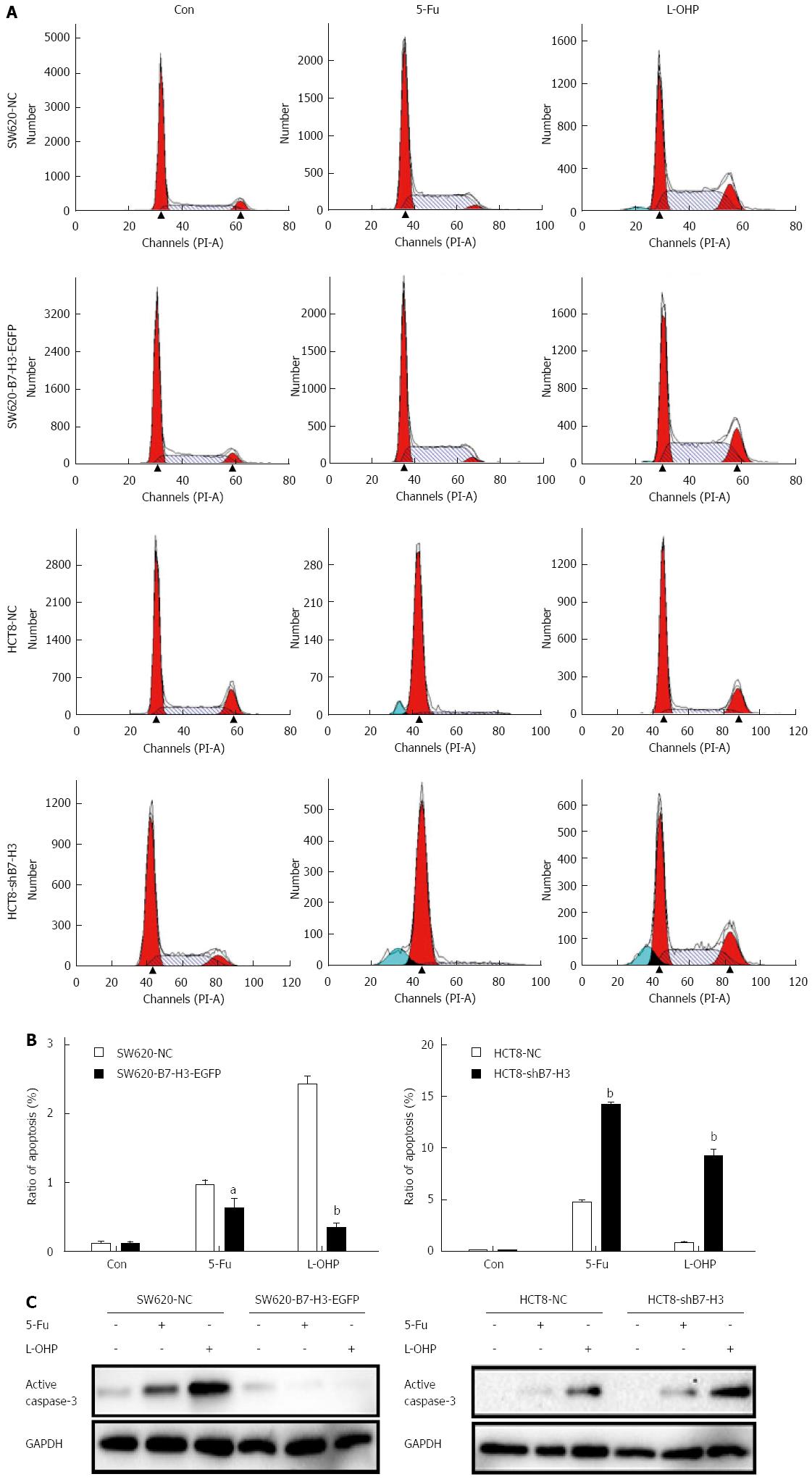
We described the increased anti-apoptotic effect in the B7-H3 overexpressing cells above. To investigate the exact response to apoptosis resulting from chemotherapeutic treatment, we treated SW620-NC, SW620-B7-H3-EGFP, HCT8-NC and HCT8-shB7-H3 cells with a high concentration (50 μg/mL) of 5-Fu or L-OHP for 48 h. A cell cycle assay was used to detect the rate of apoptosis in each cell line, according to the sub-G1 peak (Figure 3A and B). We found that SW620-B7-H3-EGFP had stronger resistance to 5-Fu or L-OHP and less apoptosis compared with SW620-NC (P < 0.05). Similar results were found in the HCT8 cells (P < 0.01). We used western blotting to detect active caspase-3 and compare apoptosis rates pairwise (Figure 3C). Similarly, the expression of active caspase-3 in B7-H3 overexpressing cells was less than that seen in cells with downregulated B7-H3. We concluded that overexpression of B7-H3 could inhibit apoptosis in CRC cell lines.
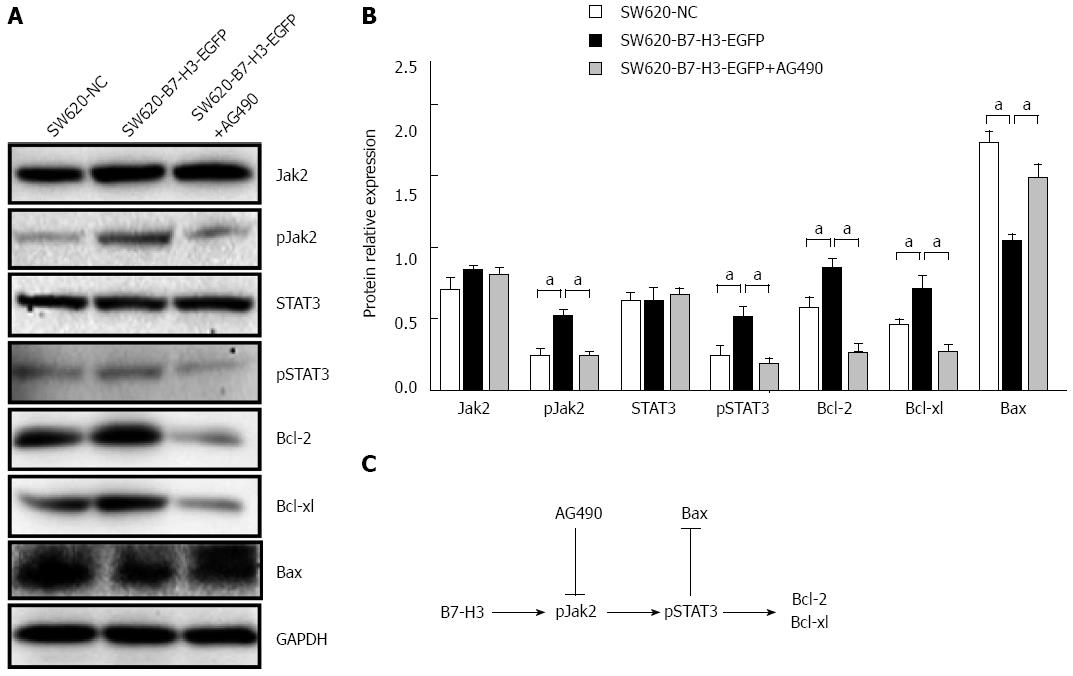
We observed chemoresistance accompanied by decreased apoptosis in 5-Fu or L-OHP treated B7-H3 overexpressing cells; therefore, we investigated which signaling pathway was involved in the apoptotic response. The Jak2-STAT3 pathway was reported to regulate anti-apoptotic molecules downstream of B7-H3 in breast cancer cells[16]. As can be seen in Figure 1A, the overexpression of B7-H3 indeed upregulated the expression of anti-apoptotic proteins. We therefore asked whether the Jak2-STAT3 pathway played the same role in CRC cells. We treated SW620-B7-H3-EGFP cells with the Jak2-specific inhibitor AG490 for 24 h at final concentration of 1 μmol/L. We performed western blotting with whole-cell lysates from SW620 cells to detect the expression of Jak2, STAT3 and their phosphorylated forms. To investigate the involvement of the Jak2-STAT3 pathway in the anti-apoptotic effect of B7-H3, we also assayed the expression of downstream apoptotic regulator proteins by western blotting (Figure 4). We observed that the phosphorylation levels of Jak2 and STAT3 increased following B7-H3 overexpression (P < 0.05). After AG490 treatment, the phosphorylation level of STAT3 was almost abolished because of the inhibition of Jak2 tyrosine phosphorylation (P < 0.05). This result indicated that the effect of B7-H3 on STAT3 occurred through Jak2. The expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl decreased with reduced expression of phosphorylation level of Jak2 and STAT3 (P < 0.05), and the expression of the pro-apoptotic protein Bax increased correspondingly (P < 0.05). In summary, these results suggested that B7-H3 overexpression increased Jak2 phosphorylation, leading to higher STAT3 phosphorylation, which, in turn, led to the increased expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl. Thus, we confirmed that the Jak2-STAT3 signaling pathway played an important role in regulating the anti-apoptotic ability of B7-H3.
In this study, the role of B7-H3 in apoptosis in colorectal cancer cell lines was investigated. Overexpression of B7-H3 increased the anti-apoptotic ability and resistance to chemotherapeutics, whereas knockdown of B7-H3 led to increased sensitivity to drug-induced apoptosis. Furthermore, we proved that B7-H3 regulated the expression of Bcl-2, Bcl-xl and Bax via the Jak2-STAT3 signaling pathway to affect the anti-apoptotic ability of cancer cells.
First, we examined the B7-H3 expression level in CRC cell lines. B7-H3 was expressed at different levels in all cell lines. SW620 had lower B7-H3 expression, while HCT8 had higher expression. SW620 was therefore chosen to generate a B7-H3 upregulated stably transfected derivative cell line, and HCT8 was chosen to construct a B7-H3 downregulated stably transfected derivative cell line. Four stably transfected CRC cell lines were generated: the B7-H3 overexpressing cell line SW620-B7-H3-EGFP, the B7-H3 knockdown cell line HCT8-shB7-H3, and the control cell lines SW620-NC and HCT8-NC.
The apoptosis regulator Bcl-2 is a member of a family of evolutionarily related proteins. These proteins can be either pro-apoptotic (including Bax, Bad, Bak and Bok) or anti-apoptotic (including Bcl-2 proper, Bcl-xl, and Bcl-w). Bcl-2 is one of the most important oncogenes in apoptosis research[17,18]. In our study, overexpression of B7-H3 caused the CRC cells to be more resistant to apoptosis because of the upregulation of Bcl-2 and Bcl-xl and the downregulation of Bax, thus reducing the sensitivity of cells to chemotherapeutics. In contrast, knockdown of B7-H3 increased drug-induced apoptosis. This is consistent with Zhao’s investigation, in which silencing of B7-H3 increased the sensitivity of the human pancreatic carcinoma cell line Patu8988 to gemcitabine as a result of enhanced drug-induced apoptosis[19]. This suggested that overexpression of B7-H3 in CRC patients made them inappropriate candidates for treatment with chemotherapeutics. This may also explain why the expression of B7-H3 is associated with poor prognosis in CRC[11]. It may be important to downregulate the expression of B7-H3 in patients for them to benefit from drug-induced apoptosis.
STAT3 is a transcription factor that mediates the expression of a variety of genes in response to cell stimuli[20,21], and thus plays a key role in many cellular processes, such as cell growth[22] and apoptosis[23]. It is activated through phosphorylation by the non-receptor tyrosine kinase Jak2, and high activity has been shown to predict resistance to chemotherapeutics resulting from the upregulation of anti-apoptotic proteins[24]. Jak2-STAT3 signaling, while regulating many aspects of cancer development and progression, promotes invasion and metastasis[25]. Interestingly, we found that upregulation of B7-H3 activated the phosphorylation of both Jak2 and STAT3, which led to the increased expression of Bcl-2 and Bcl-xl. This may explain why B7-H3 overexpressing cells were more resistant to drug-induced apoptosis. In a previous study, Liu et al[16] reported a similar finding in breast cancer. In that study, they only generated a B7-H3 knockdown model, and lacked a functional recruitment experiment. Our results confirmed the relationship between B7-H3 and the Jak2-STAT3 signal transduction pathway by both B7-H3 downregulation and overexpression models. Furthermore, the blockage of Jak2 phosphorylation by its specific inhibitor AG490 resulted in a reduction in STAT3 phosphorylation and expression of anti-apoptotic proteins in B7-H3 overexpressing cells. AG490, a specific Jak2 inhibitor, could block B7-H3 regulation of apoptosis related proteins, providing more insight into Jak2-STAT3 signal transduction and B7-H3.
A great deal of evidence exists that reinforces the link between inflammation and colorectal cancer[26-28]. The molecular pathobiology of CRC implicates pro-inflammatory conditions in promoting the progression of tumor malignancy, invasion and metastasis[29]. Patients with inflammatory bowel disease are at higher risk of CRC[30]. Furthermore, the Jak2-STAT3 signaling pathway mediates the progression of inflammation[31,32]. Activators of the Jak2-STAT3 signaling pathway are important factors released during inflammation[33]. In particular, anti-inflammatory cytokines such as IL-10 activate STAT3 phosphorylation via Jak2[34]. Oxidative stress and cytokines such as IL-6 also activate STAT3 by a Jak2-dependent mechanism[35]. The primary consequence of the activation of this pathway is to promote inflammation-associated gene expression; however, pathway activation also regulates survival-associated gene expression[36]. The role of B7-H3 overexpression in tumor cells in activating the Jak2-STAT3 signaling pathway augmented the activation of inflammation by Jak2-STAT3. This partially explained the reports of the negative relationship between B7-H3 and prognosis of CRC.
In summary, our study investigated the impact of the overexpression of B7-H3 in resistance to apoptosis mediated by the Jak2-STAT3 signaling pathway. We focused on the non-immunological function of B7-H3 in CRC. These results suggest that new CRC treatments could target B7-H3 overexpression or associated signaling pathways in tumors as a novel approach to weaken drug resistance.
Expression of B7-H3 has been positively correlated with poor prognosis in colorectal cancer (CRC). Previous studies have revealed the relationship between B7-H3 and tumor invasion and metastasis. However, the function of B7-H3 in apoptosis and the molecular mechanism involved in remains obscure.
This study was performed to explore the role of B7-H3 in apoptosis in CRC cell lines through cellular and molecular biological methods. CRC cell lines that either up- or downregulated B7-H3 expression were constructed to detect the related indicators of apoptosis, such as the expression of apoptosis regulator proteins, the cell cycle and the expression of active Caspase-3 with drug treatment. Furthermore, the molecular mechanism of B7-H3 in regulating apoptosis was also discussed in detail.
This study showed that overexpression of B7-H3 induces resistance to apoptosis in issue 7 cell lines by upregulating the Jak2-STAT3 signaling pathway.
These results provide a new paradigm for designing treatment strategies targeting B7-H3 and signaling pathways in CRC treatment.
This study provides novel and interesting insights into the function of B7-H3 on tumor cells in addition to its co-inhibitory role in T cell activation.
P- Reviewer: Pfistershammer K S- Editor: Yu J L- Editor: Stewart GJ E- Editor: Ma S
| 1. | Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 791] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 2. | Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci USA. 2008;105:10277-10278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271-5279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 272] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Yuan H, Wei X, Zhang G, Li C, Zhang X, Hou J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol. 2011;186:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong F, Zhang ZX, Zhang GB, Zhang XG, Zhao H. B7-H3 overexpression in pancreatic cancer promotes tumor progression. Int J Mol Med. 2013;31:283-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Liu C, Liu J, Wang J, Liu Y, Zhang F, Lin W, Gao A, Sun M, Wang Y, Sun Y. B7-H3 expression in ductal and lobular breast cancer and its association with IL-10. Mol Med Rep. 2013;7:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S. B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci. 2011;102:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Katayama A, Takahara M, Kishibe K, Nagato T, Kunibe I, Katada A, Hayashi T, Harabuchi Y. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int J Oncol. 2011;38:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Ingebrigtsen VA, Boye K, Nesland JM, Nesbakken A, Flatmark K, Fodstad Ø. B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer. 2014;14:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131:2528-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Lee H, Kim JH, Yang SY, Kong J, Oh M, Jeong DH, Chung JI, Bae KB, Shin JY, Hong KH. Peripheral blood gene expression of B7 and CD28 family members associated with tumor progression and microscopic lymphovascular invasion in colon cancer patients. J Cancer Res Clin Oncol. 2010;136:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, Encke J. Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep. 2007;18:745-748. [PubMed] |
| 15. | Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 757] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 16. | Liu H, Tekle C, Chen YW, Kristian A, Zhao Y, Zhou M, Liu Z, Ding Y, Wang B, Mælandsmo GM. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther. 2011;10:960-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Gómez-Fernández JC. Functions of the C-terminal domains of apoptosis-related proteins of the Bcl-2 family. Chem Phys Lipids. 2014;183:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 2342] [Article Influence: 212.9] [Reference Citation Analysis (0)] |
| 19. | Zhao X, Zhang GB, Gan WJ, Xiong F, Li Z, Zhao H, Zhu DM, Zhang B, Zhang XG, Li DC. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol Lett. 2013;5:805-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Nguyen AV, Wu YY, Lin EY. STAT3 and sphingosine-1-phosphate in inflammation-associated colorectal cancer. World J Gastroenterol. 2014;20:10279-10287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Qi QR, Yang ZM. Regulation and function of signal transducer and activator of transcription 3. World J Biol Chem. 2014;5:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (1)] |
| 22. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 455] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 23. | Kim SM, Lee JH, Sethi G, Kim C, Baek SH, Nam D, Chung WS, Kim SH, Shim BS, Ahn KS. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014;354:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Teng Y, Ross JL, Cowell JK. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT. 2014;3:e28086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20:9872-9881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 274] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 27. | Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 28. | Wimberly AL, Forsyth CB, Khan MW, Pemberton A, Khazaie K, Keshavarzian A. Ethanol-induced mast cell-mediated inflammation leads to increased susceptibility of intestinal tumorigenesis in the APC Δ468 min mouse model of colon cancer. Alcohol Clin Exp Res. 2013;37 Suppl 1:E199-E208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Cario E. The human TLR4 variant D299G mediates inflammation-associated cancer progression in the intestinal epithelium. Oncoimmunology. 2013;2:e24890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Janakiram NB, Rao CV. The role of inflammation in colon cancer. Adv Exp Med Biol. 2014;816:25-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Saravanan S, Islam VI, Babu NP, Pandikumar P, Thirugnanasambantham K, Chellappandian M, Raj CS, Paulraj MG, Ignacimuthu S. Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur J Pharm Sci. 2014;56:70-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Zhang ZH, Yu LJ, Hui XC, Wu ZZ, Yin KL, Yang H, Xu Y. Hydroxy-safflor yellow A attenuates Aβ-42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway. Brain Res. 2014;1563:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Gyurkovska V, Stefanova T, Dimitrova P, Danova S, Tropcheva R, Ivanovska N. Tyrosine kinase inhibitor tyrphostin AG490 retards chronic joint inflammation in mice. Inflammation. 2014;37:995-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1: STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Planas AM, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc Trans. 2006;34:1267-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S. The role of JAK-STAT signaling within the CNS. JAKSTAT. 2013;2:e22925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |