Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13555
Peer-review started: April 24, 2015
First decision: July 10, 2015
Revised: July 23, 2015
Accepted: September 28, 2015
Article in press: September 30, 2015
Published online: December 28, 2015
Processing time: 245 Days and 15.5 Hours
AIM: To assess the association of a surrogate of fatty liver disease (FLD) with incident type-2 diabetes, coronary heart disease, and all-cause mortality.
METHODS: In a prospective population-based study on 1822 middle-aged adults, stratified to gender, we used an algorithm of fatty liver index (FLI) to identify associations with outcomes. An index ≥ 60 indicated the presence of FLD. In Cox regression models, adjusted for age, smoking status, high-density lipoprotein cholesterol, and systolic blood pressure, we assessed the predictive value of FLI for incident diabetes, coronary heart disease (CHD), and all-cause mortality.
RESULTS: At a mean 8 year follow-up, 218 and 285 incident cases of diabetes and CHD, respectively, and 193 deaths were recorded. FLD was significantly associated in each gender with blood pressure, total cholesterol, apolipoprotein B, uric acid, and C-reactive protein; weakly with fasting glucose; and inversely with high-density lipoprotein-cholesterol and sex hormone-binding globulin. In adjusted Cox models, FLD was (with a 5-fold HR) the major determinant of diabetes development. Analyses further disclosed significant independent prediction of CHD by FLD in combined gender [hazard ratio (HR) = 1.72, 95% confidence interval (CI): 1.17-2.53] and men (HR = 2.35, 95%CI: 1.25-4.43). Similarly-adjusted models for all-cause mortality proved, however, not to confer risk, except for a tendency in prediabetics and diabetic women.
CONCLUSION: A surrogate of FLD conferred significant high risk of diabetes and coronary heart disease, independent of some metabolic syndrome traits. All-cause mortality was not associated with FLD, except likely in the prediabetic state. Such a FLI may reliably be used in epidemiologic studies.
Core tip: We prospectively assessed in 1822 adults the association between a validated surrogate of fatty liver disease (FLD) and the incidence of type-2 diabetes, coronary heart disease (CHD), and all-cause mortality by stratifying to gender and using adjusted Cox regression models. At a mean 8 year follow-up, FLD was the major determinant of developing diabetes and was a significant predictor of CHD. Similarly-adjusted models for all-cause mortality did not confer risk, except for slightly in prediabetics and diabetic women. Involvement of circulating lipoprotein(a) in autoimmune activation may be an underlying mechanism. Such a FLD surrogate may be used in epidemiologic studies.
- Citation: Onat A, Can G, Kaya A, Akbaş T, Özpamuk-Karadeniz F, Şimşek B, Çakır H, Yüksel H. Fatty liver disease: Disparate predictive ability for cardiometabolic risk and all-cause mortality. World J Gastroenterol 2015; 21(48): 13555-13565
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13555
Steatohepatitis designates fatty infiltration and inflammation of the liver and has features closely associated with the metabolic syndrome (MetS)[1]. Liver biopsy, ultrasonography, serum liver enzymes and, more recently, an algorithm-based surrogate have been commonly used in identifying the presence of non-alcoholic fatty liver disease (NAFLD) and its relationship to adverse outcomes. As a growing public health issue, NAFLD has been demonstrated in the past decade to be associated with MetS[2], type-2 diabetes[3-5], cardiovascular events[6-9], and chronic kidney disease[10-12]. A complex bidirectional relationship between the development of diabetes and progression to non-alcoholic steatohepatitis promoting hepatic fibrogenesis and insulin resistance has been identified[1]. Risk of all-cause death is also predicted by NAFLD[13-15], but conflicting results have been reported[9,16] regarding overall and cause-specific mortality. Despite an increased association with independent cardiovascular disease (CVD) prevalence and NAFLD in United States adults (The National Health and Nutrition Examination Survey (NHANES)-III), NAFLD did not predict mortality over a 14 year period[16].
The complex inter-relationships between NAFLD, visceral obesity, and insulin resistance[17] require further elucidation. Since most of the prospective studies on NAFLD have been performed in population samples of Western Europe, United States, and East Asia; investigation of different ethnicities is necessary to clarify better variation in the relationship. The controversial relationship between NAFLD and risk of overall mortality, as compared to that of diabetes and CVD, may be highly relevant for the pathophysiology of the associations and possibly related to ethnic differences.
Turkish adults are prone to MetS[18], diabetes mellitus[19], and chronic hepatitis. Cardiovascular risk profiles are characterized by a high prevalence of abdominal obesity in males, overall obesity in females, low high-density lipoprotein (HDL)-cholesterol, high triglyceride, and intermediate total cholesterol levels. Current smoking protects against abdominal obesity[18]. One-fifth of non-diabetic Turks exhibit impaired fasting glucose[20]. On one hand, evidence is growing that microbiota contribute to the pathogenesis of insulin resistance, abdominal obesity, and progression of NAFLD[1,21]. On the other hand, autoimmune activation based on enhanced proinflammatory state may be a common mechanism underlying these diseases in middle-aged and elderly Turkish adults[22]. Prospective evaluation of the same sample regarding the relationships among fatty liver disease (FLD), cardiometabolic disease risk, and all-cause death might reveal novel information.
Clinical and epidemiological studies using an algorithm-based surrogate of FLD to investigate related outcomes have been published[13,23]. We, therefore, aimed in this study to assess prospectively and simultaneously the impact of an algorithm-derived surrogate of FLD on diabetes, CHD, and overall mortality in a population-based sample representative of middle-aged Turkish adults at a lengthy follow-up period.
Population sample
The Turkish Adult Risk Factor Study is a prospective survey on the prevalence of cardiac disease and risk factors in adults in Turkey that has been carried out periodically, almost biennially, since 1990 in 59 communities scattered throughout all geographical regions of the country[24]. It comprises a representative sample of the Turkish adult population. Serum γ-glutamyltransferase (GGT) determinations were made in the 2003/04 survey, during which GGT was measured in all 1822 participants who attended the survey (examination in 60%) out of an eligible 3037 participants. Follow-up extended to the 2012/13 survey.
The survey conformed to the principles embodied in the Declaration of Helsinki and was approved by the Istanbul University Ethics Committee. All individuals gave written consent to participation. Data were obtained by history questionnaire, physical examination of the cardiovascular system, sampling of blood, and recording of a resting electrocardiogram.
