Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1775
Peer-review started: June 10, 2014
First decision: August 6, 2014
Revised: August 24, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 14, 2015
Processing time: 247 Days and 14.6 Hours
AIM: To investigate whether nitrite administered prior to ischemia/reperfusion (I/R) reduces liver injury.
METHODS: Thirty-six male Sprague-Dawley rats were randomized to 3 groups, including sham operated (n = 8), 45-min segmental ischemia of the left liver lobe (IR, n = 14) and ischemia/reperfusion (I/R) preceded by the administration of 480 nmol of nitrite (n = 14). Serum transaminases were measured after 4 h of reperfusion. Liver microdialysate (MD) was sampled in 30-min intervals and analyzed for glucose, lactate, pyruvate and glycerol as well as the total nitrite and nitrate (NOx). The NOx was measured in serum.
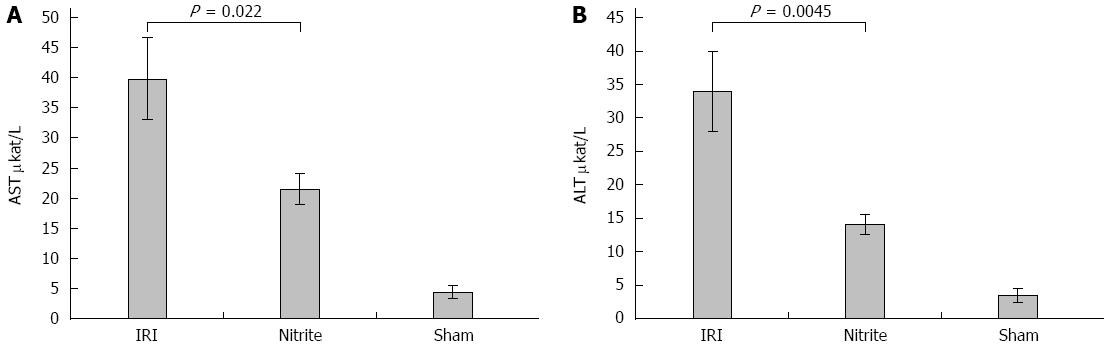
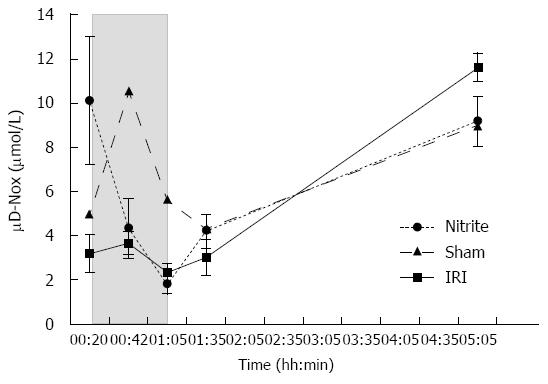
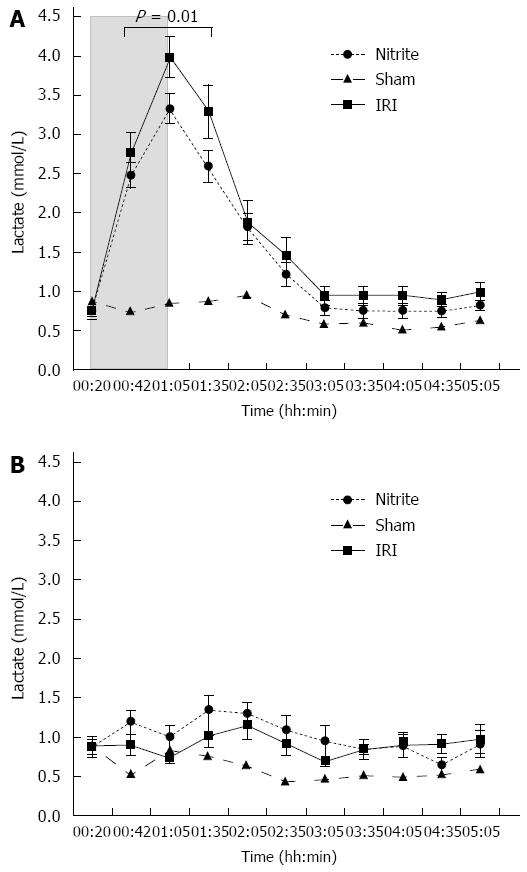
RESULTS: Aspartate aminotransferase (AST) at the end of reperfusion was higher in the IR group than in the nitrite group (40 ± 6.8 μkat/L vs 22 ± 2.6 μkat/L, P = 0.022). Similarly, alanine aminotransferase (ALT) was also higher in the I/R group than in the nitrite group (34 ± 6 μkat vs 14 ± 1.5 μkat, P = 0.0045). The NOx in MD was significantly higher in the nitrite group than in the I/R group (10.1 ± 2.9 μmol/L vs 3.2 ± 0.9 μmol/L, P = 0.031) after the administration of nitrite. During ischemia, the levels decreased in both groups and then increased again during reperfusion. At the end of reperfusion, there was a tendency towards a higher NOx in the I/R group than in the nitrite group (11.6 ± 0.7 μmol/L vs 9.2 ± 1.1 μmol/L, P = 0.067). Lactate in MD was significantly higher in the IR group than in the nitrite group (3.37 ± 0.18 mmol/L vs 2.8 ± 0.12 mmol/L, P = 0.01) during ischemia and the first 30 min of reperfusion. During the same period, glycerol was also higher in the IRI group than in the nitrite group (464 ± 38 μmol/L vs 367 ± 31 μmol/L, P = 0.049). With respect to histology, there were more signs of tissue damage in the I/R group than in the nitrite group, and 29% of the animals in the I/R group exhibited necrosis compared with none in the nitrite group. Inducible nitric oxide synthase transcription increased between early ischemia (t = 15) and the end of reperfusion in both groups.
CONCLUSION: Nitrite administered before liver ischemia in the rat liver reduces anaerobic metabolism and cell necrosis, which could be important in the clinical setting.
Core tip: Nitric oxide has an important protective effect against liver ischemia/reperfusion injury (IRI), although large levels of the substance may increase IRI. The total levels of nitrite and nitrate decrease in the liver tissue during ischemia. Because nitrite can be reduced to nitric oxide (NO) in an acidic environment, this decrease may be due to NO formation. In this study, the administration of nitrite before liver ischemia/reperfusion reduced the IRI per evaluation with transaminases, liver microdialysis and histology.
- Citation: Björnsson B, Bojmar L, Olsson H, Sundqvist T, Sandström P. Nitrite, a novel method to decrease ischemia/reperfusion injury in the rat liver. World J Gastroenterol 2015; 21(6): 1775-1783
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1775
As liver surgery continues to evolve and expand with increasingly complicated resections being performed, vascular inflow control (Pringle’s maneuver) is occasionally used to reduce bleeding associated with liver transection. Obstructing the vascular inflow to the liver inevitably leads to some degree of ischemia/reperfusion injury (IRI)[1].
It has been shown that when the availability of nitric oxide (NO) is decreased during IRI in the rat liver, there is more severe injury[2]. On the other hand, excessive NO levels, such as those found after activation of inducible nitric oxide synthase (iNOS), seem to be detrimental to the liver during IRI[3]. Although nitrite has mainly been considered an inert product of NO oxidation, nitrite can be reduced to NO in an acidic environment[4]. More recently, this process has been shown to occur, which is catalyzed by deoxyhemoglobin, at physiological oxygen levels[5]. The reduction of nitrite to NO may increase the bioavailability of NO in settings where nitric oxide synthase (NOS) does not provide NO, such as when oxygen is lacking. The administration of 48 nmol of nitrite (via intraperitoneal injection) before reperfusion decreases liver IRI (measured as the transaminase level at 24 h of reperfusion) in a mouse model[6]. Further studies in isolated rat mitochondria have shown that the administration of nitrite may modulate mitochondrial electron transfer in a way that slows electron transport and reduces the formation of reactive oxygen species during reperfusion[7]. This finding is consistent with the known inhibitory effects of NO on the respiratory chain[8-11]. The protective effect of nitrite on the mouse liver has further been supported by a study on the preservation of the mouse liver in University of Wisconsin solution or Lactated Ringer’s solution with or without the addition of nitrite[12]. In cardiac ischemia, nitrite has been shown to decrease the infarct size, reduce apoptosis and improve contractility[13]. Inhaled NO has been investigated in human liver transplantation and shown to have some protective effect[14,15]. In a previous study, our group showed that during liver ischemia, the total level of nitrite and nitrate (NOx) is reduced in the ischemic liver tissue and that iNOS transcription is less activated during early reperfusion in rats subjected to ischemic preconditioning (IPC) than those subjected to ischemia and reperfusion alone[16].
The main hypothesis of this study was that intravenously administered nitrite before ischemia reduces IRI as measured by transaminase levels, microdialysis and histology during early (4 h) reperfusion. Additional hypotheses were that this reduction could be related to the reduced anaerobic metabolism that was detectable using microdialysis and that the treatment reduces iNOS transcription during the early phase of reperfusion.
The study protocol was approved by the local ethics committee for animal experiments at Linköping University, Linköping, Sweden. Thirty-six male Sprague-Dawley rats (313-444 g, Scandjur, Sollentuna, Sweden) were randomized into the following 3 groups: (1) ischemia (n = 14); (2) nitrite administered before ischemia (n = 14); and (3) sham operated (n = 8). Two minutes before a 45-min period of segmental ischemia, 480 nmol of sodium nitrite (Cayman Chemical Company; Ann Arbor, MI, United States) dissolved in saline was administered to the animals in the nitrite group by injection into the caval vein. The animals in the sham group were not subjected to ischemia, but their livers were mobilized as in the other groups. The animals were acclimatized for 1 wk at 21 °C on a 12-h light/dark cycle with free access to standard rat food pellets and tap water before the experiment.
The animals were anesthetized with isoflurane (Baxter Medical AB, Kista, Sweden), and 0.05 mg/kg of buprenorphine (Temgesic®, Schering-Plough, Stockholm, Sweden) was administered sc for pain relief. The animals were intubated with a 16 G intravenous catheter (BD Venflon, Becton, Dickinson and Company, Franklin Lakes, NJ, United States) and ventilated with a Hallowell EMC Microvent 1 respirator (Hallowell EMC Pittsfield, MA, United States) during the experiment. The rats were monitored using a SurgiVet V3304 (Smiths Medical PM, Inc., Norwell, MA, United States), and their body temperatures were maintained within 38 °C-39 °C through the use of Animaltemp 1070 heating pads (Elmedex Elektronik HB, Uppsala, Sweden). Laparotomy was performed via a midline incision, and the ligament attachments of the liver were divided. Immediately after intubation and at every hour throughout the experiment, the animals received 5 mL of warm (38 °C) Ringer’s acetate (Baxter Medical AB, Kista, Sweden) sc.
Microdialysis was carried out during the entire experiment from two separate lobes (ischemia and control) of the liver. After 15 and 40 min of ischemia and at the end of reperfusion, biopsies were taken from the ischemic lobe and stored in liquid nitrogen for later analysis. A sample of the tissue collected at the end of reperfusion was stored in formalin.
CMA 20 Elite Microdialysis Probes (CMA/Microdialysis AB, Stockholm, Sweden) were inserted in both the left lateral and right lateral (control) liver lobes and perfused at a rate of 1.0 μL/min with Perfusion fluid T1 (147 mmol/L of Na, 4 mmol/L of K, 2.3 mmol/L of Ca, and 156 mmol/L of Cl at a pH of 6 and an osmolality of 290 mosm/kg) (CMA/Microdialysis AB) from a CMA 400 Syringe Pump (CMA/Microdialysis AB). Before insertion, the probes were preperfused. After inserting the probe, 35 min were allowed for the steady state to be reached, and 20 min of baseline sampling of microdialysate was performed before ischemia was induced. During ischemia, the microdialysate was sampled in 22.5-min intervals; thereafter, the intervals were 30 min. Analyses of glucose, glycerol, lactate and pyruvate were performed instantly using the clinical bedside analyzer ISCUS (CMA/Microdialysis AB).
The sum of NO2- and NO3- (NOx) was analyzed in the serum and microdialysate following the instructions provided in the commercial “Nitrite/Nitrate Fluorometric Assay Kit” (Cayman Chemical Company, Ann Arbor, MI, United States). Before analysis, the serum was ultrafiltrated through a 10-kD cut-off filter (Millipore, Solna, Sweden). The microdialysate, conversely, was directly analyzed. Standard curves were plotted. For nitrate, 10 μL of each sample was diluted in 70 μL of assay buffer. Aliquots of 10 μL of enzyme cofactor and 10 μL of nitrate reductase mixture were added to the buffered sample. After 30 min of incubation at room temperature, 10 μL of DAN (2,3-diaminonaphtalene) was added and incubated for another 10 min. For nitrite, 10 μL of each sample was diluted with 90 μL of assay buffer, and 10 μL of DAN was added. Both the nitrate and nitrite samples were then incubated for 10 min, and 20 μL of NaOH was added. All samples were read in threes with fluorometry using an excitation wavelength of 355 nm and an emission wavelength of 430 nm.
Ten milligrams of liver tissue (dry weight) was disrupted and homogenized in a Micro-Dismembranator (Braun Biotech, Allentown, PA, United States) at 2900 rpm for 30 s. RNA extraction was performed according to the manufacturer’s protocol (Qiagene, Valencia, CA, United States); however, β-mercaptoethanol was not used. The RNA was eluted in 300 μL of RNAse-free water; the RNA concentration was measured at 260 nm, and its purity (approximately 2.0) was read at 260/280 nm (NanoDrop, Thermo Scientific, Erembodegem, Belgium). The RNA was further stored at -70 °C until assayed. Reverse transcriptase cDNA conversion with a High Capacity cDNA Reverse Transcription Kit was used on 0.5 μg RNA in a total volume of 20 μL according to the manufacturer’s protocol (Applied Biosystems, Foster City CA, United States). cDNA samples were stored at -20 °C until assayed. Samples of 2 μL containing 50 ng cDNA (0.025 μg/μL) were used in a total reaction volume of 20 μL for the real-time PCR reaction. Samples were analyzed in triplicate with a Fast Master Mix and TaqMan Gene Expression Assay (Applied Biosystems) in a 7500 Fast Instrument (Applied Biosystems). The mean of the samples’ Ct is normalized in the results against the mean Ct for glyceraldehyde-3-phosphate-dehydrogenase, which is presented as ΔCt.
Liver sections (2 μm) were hematoxylin-eosin stained and coded before examination by a pathologist who was blind to the experimental design. The degree of liver injury was estimated using a scoring system that evaluated the sinusoidal congestion (0-4), cytoplasmic vacuolation (0-4) and necrosis (0-2)[17].
The data are presented as the mean ± SE unless otherwise stated. A P value < 0.05 was considered statistically significant. t-tests were used to compare the treatment groups. No statistical comparisons were made between the study groups and sham group. Statistica 8.0 software (StatSoft Inc., Tulsa, OK, United States) was used for all statistical calculations.
Liver transaminases: After 4 h of reperfusion, higher serum AST levels (Figure 1A) were found in the I/R group, 40 ± 6.8 μkat/L, compared with the nitrite group, 22 ± 2.6 μkat/L (P = 0.022). Similarly, the ALT levels (Figure 1B) at 4 h of reperfusion were significantly higher in the IRI group, 34 ± 6 μkat/L, than in the nitrite group, 14 ± 1.5 μkat/L, (P = 0.0045). In the sham group, the AST and ALT levels were near normal when measured at the end of the experiment (4.4 and 3.5, respectively).
NOx: The sum of nitrite and nitrate (NOx) was measured in the serum at the end of reperfusion and was not significantly different between the groups (data not shown).
NOx: After the administration of nitrite, NOx in the liver tissue was higher in the nitrite group, 10.1 ± 2.9 μmol/L, than in the I/R group, 3.2 ± 0.9 μmol/L (P = 0.031) (Figure 2). During ischemia, the levels of NOx decreased in both groups and thereafter increased during reperfusion. At the end of 4 h of reperfusion, there was a trend towards higher levels of NOx in the I/R group, 11.6 ± 0.7, than in the nitrite group, 9.2 ± 1.1 (P = 0.067).
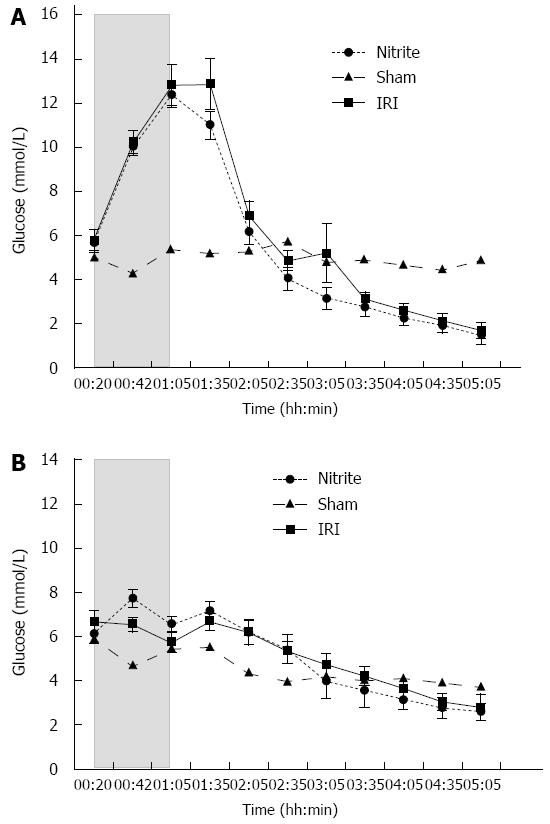
Glucose: In both the I/R and nitrite groups, glucose increased approximately 2-fold (range: 6-12 mmol/L) in the ischemic lobe compared with either the control lobe or the pre-ischemic value in the ischemic lobe. No significant difference was found between the two ischemic lobes (Figure 3A). In the control lobes of the nitrite group, a significant (P < 0.001) increase (range: 6.1-7.7 mmol/L) in glucose was observed after nitrite administration. At t = 00:43 and during the first 95 min of the experiment, the glucose level was higher in the control lobes of the nitrite group compared with the I/R group (P = 0.049 and P = 0.02, respectively). Otherwise, there was a constant decrease in glucose during the experiment (Figure 3B).
Pyruvate: In the I/R group, pyruvate in the ischemic segments fell to approximately 40% of the level in the control segments at the end of ischemia. In the nitrite group, the corresponding value was 15%. In the early phase of reperfusion, there was a transient increase in the parenchymal pyruvate in both groups. There were, however, large interindividual variations in the pyruvate levels, and no significant differences were noted.
In the sham group, there were no significant differences between the two lobes in the microdialysate (data not shown).
Lactate: The lactate levels increased more than four-fold in the ischemic lobes (4 mmol/L) compared with the control lobes (0.7 mmol/L) in the I/R group during the ischemic phase; the same pattern was observed in the nitrite group (3.3 and 1 mmol/L, respectively), although the increase had a smaller in magnitude. During ischemia and the first 30 min of reperfusion, the lactate levels were significantly higher in the IRI group than in the nitrite group (P = 0.01). At the end of ischemia (t = 01:05) and after 30 min of reperfusion (t = 01:35), there was a trend towards less lactate in the nitrite group compared with the I/R group (P = 0.053 and 0.08, respectively) (Figure 4A).
Lactate increased from 0.9 to 1.2 mmol/L (P = 0.012) in the control lobes in the nitrite group after the administration of nitrite, which was not observed in the I/R group. Furthermore, the lactate levels were significantly (P = 0.01) higher during the first 95 min of the experiment in the control lobes in the animals treated with nitrite than in those subjected to I/R alone (Figure 4B).
In the sham group, the lactate levels remained low and stable throughout the experiment.
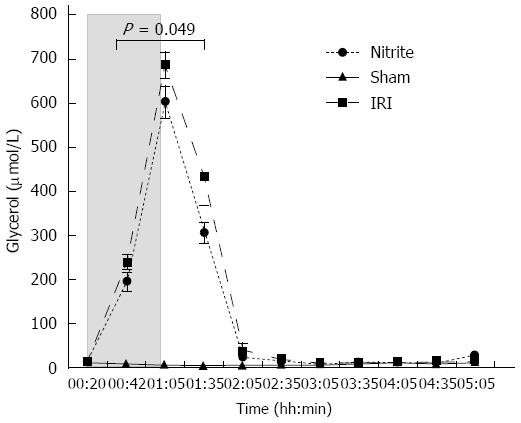
Glycerol: The increase in glycerol in the ischemic lobes (range: 18-686 μmol/L) compared with the control lobes was approximately 38-fold in the I/R group during the ischemic phase. The nitrite group followed a similar pattern, but the increase (range: 25-602 μmol/L) was less pronounced than in the I/R group (24-fold). During ischemia and the first 30 min of reperfusion, the glycerol levels were significantly higher in the IRI group than in the nitrite group (P = 0.049). At the end of ischemia (t = 01:05), there was a trend towards higher glycerol in the ischemic lobes in the I/R group compared with the nitrite group (P = 0.079); a similar trend was noticed after 30 min of reperfusion (t = 01:35, P = 0.069). In the ischemic lobes, the glycerol levels returned to the levels observed in the control lobes after approximately 60 min of reperfusion. No significant changes were observed in the sham group or in the control lobes (Figure 5).
iNOS mRNA: There was no difference in the iNOS mRNA between the groups 15 min into ischemia (IRI ΔCt = 9.3 ± 0.72, ΔCt nitrite = 8.6 ± 0.24). In both groups, transcription was increased at the end of the experiment to ΔCt = 3.8 ± 0.52 in the I/R group and 3.4 ± 0.26 in the nitrite group (P < 0.01 in both groups). There was, however, no significant difference between the groups.
Histology: The degree of liver injury was evaluated in terms of sinusoidal congestion (0-4), cytoplasmic vacuolation (0-4) and necrosis (0-2)[17].
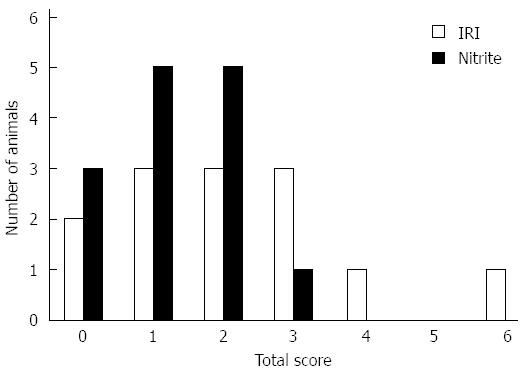
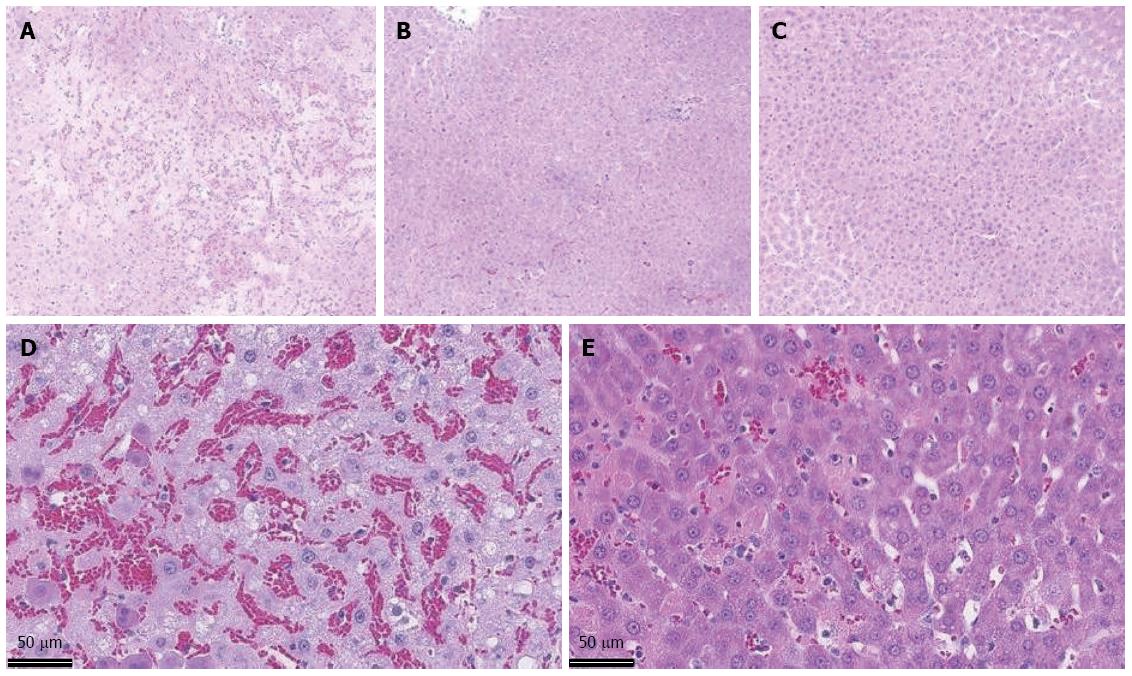
For the sham animals, all scores were zero, except for 1 animal that scored 1 (10%-40% of hepatic tissue was affected) for sinusoidal congestion. In the I/R group (n = 14), the mean score for sinusoidal congestion was 1.29 compared with 0.86 in the nitrite group (n = 14). Five animals (36%) scored 2 or higher in the I/R group compared with 1 (7%) in the nitrite group. Similarly, the mean score for cytoplasmic vacuolization was 0.71 in the I/R group and 0.43 in the nitrite group, and 5 (36%) of the animals in the IRI group did not show signs of vacuolation compared with 8 (57%) in the nitrite group. Of the 14 animals in the I/R group, 4 (29%) had signs of necrosis by histology, whereas none were found in the nitrite group. Together, the median total score for the I/R group was 2 (0-6), and this value for the nitrite group was 1 (0-3); Figure 6 shows the distribution of the scores and Figure 7 shows the representative histologic slides for each group.
This is the first experimental study to investigate the effect of nitrite administered intravenously before a period of segmental liver I/R in rats. This study shows that nitrite reduces the systemic levels of AST and ALT after 4 h of reperfusion, improves anaerobic metabolism in the ischemic lobe (measured via microdialysis) and reduces the histological signs of IRI.
The administration of nitrite resulted in significantly higher NOx in the microdialysate before ischemia, indicating that the administered nitrite reached the liver parenchyma. The total NOx was markedly reduced in the liver parenchyma during ischemia in both groups. This reduction had previously been shown in animals that were not treated with nitrite in this model[16]. The tendency towards higher NOx in the IRI group at the end of the experiment, although not statistically significant, may reflect more endogenous NO production in the I/R group during reperfusion. In the sham group, an increase in the MD NOx was noted during the experiment, which is most likely consistent with the inflammatory response to laparotomy. In the serum analysis, no differences could be found between the groups, which is consistent with our previous results and with published data from others[6,16,18]. These findings may be explained by data indicating that nitrite is converted to NO and is bound to thiols and metals[5,19]. Therefore, the administration of nitrite may increase the bioavailability of NO and, in that way, may reduce IRI.
Reduced iNOS transcription has been found to accompany reduced IRI when IPC and R-IPC are compared[20]. In the present study, no significant difference was noted, although there was a tendency towards a larger increase of transcription in the I/R group. Keeping in mind that almost 30% of the liver cells showed signs of necrosis in the I/R group and, therefore, may not have been capable of inducing transcription, this could be a false-negative finding. Another possible explanation is that the protective effects of nitrite found in this study are not related to a reduction in iNOS transcription.
In previous studies, lower glucose levels in microdialysate from the ischemic liver were associated with diminished signs of IRI[21,22]. In this study, this phenomenon was not confirmed, although the glucose levels tended to return to normal earlier in the nitrite group than they did in the I/R group (Figure 3A). Lactate was significantly higher in the ischemic lobes in the I/R group than in the nitrite group, indicating that there is reduced anaerobe metabolism when nitrite is administered. The opposite was observed in the control lobes, a finding that has not been reported in earlier IRI studies, including those using microdialysis. The increase in lactate observed in the control lobes after nitrite administration may be related to a dampening of the electron transport chain, which is in agreement with previous studies[7,8]. Dampening of the electron transport chain will decrease the oxidative stress and may therefore be important in reducing IRI. This potential explanation is further supported by the increase in hepatic glucose found in the same control lobes, which may indicate reduced metabolism. This subtle difference was not observed in the ischemic lobe, which is perhaps due to the increased anaerobic metabolism.
Glycerol was found to be significantly higher in the I/R group than in the nitrite group during ischemia and the first 30 min of reperfusion. Although the degrees of correlation with the AST (r = 0.3) and ALT (r = 0.27) levels were low and non-significant, they are consistent with studies on IRI that have included microdialysis and indicate damage to the cellular membranes[21,23]. Furthermore, the histological findings also indicate that the IRI is reduced with nitrite treatment administered before ischemia, and there is no necrosis in the treated animals, compared with almost one-third of the untreated animals.
Together, these results indicate that nitrite administered before I/R reduces the IRI, and they further corroborate an earlier study on mice that showed that nitrite administered before reperfusion reduces the IRI in a dose-dependent manner[6]. When interpreting these findings, one has to consider that the results are obtained after a short period of reperfusion; therefore, it is unclear whether the damage continues during the later phase.
Because the administration of nitrite during ischemia in the setting of liver resection would be impractical, it is important to also evaluate the effects when nitrite is administered prior to an ischemic insult, as was performed in this study. The protective effect of oral nitrite 24 h before hepatic ischemia in mice has already been established; until now, however, intravenous administration immediately prior to ischemia has not been investigated[7]. The results presented herein support that nitrite may have a protective role against IRI when administered before an ischemic period. This could have clinical implications insofar as one of the methods used to lower the central venous pressure during liver resections in humans involves the intravenous administration of nitroglycerine[24]. Nitroglycerine has a short half-life, and it is metabolized in the liver to nitrite, which may constitute a pool of substrate for NO production without NOS involvement[25]. In a small, randomized study on the effect of low central venous pressure (CVP) on bleeding during hepatectomy, nitroglycerin was among the methods used to lower the CVP. The low CVP group was had lower (although nonsignificant) ALT levels postoperatively than controls, which may be attributed to the aforementioned effects of nitrite[26].
In conclusion, the administration of nitrite before I/R reduces the biochemical and pathological signs of the IRI. This effect may have clinical implications and should be further investigated in the clinical setting.
When the circulation to the liver is temporarily closed (ischemia/reperfusion), as is occasionally necessary during liver surgery, there is inevitably damage to the liver. It has been shown that nitric oxide plays an important role in this process although the exact mechanisms are unknown. Furthermore, the methods to prevent this damage seem to influence the nitric oxide system.
Nitrite administered into the abdomen during ischemia/reperfusion has been shown to reduce the liver damage in the mice. Furthermore, nitrite administered by the oral route in mice 24 h prior to ischemia/reperfusion has a similar effect. This novel treatment could later prove to be of value in humans, but further studies in animals are needed.
Earlier research has shown that nitrite can be reduced to nitric oxide, particularly in the acidic environment. Furthermore, nitrite and nitrate are reduced in the liver parenchyma during ischemia. This, along with findings indicating that nitrite may reduce liver damage when administered during liver ischemia/reperfusion, may indicate that pre-treatment with nitrite can prevent the damage. Until now, this has only been shown for mice. The current study supports earlier findings as the route of administration, and the animal strain differs from the previous reports.
The findings of this basic research article add to the authors’ understanding of the value of nitrite as a protective treatment against damage to the liver that is related to ischemia/reperfusion. Showing that this effect occurs in rats as well as in mice (previously shown) may serve as the foundation for further research in the area. It should be noted, however, that such treatment would not immediately be adaptable to the clinical setting.
Ischemia/reperfusion injury (IRI) is an ill-defined term that refers to the damage in the liver when the circulation is closed and then reopened. The extent of the damage is typically measured by the levels of liver transaminases in the serum.
In this paper, authors aim to investigate whether nitrite administration prevents liver IRI. To reach their scope, the Authors used a rat model of liver IRI. In the animal group treated with nitrates before IRI transaminases were significantly lower, as well as anaerobic metabolism markers, than in the control or the sham-operated group. Accordingly, histopathological liver tissue sections demonstrated less injury in the treated group compared to controls.
P- Reviewer: Esposito C, Touil-Boukoffa C S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 245] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Köken T, Inal M. The effect of nitric oxide on ischemia-reperfusion injury in rat liver. Clin Chim Acta. 1999;288:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Tsuchihashi S, Kaldas F, Chida N, Sudo Y, Tamura K, Zhai Y, Qiao B, Busuttil RW, Kupiec-Weglinski JW. FK330, a novel inducible nitric oxide synthase inhibitor, prevents ischemia and reperfusion injury in rat liver transplantation. Am J Transplant. 2006;6:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Zhang Z, Naughton DP, Blake DR, Benjamin N, Stevens CR, Winyard PG, Symons MC, Harrison R. Human xanthine oxidase converts nitrite ions into nitric oxide (NO). Biochem Soc Trans. 1997;25:524S. [PubMed] |
| 5. | Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1396] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 6. | Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 511] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 7. | Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089-2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 8. | Shiva S, Gladwin MT. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res Cardiol. 2009;104:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 914] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 10. | Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994;204:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 240] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 763] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 12. | Li W, Meng Z, Liu Y, Patel RP, Lang JD. The hepatoprotective effect of sodium nitrite on cold ischemia-reperfusion injury. J Transplant. 2012;2012:635179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 15. | Lang JD, Smith AB, Brandon A, Bradley KM, Liu Y, Li W, Crowe DR, Jhala NC, Cross RC, Frenette L. A randomized clinical trial testing the anti-inflammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One. 2014;9:e86053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Björnsson B, Winbladh A, Bojmar L, Sundqvist T, Gullstrand P, Sandström P. Conventional, but not remote ischemic preconditioning, reduces iNOS transcription in liver ischemia/reperfusion. World J Gastroenterol. 2014;20:9506-9512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Calabrese F, Valente M, Pettenazzo E, Ferraresso M, Burra P, Cadrobbi R, Cardin R, Bacelle L, Parnigotto A, Rigotti P. The protective effects of L-arginine after liver ischaemia/reperfusion injury in a pig model. J Pathol. 1997;183:477-485. [PubMed] |
| 18. | Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308-4313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 315] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder SJ, Jones HP. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987;79:1564-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 255] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Björnsson B, Winbladh A, Bojmar L, Trulsson LM, Olsson H, Sundqvist T, Gullstrand P, Sandström P. Remote or conventional ischemic preconditioning--local liver metabolism in rats studied with microdialysis. J Surg Res. 2012;176:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Winbladh A, Björnsson B, Trulsson L, Bojmar L, Sundqvist T, Gullstrand P, Sandström P. N-acetyl cysteine improves glycogenesis after segmental liver ischemia and reperfusion injury in pigs. Scand J Gastroenterol. 2012;47:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Nowak G, Ungerstedt J, Wernerson A, Ungerstedt U, Ericzon BG. Hepatic cell membrane damage during cold preservation sensitizes liver grafts to rewarming injury. J Hepatobiliary Pancreat Surg. 2003;10:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 367] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Govoni M, Tocchetti P, Lundberg JO. Metabolism and pathways for denitration of organic nitrates in the human liver. J Pharmacol Exp Ther. 2013;346:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935-939. [PubMed] |