Blood pressure (BP) was measured in the sitting position on the right arm, and the mean of two recordings at least 5 min apart was recorded. Waist circumference was measured with a tape (Roche LI95 63B 00, Basel, Switzerland) with the subject standing and wearing only underwear at the level midway between the lower rib margin and the iliac crest. Self-reported cigarette smoking was categorized into never smokers, former smokers (discontinuance for 3 mo or longer), and current smokers (regularly 1 or more cigarettes daily). Anyone who consumed alcohol at least once a week was considered a user of alcoholic drinks.
Biochemical parameters were assayed in a central laboratory. Blood samples were shipped to Istanbul and stored in deep-freeze at -75 °C until analyzed. Serum concentrations of total and HDL-cholesterol (directly without precipitation) and triglycerides were determined using enzymatic kits from Roche Diagnostics with a Hitachi 902 analyzer (Tokyo, Japan). Concentrations of sex hormone-binding globulin (SHBG) and total testosterone were determined by the electrochemiluminescence immunoassay ECLIA on Roche Elecsys 2010 (Roche Diagnostics). Serum concentrations of apolipoprotein (apo) A-I, apo B, lipoprotein (Lp)(a) and high-sensitivity C-reactive protein (CRP) was measured with nephelometry by BN ProSpec analyzer (Siemens Healthcare Diagnostics, Munich, Germany). Serum GGT activity was assayed by Cobas c 501 analyzer (Roche Diagnostics GmbH). Plasma fibrinogen was assayed by the modified Clauss method using Fibrintimer II coagulometer and Multifibren U kit (Siemens Healthcare Diagnostics).
Individuals with diabetes were diagnosed with criteria of the American Diabetes Association[25], namely plasma fasting glucose ≥ 126 mg/dL (or 2 h postprandial glucose > 200 mg/dL) and/or the current use of diabetes medication. Prediabetes was identified by fasting glucose of 100-125 mg/dL. Individuals with MetS were identified when three out of the five criteria of the National Cholesterol Education Program (ATP III) were met, modified for prediabetes and for abdominal obesity using ≥ 95 cm as cutoff point in men, as assessed in the Turkish Adult Risk Factor study[18]. For women, the cutoff point ≥ 88 cm was retained based on our own prospective analyses. Homeostatic model assessment (HOMA) was estimated by the standard equation using fasting glucose and insulin levels.
Identification of CHD was based on the presence of angina pectoris, a history of myocardial infarction with or without accompanying Minnesota codes of the electrocardiogram (ECG)[26], or a history of myocardial revascularization. Typical angina and, in women, age > 45 years were prerequisite for a diagnosis when angina was isolated. ECG changes of “ischemic type” greater than minor degree (Codes 1.1-2, 4.1-2, 5.1-2, 7.1) were considered as myocardial infarct sequelae or myocardial ischemia, respectively. Death was identified via the information from first-degree relatives, records of local health personnel, and/or the nation-wide Identity Participation System.
We used a previously reported algorithm to detect fatty liver based on body mass index (BMI), waist circumference, triglycerides, and GGT[27] using the following equation.
= (e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference - 15.745)/(1 + e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference - 15.745) × 100
In agreement with the authors, we used an index < 30 to indicate absence of FLD, ≥ 60 for presence of FLD, and 30-59 for probable presence of FLD.
Descriptive parameters are shown as mean ± standard deviation or in percentages. Two-sided t-tests and Pearson’s χ2 tests were used to analyze the differences in means and proportions between groups. Due to the skewed distribution, log-transformed values were used for triglycerides, CRP, GGT, and Lp(a) for analyses. Analysis of variance (ANOVA) was used to detect difference across multiple groups, whereby a difference between two groups was determined using Bonferroni corrections. Estimates [and 95% confidence intervals (CI)] for relative risk (RR) of the dependent variable were obtained by use of Cox proportional hazard regression analyses in models that controlled for potential confounders, including cardiovascular risk factors and HOMA. A value of P < 0.05 on the two-sided test was considered statistically significant. Statistical analyses were performed using SPSS-10 for Windows (SPSS Inc., Chicago, IL, United States).
The study sample consisted of 1822 middle-aged adults (877 men and 945 women). CHD in 93 and diabetes in 103 subjects were identified at baseline. FLD was detected in 48% and there was no FLD in one-quarter of the sample at baseline. Follow-up averaged 8.0 ± 2.7 and 7.8 ± 2.8 years for mortality and incident CHD, respectively, with similar gender distribution (P = 0.52), yielding 14540 person-years for mortality. Fourteen percent of men and 1% of women were categorized as alcohol users. Six percent of males used alcohol at a daily mean equivalent to 19 mL ethanol and the remainder much less. Liver diseases of specific causes were not reported.
Table 1 shows the characteristics of the study sample at baseline, stratified to gender and FLD status. Significant differences in values across the sex-specific categories are noted in virtually all variables, except creatinine and testosterone, as well as age, fasting glucose, and Lp(a) in men. Notably, MetS traits were increased in subjects with FLD (blood pressure, HDL-cholesterol and glucose [borderline in males]), and insulin, apoB, SHBG, uric acid, and CRP levels were elevated as well. A significantly higher proportion of participants with no FLD were current smokers.
| n | Men (n = 877) | Women (n = 945) | |||||||
| No NAFLD | Probable NAFLD | NAFLD | ANOVAP value | No NAFLD | Probable NAFLD | NAFLD | ANOVA P value | ||
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | ||||
| n = 198 | n = 263 | n = 416 | n = 252 | n = 241 | n = 452 | ||||
| Age, yr | 1822 | 52.8 ± 12.7 | 53.4 ± 12 | 51.7 ± 9.3 | 0.16 | 47.5 ± 10.3 | 51.92± 10.5 | 54.8 ± 9.9 | < 0.001 |
| Waist circumference, cm | 1811 | 83.3 ± 8.1 | 92.42± 7.4 | 100.6 ± 9 | < 0.001 | 79 ± 9 | 892± 7.5 | 99.4 ± 9.7 | < 0.001 |
| Body mass index, kg/m2 | 1808 | 24.1 ± 3.3 | 27.22± 2.7 | 30.9 ± 4 | < 0.001 | 26.2 ± 4.4 | 30.22± 3.8 | 34.5 ± 5.5 | < 0.001 |
| Systolic BP, mmHg | 1822 | 120 ± 18 | 1262± 21 | 132.5 ± 23 | < 0.001 | 121 ± 19 | 1322± 24 | 142 ± 27 | < 0.001 |
| Diastolic BP, mmHg | 1822 | 75 ± 11 | 802± 11 | 85.6 ± 13.5 | < 0.001 | 77 ± 12 | 832± 13 | 88 ± 14 | < 0.001 |
| Total cholesterd, mg/dL | 1822 | 168 ± 34 | 1782± 34 | 192 ± 39 | < 0.001 | 174 ± 34 | 1902± 37 | 201 ± 41 | < 0.001 |
| LDL cholesterd, mg/dL | 1424 | 108 ± 32 | 112 ± 29 | 1162± 33 | 0.037 | 1052± 28 | 120 ± 33 | 126 ± 36 | < 0.001 |
| HDL cholesterd mg/dL | 1820 | 422± 12 | 37 ± 11 | 35.3 ± 11 | < 0.001 | 47.7 ± 13 | 46 ± 13 | 432± 12 | < 0.001 |
| Lipoprotein(a)1, mg/dL | 1186 | 8.3 × 2.74 | 7.76 × 2.77 | 9.12 × 3.14 | 0.34 | 11.8 × 2.76 | 12.7 × 3.13 | 10.47 × 2.9 | 0.11 |
| Fasted glucose, mg/dL | 1822 | 99 ± 27 | 100 ± 30 | 104 ± 37 | 0.099 | 97 ± 19 | 100 ± 31 | 1052± 35 | 0.001 |
| F. triglyceride1, mg/dL | 1413 | 95 × 1.48 | 1262× 1.55 | 174 × 1.72 | < 0.001 | 87 × 1.47 | 1102× 1.53 | 148 × 1.69 | < 0.001 |
| γ-glutamyl transferase1, U/L | 1822 | 17 × 1.48 | 21.92× 1.59 | 37.2 × 1.87 | < 0.001 | 12.9 × 1.62 | 15.82× 1.65 | 24.5 × 1.87 | < 0.001 |
| Fasted insulin, mIU/L | 1636 | 6.94 × 2.36 | 8.00 × 2.00 | 11.02× 2.02 | < 0.001 | 6.98 × 1.87 | 8.752× 1.97 | 11.0 × 1.87 | < 0.001 |
| Apolipoprotein A-I, g/L | 1740 | 1.40 ± 0.24 | 1.35 ± 0.24 | 1.3462± 0.24 | 0.042 | 1.55 ± 0.30 | 1.51 ± 0.26 | 1.50 ± 0.28 | 0.082 |
| Apolipoprotein B, g/L | 1759 | 0.942± 0.26 | 1.05 ± 0.19 | 1.09 ± 0.29 | < 0.001 | 1.03 ± 0.27 | 1.04 ± 0.28 | 1.132± 0.31 | < 0.001 |
| Creatinine, mg/dL | 1504 | 0.937± 0.17 | 0.976 ± 0.26 | 1.01 ± 0.37 | 0.023 | 0.80 ± 0.42 | 0.80 ± 0.44 | 0.805 ± 0.20 | 0.98 |
| SHBG1, nmol/L | 1304 | 43.8 × 1.7 | 39 × 1.6 | 33.42× 1.63 | < 0.001 | 55.4 × 1.7 | 46.22× 1.7 | 48.2 × 1.73 | < 0.001 |
| Testosterone1, nmol/L | 1412 | 19.2 × 4.2 | 15.8 × 3.3 | 15.1 × 3.3 | 0.16 | 0.79 × 3.66 | 0.70 × 3.1 | 0.79 × 3.8 | 0.50 |
| Uric acid, mg/dL | 1821 | 5.51 ± 1.3 | 5.82 ± 1.3 | 6.362± 1.6 | < 0.001 | 4.23 ± 1.2 | 4.62± 1.1 | 5.15 ± 1.4 | < 0.001 |
| C-reactive protein1, mg/L | 1788 | 1.642× 3.13 | 1.93 × 3.0 | 2.39 × 2.75 | < 0.001 | 1.41 × 3.15 | 2.532× 2.8 | 3.75 × 2.65 | < 0.001 |
| Current; past smokers, % | 1817 | 59.42; 18.8 | 47.5; 23.4 | 46.1; 25.4 | 0.037 | 27.42 ; 3.2 | 14.1; 3.3 | 10.2; 4.4 | < 0.001 |
| CHD prevalence, n (%) | 1820 | 5 (2.5) | 11 (4.2) | 31 (7.52) | 0.02 | 6 (2.4) | 11 (4.6) | 29 (6.4) | 0.056 |
Pearson correlations of the fatty liver index (FLI) with relevant variables in males and females are provided in Table 2. BP, fasting insulin, total cholesterol, apoB, uric acid, and CRP were positively correlated, and SHBG and HDL-cholesterol were inversely correlated, in each gender. Age and fasting glucose were positive correlates in women alone, and alcohol usage was a positive correlate in men, while apoA-I, Lp(a), creatinine, and testosterone were not correlated with the FLI.
| Men | Women | |||
| n | r | n | r | |
| Age | 877 | -0.04 | 945 | 0.28 |
| Body mass index | 869 | 0.71 | 939 | 0.68 |
| Systolic BP | 877 | 0.27 | 945 | 0.38 |
| Diastolic BP | 877 | 0.34 | 945 | 0.36 |
| Total cholesterol | 877 | 0.27 | 945 | 0.28 |
| HDL cholesterol | 877 | -0.22 | 945 | -0.19 |
| C-reactive protein1 | 864 | 0.16 | 924 | 0.41 |
| Apo A-I | 841 | -0.08 | 899 | -0.07 |
| Apo B | 844 | 0.18 | 915 | 0.19 |
| Lipoprotein(a)1 | 549 | 0.02 | 637 | -0.05 |
| Fasting glucose | 877 | 0.09 | 945 | 0.12 |
| Sex h-b globulin1 | 623 | -0.23 | 681 | -0.26 |
| Testosterone1 | 642 | -0.08 | 708 | 0.01 |
| Uric acid | 869 | 0.23 | 938 | 0.20 |
| Creatinine | 735 | 0.04 | 785 | 0.00 |
| Fasting insulin | 775 | 0.33 | 861 | 0.33 |
| Alcohol usage | 873 | 0.11 | 941 | -0.06 |
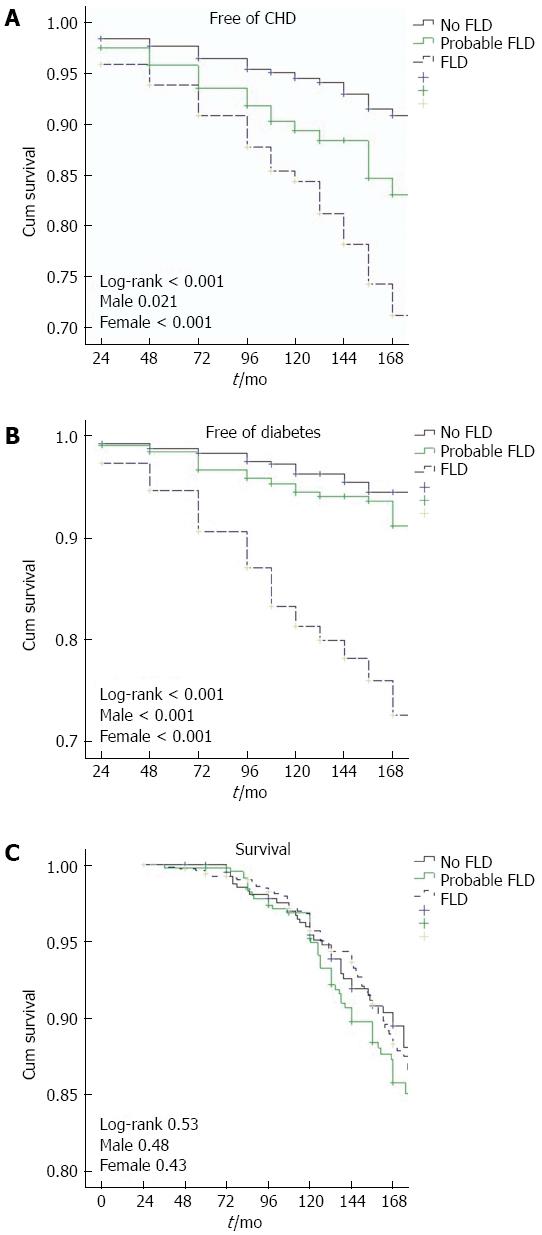
Kaplan-Meier plots were constructed for survival and survival free of diabetes/CHD, as seen in Figure 1. These demonstrated significantly lower survival free of diabetes and of incident CHD for participants with FLD at baseline. Subjects categorized as probable FLD also separated from those with no FLD in regard to CHD. However, overall survival curves were similar in the three groups.
Table 3 displays findings of Cox regression analyses for the prediction by FLD of diabetes mellitus and CHD, adjusted for five other conventional cardiovascular risk factors and stratified to gender. It is evident that FLD was (with a 5-fold relative risk) the major determinant of the development of diabetes, besides (inversely) serum uric acid. In regard to incident CHD, FLD proved to be a significant predictor [hazard ratio (HR) = 1.72, 95%CI: 1.17-2.53] independent of age, presence of diabetes, systolic BP, and current smoking.
| Total | Men | Women | ||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | |
| Diabetes n = | 203/14901 | 110/7071 | 93/7831 | |||
| Gender, female | 0.82 | 0.56; 1.20 | ||||
| Age, 11 yr2 | 1.14 | 0.97; 1.34 | 1.13 | 0.90; 1.41 | 1.14 | 0.90; 1.46 |
| Current smoking, n = 480 | 1.38 | 0.94; 2.00 | 1.35 | 0.84; 2.16 | 1.39 | 0.74; 2.69 |
| Former smoking, n = 183 | 1.32 | 0.83; 2.08 | 1.22 | 0.72; 2.06 | 1.98 | 0.72; 5.48 |
| HDL-cholesterol, 12 mg/dL | 0.91 | 0.78; 1.06 | 0.96 | 0.77; 1.21 | 0.89 | 0.71; 1.10 |
| Systolic BP, 25 mmHg | 1.16 | 1.00; 1.38 | 1.28 | 1.03; 1.60 | 1.08 | 0.88; 1.35 |
| Uric acid, 1.3 mg/dL | 0.87 | 0.76; 0.996 | 0.81 | 0.67; 0.97 | 0.94 | 0.76; 1.15 |
| Probable FLD, n = 423 | 1.44 | 0.80; 2.57 | 1.57 | 0.72; 3.43 | 1.28 | 0.52; 3.12 |
| FLD, n = 673 | 4.93 | 2.98; 8.14 | 4.80 | 2.40; 9.59 | 5.33 | 2.53; 11.2 |
| DM incid. per 1000 person-yr | 16.6 | 18.8 | 14.5 | |||
| CHD: Diabetic sample n = | 88/4091 | 45/1921 | 43/2171 | |||
| Gender, female | 0.95 | 0.55; 1.63 | ||||
| Age, 11 years | 1.18 | 0.94; 1.49 | 1.27 | 0.90; 1.80 | 1.10 | 0.79; 1.56 |
| Current smoking | 1.12 | 0.64; 1.95 | 1.46 | 0.68; 3.15 | 0.66 | 0.25; 1.75 |
| Former smoking | 1.13 | 0.56; 2.31 | 1.36 | 0.59; 3.13 | 1.12 | 0.15; 8.52 |
| Alcohol usage, yes/no | 0.50 | 0.12; 2.10 | 0.58 | 0.14; 2.45 | No user | |
| HDL-cholesterol, 12 mg/dL | 0.78 | 0.61; 1.00 | 0.90 | 0.62; 1.28 | 0.69 | 0.48; 0.99 |
| Systolic BP, 25 mmHg | 0.95 | 0.72; 1.28 | 0.98 | 0.60; 1.60 | 0.93 | 0.65; 1.31 |
| Uric acid, 1.3 mg/dL | 0.98 | 0.79: 1.23 | 0.73 | 0.54: 0.999 | 1.35 | 0.99: 1.84 |
| Probable FLD | 1.45 | 0.66; 3.18 | 1.68 | 0.56; 5.05 | 1.32 | 0.42; 4.11 |
| FLD | 3.59 | 1.78; 7.23 | 4.61 | 1.72; 12.4 | 3.12 | 1.17; 8.35 |
| CHD inciden. per 1000 person-yr | 25.3 | 27.4 | 23.4 | |||
| CHD: Whole sample n = | 237/15051 | 104/716 | 133/7891 | |||
| Gender, female | 1.34 | 0.96; 1.86 | ||||
| Age, 11 yr | 1.48 | 1.27; 1.71 | 1.54 | 1.26; 2.00 | 1.43 | 1.17; 1.73 |
| Current smoking | 1.50 | 1.06; 2.13 | 1.80 | 1.12; 2.90 | 1.13 | 0.64; 1.98 |
| Former smoking | 1.02 | 0.64; 1.63 | 0.92 | 0.51; 1.64 | 1.82 | 0.79; 4.17 |
| Alcohol usage, yes/no | 0.80 | 0.37; 1.74 | 0.79 | 0.36; 1.75 | Too few | |
| HDL-cholesterol, 12 mg/dL | 0.90 | 0.78; 1.02 | 1.04 | 0.84; 1.28 | 0.81 | 0.69; 0.98 |
| Systolic BP, 25 mmHg | 1.25 | 1.08; 1.42 | 1.31 | 1.12; 1.27 | 1.25 | 1.05; 1.45 |
| Presence of diabetes | 1.44 | 0.98; 2.13 | 1.81 | 1.04; 3.17 | 1.13 | 0.65; 1.95 |
| Probable FLD | 1.26 | 0.83; 1.92 | 1.79 | 0.92; 3.47 | 0.99 | 0.57; 1.73 |
| FLD | 1.72 | 1.17; 2.53 | 2.35 | 1.25; 4.43 | 1.42 | 0.86; 2.35 |
| CHD incid. per 1000 person-yr | 18.9 | 17.5 | 19.7 | |||
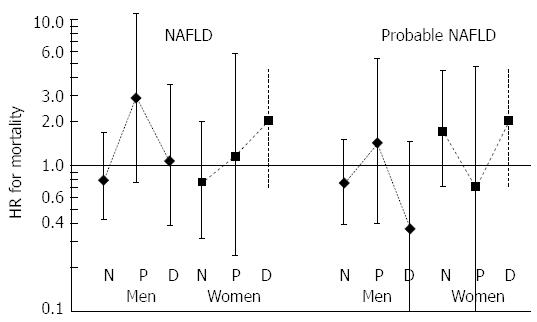
With respect to overall mortality, however, age, diabetes, and -in non-diabetic men- current smoking were determinants, whereas FLD and HOMA index did not emerge as independent predictors (Table 4). We further analyzed similar regression models for mortality stratifying to glucose categories (normoglycemia, impaired fasting glucose and diabetes). Tendency to excess independent mortality risk for FLD (HR = 2.69, 95%CI: 0.42-17) and HOMA index was restricted to prediabetic men in whom age had an exceptionally high HR. In women - albeit non-significant - increasing HRs were noted for both FLD and HOMA index from normoglycemia to diabetes categories.
| Total | Men | Women | ||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | |
| Diabetic sample n = | 30/1181 | 20/591 | 10/591 | |||
| Gender, female | 0.71 | 0.25; 2.00 | ||||
| Age, 11 yr | 2.74 | 1.43; 5.22 | 2.61 | 1.19; 5.73 | 3.48 | 1.02; 12.0 |
| Current vs non-smoker | 0.94 | 0.30; 3.02 | 1.38 | 0.37; 5.18 | n = 2 | protecting |
| Former smoking, n = 170 | 1.17 | 0.40; 3.40 | 2.20 | 0.61; 7.85 | n = 2 | Too few |
| HDL-cholesterol, 12 mg/dL | 0.81 | 0.55; 1.20 | 0.72 | 0.41; 1.24 | 0.77 | 0.43; 1.36 |
| Systolic BP, 25 mmHg | 1.16 | 0.74; 1.81 | 0.82 | 0.40; 1.72 | 1.25 | 0.62; 2.60 |
| HOMA index, 2-fold | 1.15 | 0.89; 1.49 | 1.03 | 0.75; 1.41 | 1.47 | 0.95; 2.27 |
| Probable FLD, n = 25 | 0.48 | 0.15; 1.55 | 0.35 | 0.09; 1.34 | n = 12 | risk- |
| FLD, n = 66 | 0.74 | 0.24; 2.24 | 0.72 | 0.20; 2.57 | n = 36 | conferring |
| Death rate per 1000 person-yr | 30.2 | 38.2 | 22.4 | |||
| Prediabetic sample2n = | 32/2681 | 17/1191 | 15/1491 | |||
| HOMA index, 2-fold | 1.25 | 0.93; 1.68 | 1.33 | 0.80; 2.23 | 1.32 | 0.87; 2.00 |
| Probable FLD n = 57 | 1.05 | 0.25; 4.40 | 0.70 | 0.10; 4.77 | 1.19 | 0.10; 14.5 |
| FLD n = 116 | 2.07 | 0.52; 8.19 | 2.69 | 0.42; 17.0 | 1.30 | 0.12; 14.7 |
| Death rate per 1000 person-yr | 14.0 | 17.2 | 11.8 | |||
| Normoglycemic sample n = | 89/12681 | 54/6101 | 35/6581 | |||
| Gender, female | 0.77 | 0.42; 1.40 | ||||
| Age, 11 yr | 3.76 | 2.85; 4.97 | 3.91 | 2.69; 5.68 | 3.41 | 2.24; 5.17 |
| Current vs never smoking | 2.39 | 1.27; 4.51 | 2.99 | 1.36; 6.56 | 0.98 | 0.22; 4.38 |
| Former smoking, n = 170 | 1.04 | 0.46; 2.34 | 1.16 | 0.47; 2.87 | n = 15 | Too few |
| HDL-cholesterol, 12 mg/dL | 1.05 | 0.84; 1.31 | 1.07 | 0.78; 1.46 | 1.07 | 0.78; 1.51 |
| Systolic BP, 25 mmHg | 1.03 | 0.80; 1.31 | 1.13 | 0.80; 1.64 | 0.98 | 0.67; 1.38 |
| HOMA index, 2-fold | 1.02 | 0.88; 1.17 | 1.10 | 0.94; 1.29 | 0.79 | 0.62; 1.007 |
| Probable FLD n = 312 | 0.89 | 0.47; 1.68 | 0.55 | 0.24; 1.31 | 2.02 | 0.75; 5.39 |
| FLD n = 500 | 0.68 | 0.34; 1.36 | 0.58 | 0.24; 1.40 | 0.96 | 0.32; 2.88 |
| Death rate per 1000 person-yr | 8.1 | 10.0 | 6.3 | |||
| Whole sample n = | 151/16541 | 91/7881 | 60/8661 | |||
| Gender, female | 0.73 | 0.46; 1.17 | ||||
| Age, 11 yr3 | 3.38 | 2.69; 4.19 | 3.48 | 2.60; 4.65 | 3.34 | 2.36; 4.74 |
| Current vs never smoking, n = 414 | 1.67 | 1.03; 2.70 | 2.01 | 1.13; 3.57 | 0.96 | 0.29; 3.17 |
| Former smoking, n = 170 | 0.92 | 0.52; 1.64 | 0.99 | 0.52; 1.88 | n = 22 | Too few |
| Alcohol usage, yes/no, n = 116 | 0.84 | 0.37; 1.90 | 0.73 | 0.32; 1.67 | n = 2 | Too few |
| HDL-cholesterol, 12 mg/dL | 1.02 | 0.87; 1.11 | 1.09 | 0.85; 1.38 | 0.94 | 0.73; 1.22 |
| Systolic BP, 25 mmHg | 1.03 | 0.86; 1.25 | 1.00 | 0.78; 1.31 | 1.08 | 0.84; 1.42 |
| Prediabetes, n = 220 | 1.30 | 0.82; 2.07 | 1.08 | 0.56; 2.09 | 1.73 | 0.89; 3.35 |
| Presence of diabetes, n = 104 | 2.70 | 1.73; 4.22 | 2.22 | 1.52; 4.86 | 2.76 | 1.32; 5.76 |
| HOMA index, 2-fold | 1.08 | 0.97; 1.21 | 1.12 | 0.99; 1.27 | 0.98 | 0.80; 1.20 |
| Probable FLD, n = 394 | 0.79 | 0.48; 1.31 | 0.58 | 0.31; 1.11 | 1.42 | 0.57; 3.50 |
| FLD n = 681 | 0.84 | 0.50; 1.39 | 0.83 | 0.44; 1.56 | 1.03 | 0.41; 2.59 |
| Death rate per 1000 person-yr | 10.8 | 13.3 | 7.9 | |||
In order to assess whether some of the components of the FLI, rather than the overall algorithm, were determinants of outcomes, we analyzed the Cox models separately with the four components (Table 5). These demonstrated a greater impact of triglycerides and GGT levels and - to some extent - of abdominal obesity but not of overall obesity, which interestingly and significantly protected against risk of death.
| Total | Men | Women | ||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | |
| Diabetes | ||||||
| Waist circumference, 12 cm | 1.70 | 1.33; 2.15 | 1.64 | 1.13; 2.38 | 1.78 | 1.27; 2.46 |
| Body mass index, 5 kg/m2 | 1.10 | 0.91; 1.33 | 1.06 | 0.80; 1.40 | 1.11 | 0.86; 1.44 |
| Triglycerides, 90 mg/dL | 1.22 | 1.06; 1.40 | 1.09 | 0.91; 1.31 | 1.43 | 1.20; 1.71 |
| γ-glutamyltransferase, 1.7-fold | 2.19 | 1.61; 2.99 | 2.44 | 1.52; 3.92 | 2.22 | 1.44; 3.43 |
| CHD | ||||||
| Waist circumference, 12 cm | 1.21 | 0.80; 1.82 | 1.21 | 0.20; 1.82 | 1.02 | 0.78; 1.33 |
| Body mass index, 5 kg/m2 | 1.01 | 0.84; 1.20 | 0.77 | 0.52; 1.14 | 0.98 | 0.80; 1.20 |
| Triglycerides, 90 mg/dL | 1.19 | 1.03; 1.35 | 1.20 | 1.01; 1.43 | 1.12 | 0.84; 1.31 |
| γ-glutamyltransferase, 1.7-fold | 1.52 | 0.94; 2.44 | 1.52 | 0.94; 2.44 | 1.64 | 1.16; 2.31 |
| Mortality | ||||||
| Waist circumference, 12 cm | 1.31 | 1.00; 1.74 | 1.21 | 0.81; 1.80 | 1.38 | 0.90; 2.11 |
| Body mass index, 5 kg/m2 | 0.73 | 0.56; 0.96 | 0.68 | 0.45; 1.03 | 0.82 | 0.56; 1.20 |
| Triglycerides, 90 mg/dL | 1.12 | 0.96; 1.31 | 1.28 | 1.01; 1.57 | 0.91 | 0.70; 1.20 |
| γ-glutamyltransferase, 1.7-fold | 1.37 | 0.97; 1.93 | 1.28 | 0.79; 2.06 | 1.62 | 0.95; 2.77 |
Risk of death related to the three glucose categories is schematized in Figure 2.
In this follow-up analysis of a cohort representative of middle-aged and elderly Turkish adults, we examined the independent predictive value of FLD, derived from a FLI, for the risks of type-2 diabetes, CHD, and overall mortality. The FLI was correlated with MetS traits as well as with markers of enhanced low-grade systemic inflammation (total cholesterol, apoB, uric acid, CRP, and SHBG levels). FLD was a powerful predictor of incident diabetes and disclosed a nearly 2-fold relative risk for CHD compared to participants without FLD. All-cause mortality, however, was not independently related to baseline FLD, or to HOMA index, except for disclosing a tendency in prediabetic men and a tendency in women increasing in categories from normoglycemia to diabetes. These findings are in agreement with most previous reports and -regarding mortality- with studies on general population samples. We suspect the discrepancy between the relationship to outcomes (mortality vs cardiometabolic risk) in subjects with FLD is a consequence of (gender-modulated) circulating Lp(a) levels.
The close association between impaired glucose regulation and lipid metabolism with NAFLD is widely recognized[28]. It has been proposed that fatty liver represents a (novel) component of the MetS[2]. Correlation of the FLI with MetS traits in the present study is in line with this and other reports. The index was also significantly correlated with serum apoB, uric acid, and CRP and inversely correlated with circulating SHBG-all markers of proinflammatory state in our experience. Hence, male and female participants identified with FLD harbored both MetS components and markers of enhanced subclinical inflammation.
In these middle-aged and elderly adults, FLD prevailed in 48%, a substantially higher prevalence than in other population samples. In a slightly younger adult sample from the United States, ultrasonography-defined NAFLD prevalence was reported as 19.5%[16]. Current participants with FLD likely represent, moreover, a higher degree of fat accumulation and inflammation in the liver, as may be assessed from stronger HRs associated with incident cardiometabolic risk. NAFLD prevailed at a lower rate in other reports[4,5] as well.
We confirmed results of previous prospective studies documenting significant prediction of type-2 diabetes by NAFLD diagnosed by ultrasonography. In study samples exceeding 12000 subjects, Yamada et al[4] in Japanese and Sung et al[5] in South Korean people found over 5-year follow-ups that NAFLD independently predicted diabetes risk at about 2- to 2.5-fold HRs, respectively. In prior prospective studies on Japanese people with smaller sample sizes[29,30], the related HRs ranged between a non-significant value and 4.6. In this study, the predictive value of FLD for this association was over 5-fold that of individuals without FLD, independent of sex, age, smoking status, systolic blood pressure, serum HDL-cholesterol level, and uric acid level. This HR was similar to that found in French men but lower than that in women in the highest versus the lowest quartile of FLI[31].
NAFLD has been shown to predict incident CVD[6-9]. The prospective analysis over a 14-year follow-up in approximately 11600 participants of NHANES-III demonstrated an independent association between NAFLD by ultrasonography and cardiovascular disease[9], similar to our current findings. However, the strong predictive ability of FLD for CHD among our diabetic subjects was substantially attenuated in the whole male sample when diabetes was included in the adjustments and was reduced to a non-significant level in female participants. It appears that in Turkish women who are prone to autoimmune activation NAFLD and diabetes, each conferring CHD risk, emerging bidirectional changes[1] mediate each other and attenuate this risk. Thus, both the substrate (prevalent CHD or diabetes) and gender modulate this risk. In fact, in Chinese patients with suspected CHD (n = 713), significant association between FLI and CHD was not detected[32].
Reasons for the paradoxical lack of NAFLD on mortality risk remain unclear. Using the NHANES-III survey data, all-cause mortality for alanine transferase-defined NAFLD over a mean 8.7 year follow-up was marginally increased (HR = 1.37)[13], a risk confined to the age group 45-54. Women, Mexican Americans, non-smokers, and those with MetS or diabetes were more likely to have NAFLD. A more recent analysis of NHANES-III survey data confirmed that ultrasonography-defined NAFLD did not increase the risk of mortality[33]. However, NAFLD with evidence of advanced fibrosis (only one out of 30 NAFLD cases) using non-invasive marker panels was associated with increased mortality, mainly attributable to cardiovascular causes. NAFLD fibrosis score was based on an algorithm using additional data on impaired fasting glucose/diabetes, as well as inflammation-related parameters such as aspartate aminotransferase/alanine aminotransferase ratio, platelet count, and serum albumin level.
GGT, a participant in the degradation of the antioxidant glutathione and, hence, capable of inducing pro-oxidant effect, is a major component of the FLI. GGT was shown to be independently and inversely associated with the mean low density lipoprotein (LDL) particle size in asymptomatic elderly subjects with dyslipidemia[34]. Though the FLI was found to predict all-cause mortality in the Cremona study, characterized by a cohort having a high prevalence of MetS and insulin resistance, it was the significant association of the HOMA index with the FLI that emerged as a mediator of mortality risk[15]. Lp(a) constitutes a typical example of small dense LDL and was documented elsewhere[35] and in the TARF study[36] to be inversely associated with HOMA index.
Our multivariable analysis with the four components of the FLI explains in part the lack of association with risk of death, insofar as BMI emerged (especially in men) as protective against mortality risk. Moreover, on our previous findings[37] showed that a disparate independent association existed among sexes between serum GGT and Lp(a) levels, with high Lp(a) levels in men and low levels in women (reflecting autoimmune activation), and this may have been pivotal for the associations of FLI with the risks of death and, in women, with incident CHD. Diabetic status, a major confounder of and interactor with an underlying autoimmune activation, may have, therefore, largely mediated FLD [and low Lp(a) concentrations] and attenuated the outcome of mortality.
Since FLD, HOMA index, and age in current prediabetic men had higher HRs and higher Lp(a) concentrations than in the remaining two categories, serum Lp(a) may not be involved in the autoimmune complex underlying its relation to FLD and HOMA index, which is in contrast to the relatively elevated HRs for mortality. The independent contribution to the risk of death, likely via cardiorenal disease, may well be reflected in the high HR of age. In women, the persistent increase in HRs of FLD and HOMA index may be a consequence of the increasing involvement of circulating Lp(a) in autoimmune activation from normoglycemia onwards.
Our observations in men support the view that the development of diabetes from prediabetes attenuates the independent risk of death for FLD[38,39]. Age-adjusted mortality in patients with NAFLD was, indeed, reported to be associated with IFG[40].
A critical role of serum GGT in the pathogenesis of IFG was suggested in a large Korean population-based study that assessed the varying association of the enzyme level with BMI[41]. In our evaluation of subjects with IFG, we observed a parallel trend between serum Lp(a) and GGT in women regardless of the presence of MetS but in men in the absence of MetS alone[20].
FLD was defined herein not by imaging methods or histology but by an algorithm based on obesity markers, fasting triglyceride, and GGT levels. This method has been validated in several epidemiologic studies[15,23], and other methods are costly and impractical to identify FLD in large epidemiologic studies. Collinearity between FLI and metabolic factors such as Lp(a) levels or obesity cannot be ruled out. The study sample size, long follow-up, and analysis stratified to gender are strengths of the study. Concomitant investigation of diabetes, CHD, and overall mortality in the same study sample is a major strength that allowed for the detection of emerging differences in the underlying pathogenesis.
FLD, defined by a FLI, was detected in one-half of a population-based cohort. FLD was a powerful predictor of incident diabetes and disclosed a nearly 2-fold HR for the risk of CHD, compared to participants without FLD. In essential agreement with most previous reports on general population samples, all-cause mortality, however, was not independently related to baseline FLD, or to HOMA index, except for a tendency in prediabetic men as well as prediabetic and diabetic women. Associations between BMI, GGT, and Lp(a) concentrations may herein be pivotal. Further research seeking the association between FLD and mortality risk should address the impact of circulating Lp(a) in the separate glycemic states in larger population samples.
Liver biopsy, ultrasonography, serum liver enzymes, and, more recently, an algorithm-based surrogate of fatty liver disease (FLD) have been commonly used in identifying the presence of non-alcoholic fatty liver disease (NAFLD), closely associated with features of the metabolic syndrome (MetS), and its relationship to adverse outcomes. NAFLD, a growing public health issue, has been demonstrated to be associated with MetS, type-2 diabetes cardiovascular events, and chronic kidney disease, but controversy exists on its predictive ability for overall mortality.
Diabetic status, a recognized major confounder in the bidirectional relationship between FLD and cardiovascular morbidity and mortality, appears to be a major area requiring future research. Another hotspot that further research should be engaged, especially in population subgroups prone to metabolic syndrome, is the potential disparate independent association potentially existing among sexes between serum γ-glutamyltransferase (GGT) (a component of the FLI) and lipoprotein (Lp)(a) levels and their influence on outcome.
The lack of a relationship between NAFLD and risk of overall mortality as compared to its independent prediction of diabetes and cardiovascular disease has been intriguing. An algorithm consisting of body mass index, waist circumference, triglycerides, and GGT has been used elsewhere and herein to detect fatty liver. Confirmation in the present study that risk of death was essentially not predicted by FLI may be due to underlying involvement of circulating Lp(a) in autoimmune activation and the generally confounding role of diabetes, which may have largely mediated FLD and attenuated the outcome of mortality.
The previously proposed FLD index may reliably be utilized as a surrogate in population screening for the detection of fatty liver.
Steatohepatitis designates fatty infiltration and inflammation of the liver.
The authors examined prospectively in over 1800 middle-aged Turkish adults the association of a surrogate of FLD, consisting of adiposity measures, triglyceride, and GGT levels, with type-2 diabetes, coronary heart disease (CHD), and all-cause mortality. Multivariably adjusted Cox regression analyses were used. Over an average 8-year follow-up, FLD was found as the major determinant of incident diabetes at a high relative risk. CHD was significantly and independently predicted by FLD in men alone and in the whole study sample. Despite these, and in line with several previous reports on the controversial topic, the authors detected no significant excess risk of death, though a tendency to increased risk was observed in the prediabetic state. Authors attributed the lack of prediction by FLI possibly to serum Lp(a) being involved in autoimmune activation and to a confounding role of the diabetic status mediating FLD.
P- Reviewer: Abenavoli L, Daltro C, Perazzo H S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 2. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 3. | Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Yamada T, Fukatsu M, Suzuki S, Wada T, Yoshida T, Joh T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol. 2010;25:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Loria P, Lonardo A, Bellentani S, Day CP, Marchesini G, Carulli N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: an open question. Nutr Metab Cardiovasc Dis. 2007;17:684-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 8. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1487] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 9. | Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer - a narrative review. Clin Chem Lab Med. 2010;48:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Targher G, Chonchol M, Zoppini G, Abaterusso C, Bonora E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J Hepatol. 2011;54:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 15. | Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, Bosi E, Ruotolo G, Piemonti L, Perseghin G. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 18. | Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Can G. Determinants and definition of abdominal obesity as related to risk of diabetes, metabolic syndrome and coronary disease in Turkish men: a prospective cohort study. Atherosclerosis. 2007;191:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 19. | Onat A, Can G, Çiçek G, Ayhan E, Doğan Y, Kaya H. Fasting, non-fasting glucose and HDL dysfunction in risk of pre-diabetes, diabetes, and coronary disease in non-diabetic adults. Acta Diabetol. 2013;50:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Onat A, Aydın M, Can G, Cakmak HA, Köroğlu B, Kaya A, Ademoğlu E. Impaired fasting glucose: Pro-diabetic, "atheroprotective" and modified by metabolic syndrome. World J Diabetes. 2013;4:210-218. [PubMed] |
| 21. | Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 22. | Onat A, Can G. Enhanced proinflammatory state and autoimmune activation: a breakthrough to understanding chronic diseases. Curr Pharm Des. 2014;20:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Lerchbaum E, Pilz S, Grammer TB, Boehm BO, Stojakovic T, Obermayer-Pietsch B, März W. The fatty liver index is associated with increased mortality in subjects referred to coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Onat A. Risk factors and cardiovascular disease in Turkey. Atherosclerosis. 2001;156:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32 Suppl 1:S13-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1215] [Cited by in RCA: 1237] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 26. | Rose G, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. 2nd ed. Geneva, Switzerland: WHO 1982; 124-127. |
| 27. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2036] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 28. | Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Okamoto M, Takeda Y, Yoda Y, Kobayashi K, Fujino MA, Yamagata Z. The association of fatty liver and diabetes risk. J Epidemiol. 2003;13:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Fan JG, Li F, Cai XB, Peng YD, Ao QH, Gao Y. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol. 2007;22:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Balkau B, Lange C, Vol S, Fumeron F, Bonnet F. Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol. 2010;10:56. [PubMed] |
| 32. | Jiang ZY, Xu CY, Chang XX, Li WW, Sun LY, Yang XB, Yu LF. Fatty liver index correlates with non-alcoholic fatty liver disease, but not with newly diagnosed coronary artery atherosclerotic disease in Chinese patients. BMC Gastroenterol. 2013;13:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 627] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 34. | Kotani K, Tsuzaki K, Sakane N. The relationship between gamma-glutamyltransferase (GGT), bilirubin (Bil) and small dense low-density lipoprotein (sdLDL) in asymptomatic subjects attending a clinic for screening dyslipidaemias. Ann Acad Med Singapore. 2014;43:216-219. [PubMed] |
| 35. | Rainwater DL, Haffner SM. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler Thromb Vasc Biol. 1998;18:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Onat A, Çoban N, Can G, Yüksel M, Karagöz A, Yüksel H, Ademoğlu E, Erginel-Ünaltuna N. Low "quotient" Lp(a) concentration mediates autoimmune activation and independently predicts cardiometabolic risk. Exp Clin Endocrinol Diabetes. 2015;123:11-18. [PubMed] |
| 37. | Onat A, Hergenç G, Ozhan H, Kaya Z, Bulur S, Ayhan E, Can G. Lipoprotein(a) is associated with coronary heart disease independent of metabolic syndrome. Coron Artery Dis. 2008;19:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 39. | Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 40. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2129] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 41. | Hong NS, Kim JG, Lee YM, Kim HW, Kam S, Kim KY, Kim KS, Lee DH. Different associations between obesity and impaired fasting glucose depending on serum gamma-glutamyltransferase levels within normal range: a cross-sectional study. BMC Endocr Disord. 2014;14:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |