Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13490
Peer-review started: July 7, 2015
First decision: August 2, 2015
Revised: August 16, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: December 28, 2015
Processing time: 171 Days and 20.2 Hours
AIM: To compare therapeutic outcomes and adverse events in initial solitary hepatocellular carcinoma (HCC) treated with radiofrequency ablation (RFA) and CyberKnife®.
METHODS: Seventy three consecutive patients with initial solitary HCC treated with RFA (38 patients; RFA group) and CyberKnife® (35 patients; CK group) were enrolled in this study. Background factors were compared between the two groups. Local and intrahepatic distant recurrence control, and cumulative survival rates were compared between the two groups. These were determined using the Kaplan-Meier method, and the significance of differences was analyzed by log-rank test. The presence of more grade 3 on CTCAE ver. 4.0 early and late adverse events was investigated.
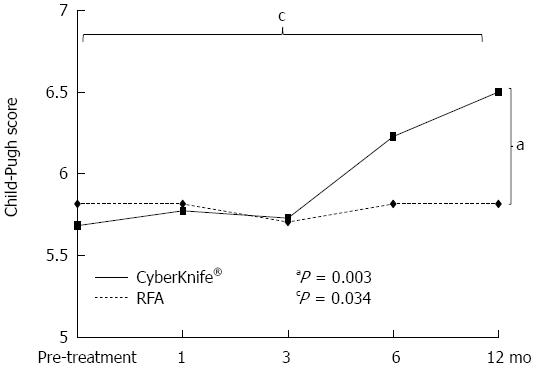
RESULTS: In background factors, age was significantly higher (P = 0.005) and the tumor diameter was significantly larger (P = 0.001) in the CK group. The 1-year local recurrence control rates were 97.4% and 97.1% in the RFA and CK groups, respectively (P = 0.71); the 1-year intrahepatic distant recurrence control rates were 85.6% and 86.1%, respectively (P = 0.91); and the 1-year cumulative survival rates were 100% and 95.2%, respectively (P = 0.075), showing no significant difference in any rate between the two groups. There were no late adverse event in the RFA group, but 11.4% in the CK group had late adverse events. In the CK group, the Child-Pugh score at 12 mo after treatment was significantly higher than that in the RFA group (P = 0.003) and significantly higher than the score before treatment (P = 0.034).
CONCLUSION: The occurrence of adverse events is a concern, but CyberKnife® treatment is likely to become an important option for local treatment of early HCC.
Core tip: To compare therapeutic outcomes and adverse events in initial solitary hepatocellular carcinoma (HCC) treated with radiofrequency ablation (RFA; 38 patients) or CyberKnife® (35 patients). The 1-year local recurrence control, the 1-year intrahepatic distant recurrence control and the 1-year cumulative survival rates were no significant difference in any rate between the two groups. In the CyberKnife® group, the Child-Pugh score at 12 mo after treatment was significantly higher than that in the RFA group and significantly higher than the score before treatment. The occurrence of adverse events is a concern, but CyberKnife® is likely to become an important option for local treatment of early HCC.
- Citation: Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kogame M, Kishimoto Y, Okubo Y, Makino H, Tsukamoto N, Igarashi Y, Sumino Y. Comparison of percutaneous radiofrequency ablation and CyberKnife® for initial solitary hepatocellular carcinoma: A pilot study. World J Gastroenterol 2015; 21(48): 13490-13499
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13490.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13490
Hepatocellular carcinoma (HCC) is a common malignancy worldwide, causing more than 500000 deaths every year. The incidence of HCC has increased globally due to the spread of hepatitis B and C virus infections[1,2]. In Japan, therapeutic policy for HCC is mainly decided based on the Evidence-based Clinical Practice Guidelines for HCC developed by the Japan Society of Hepatology (JSH)[3]. For HCC with liver damage A or B and 3 or fewer tumors with a diameter of 3 cm or smaller, liver resection and percutaneous ablation therapy are selected. However, most patients with HCC confined to the liver are not candidates for resection because of the frequent association with cirrhosis and other contraindications. Liver resection is also associated with a recurrence rate of 40%-60%[4,5]. Thus, many HCC patients are treated with percutaneous ablation therapy. Radiofrequency ablation (RFA) was introduced in Japan in 1999 and has been covered by national health insurance since April 2004. RFA is performed at many institutions because it can coagulate a wide area in one session compared to other percutaneous treatments, such as percutaneous ethanol injection therapy, and local control is high[6].
CyberKnife® (Accuray Incorporated, Sunnyvale, CA, United States) stereotactic body radiotherapy (SBRT) is image-guided robotic radiosurgery using a radiation delivery platform that can detect and correct for intrafraction tumor motion, as well as adapt to the patient’s breathing pattern by moving the linear accelerator in concert. CyberKnife® was developed in the United States in 1992, first applied clinically in 1994, and introduced in Japan in 1997. CyberKnife® can be used to perform multidirectional irradiation and disperse the dose among normal tissues due to a high degree of freedom in the direction of irradiation. Therefore, irradiation in CyberKnife® treatment is more intensive than that in SBRT using a conventional Liniac system.
The therapeutic indications of CyberKnife® originally included brain tumor and head and neck cancer. Use for cancer in the trunk, including HCC, was begun after approval in June 2008. Tumors in the trunk move with respiration, but CyberKnife® detects minute body movements and fine-tunes the irradiation angle using a seeker. Therefore, this approach has potential as a novel local treatment for HCC due to its low invasiveness and reduced burden on the patient[7,8].
In the 10 years since introduction of RFA for treatment of HCC in Japan, the therapeutic outcome and adverse events in RFA-treated HCC have been widely reported[9,10]. In contrast, there have only been a few studies on the therapeutic outcome of HCC treated with SBRT including CyberKnife®[7,8,11-13] and, to our knowledge, there has been no comparison of therapeutic outcomes and adverse events in HCC between RFA and CyberKnife®. This comparison is important in determining the indication of CyberKnife® treatment for HCC. In this study, we compared therapeutic outcomes and adverse events in patients with initial solitary HCC treated with RFA or CyberKnife® in almost the same period at our hospital and related institutions, and retrospectively investigated the efficacy of CyberKnife® for HCC treatment.
The subjects were 73 patients with initial solitary HCC without comorbidity like cardiac, pulmonary and celebral diseases, and treated with RFA or CyberKnife® between October 2011 and September 2014 at our hospital and related institutions. There were 38 consecutive patients treated with RFA (RFA group) and 35 consecutive patients treated with CyberKnife® (CK group). All patients were diagnosed with HCC using gray-scale ultrasonography (US), dynamic computed tomography (CT) and Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) (EOB-MRI) based on the new guideline of the American Association for the Study of Liver Diseases[14]. Serum α-fetoprotein (AFP), AFP-L3 fraction, and des-γ-carboxyprothrombin (DCP) levels were referred to, as needed. If a diagnosis was difficult based on these examinations, ultrasound-guided percutaneous transhepatic tumor biopsy was performed and the diagnosis was made histopathologically.
We chose treatments for all patients based on the Evidence-based Clinical Practice Guidelines for HCC published by the JSH. The RFA group comprised patients with liver damage A or B, and a solitary tumor with a diameter ≤ 3 cm, and in whom liver resection was not indicated because they were elderly, had other underlying diseases, or did not want liver resection. Among the adaptation cases of RFA, we chose CyberKnife® treatment for elderly patients and patients with respiratory disease for whom breath-holding was difficult, those for whom RFA could not be safely performed because of the location of HCC, and those who requested CyberKnife® treatment. The patients met the following indications established at related institutions based on reports on HCC treated with SBRT: (1) A performance status ≤ 2; (2) Child-Pugh classification A to B (scored 8); (3) serum T-Bil ≤ 3 mg/dL; (4) ICG 15-min ≤ 50%; (5) absence of ascites; (6) solitary tumor ≤ 5 cm; (7) tumor located ≥1 cm from the intestine; (8) tumor not in contact with the gall bladder; and (9) absence of distant metastasis. Patients in both groups were all initial (non-recurrent) cases.
RFA was performed using a Cool-tip RF System (Covidian, Boulder, CO, United States) or a CelonPower System[15] (Olympus Medical Systems, Tokyo, Japan). All patients underwent ultrasound-guided RFA. Artificial pleural or ascitic fluid (500-1000 mL of 5% glucose solution) was used to facilitate visualization of lesions with a subcapsular location or in the vicinity of the diaphragmatic dome, since these were difficult to visualize by US.
The Cool-tip RF System had a 17-gauge cooled-tip electrode with a 20- or 30-mm exposed tip. For the 20-mm exposed tip, the initial power output was 40 W. This was increased by 10 W/min to a maximum of 60 W. For the 30-mm exposed tip, the initial power output was 60 W, and this was increased by 10 W/min to a maximum of 90 or 100 W. For each tip, RF energy was delivered 1 to 3 times until impedance increased beyond the limit of the generator. After completion of ablation, the RF needle was energized at 60 W and removed while ablating the needle tract.
The CelonPower System generator had needle-type bipolar applicators with electrodes of 3 cm in length. The total energy and output were based on the standard dosimetry table. RF current was generated using automated control of the output with a resistance-controlled power function. Ablation times using 2 and 3 applicators were 17 and 16 min, respectively.
When the ranges ablated using the two RF systems were judged to be insufficient based on US findings during treatment or in dynamic CT performed 2-4 d after treatment, ablation was repeated using the same procedure on the same day or the day after dynamic CT.
SBRT was performed with CyberKnife®, a robotic image-guided whole body radiosurgery system equipped with a synchrony system for real-time respiratory tracking of target volumes that move with respiration. Overall accuracy is < 1.5 mm with synchrony for mobile targets, with a treatment accuracy of 0.3 mm. Since CyberKnife® treatment of cancer in the trunk, including HCC, cannot be performed using the skeleton as the focal point (in contrast to the skull in head and neck cancer), a gold fiducial marker was implanted percutaneously around the perimeter of the target volume using an ultrasound-guided procedure prior to acquisition of a planning CT scan. The gold fiducial marker is a coiled device (0.75 mm in diameter and 5 mm in length) that is implanted around the target lesion. For a lesion in the right or left lobe of the liver, the marker could be implanted in the right or left lobe, except for a S6 lesion.
Treatment planning CT was performed at least 7 d after fiducial placement. Patients were immobilized on a vacuum mattress or a self-expanding foam mattress in the treatment position (supine). A spiral CT scan without contrast and a three-phase scan with contrast (arterial, portal, and equilibrium) were acquired for planning. The gross tumor volume (GTV) was contoured on the contrast-enhanced lesion visible on the partial-exhale contrast-enhanced CT scan. Tumor tracking was performed during treatment using the inserted fiducial marker. The clinical target volume (CTV) was defined as the GTV with a 10-mm margin in all directions within the liver. A 1.5-mm margin was applied to the CTV to obtain the planning target volume (PTV). The total dose was 60 Gy and the dose was increased or decreased based upon the tumor size, location and residual liver function to give 95% PTV coverage. Irradiation was divided into 3 to 5 fractions. The irradiation range of the hepatic parenchyma surrounding the tumor was ≥ 17 Gy and the irradiated site was ≤ 20% of the whole liver.
Outcomes: The course was followed in both groups using changes in tumor markers and liver function in blood tests at 1, 3, 6 and 12 mo after treatment and at appropriate timepoints thereafter; the presence of local and intrahepatic distant recurrence detected on dynamic CT, contrast-enhanced US and EOB-MRI; and adverse events. Background factors of median observation periods, mean age, sex (male/female), Child-Pugh classification (A/B), and mean tumor diameter were compared between the two groups by χ2 test, Student t-test, and Mann-Whitney U-test.
Local and intrahepatic distant recurrence control, and cumulative survival rates were compared between the two groups. Local recurrence was defined as recurrence of the treated lesion and intrahepatic distant recurrence was defined as recurrence beyond 2 cm from the treated area. For instance, in large segments such as segment VII, a recurrence within the segment but beyond 2 cm from the previously treated area is considered distant recurrence, although if in the same segment. Only death from HCC or liver failure (i.e., liver disease-related death) was regarded as a fatal case. Local and intrahepatic distant recurrence control, and cumulative survival rates were determined using the Kaplan-Meier method, and the significance of differences was analyzed by log-rank test. Kaplan-Meier curves were also prepared setting the baseline at the day of treatment. Factors involved in local and intrahepatic distant recurrence, including the tumor diameter in the RFA group and the tumor diameter and total dose in the CK group, were analyzed by Student t-test.
Adverse events: The presence of grade 3 or more severe early and late adverse events was investigated based on the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. Changes in the Child-Pugh score from before treatment to 1, 3, 6 and 12 mo after treatment were analyzed by two-way repeated measures ANOVA. Adverse events that developed during treatment and within 3 mo after treatment were defined as early complications, and those that developed 4 mo to one year after treatment were defined as late complications.
P value < 0.05 was regarded as significant in all statistical analyses. The study was approved by the Ethical Review Board of Toho University Medical Center, Omori Hospital.
The median length of observation periods in the RFA and CK groups were 561 and 379 d, respectively, with no significant difference between the groups. In the RFA group, the mean age was 68.7 ± 10.5 years old, and there were 27 males and 11 females. The Child-Pugh classification was A in 31 and B in 7 cases before treatment, and the mean tumor diameter was 17.5 ± 6.1 mm. In the CK group, the mean age was 75.1 ± 8.1 years old, and there were 24 males and 11 females. The Child-Pugh classification before treatment was A in 28 and B in 7 cases, and the mean tumor diameter was 28.6 ± 11.5 mm. Age was significantly higher (P = 0.005) and the tumor diameter was significantly larger (P = 0.001) in the CK group (Table 1).
| Variable | RFA (n = 38) | CyberKnief® (n = 35) | P value |
| Observation period | 561 (range 222-1223) | 379 (range 203-1065) | 0.15 |
| Age (yr), mean ± SD | 68.7 ± 10.5 (range 42-86) | 75.1.7 ± 8.1 (range 55-89) | 0.005 |
| Gender | 0.82 | ||
| Male/female | 27/11 | 24/11 | |
| Etiology | 0.32 | ||
| HBV/HCV/alchol/other | 9/18/3/8 | 4/23/1/7 | |
| Child-Push classification, A/B | 31/7 | 28/7 | 0.86 |
| Tumor size | 17.5 ± 6.1 (range 7-29) | 28.6 ± 11.5 (range 12-50) | 0.001 |
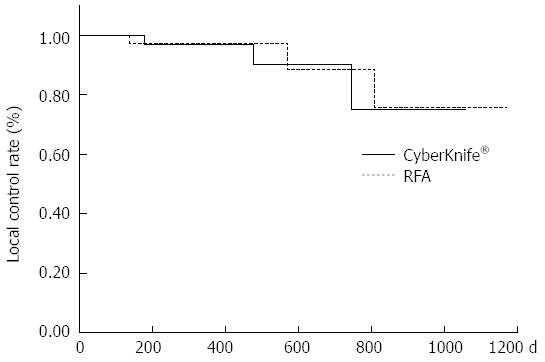
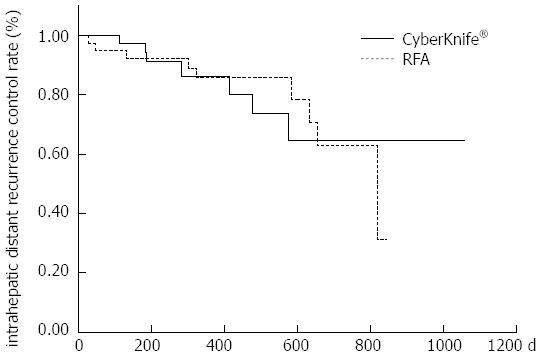
The 1-year local recurrence control rates were 97.4% and 97.1% in the RFA and CK groups, respectively (P = 0.71) (Figure 1); the 1-year intrahepatic distant recurrence control rates were 85.6% and 86.1%, respectively (P = 0.91) (Figure 2); and the 1-year cumulative survival rates were 100% and 95.2%, respectively (P = 0.075), showing no significant difference in any rate between the two groups (Figure 3).
In the RFA group, local recurrence occurred in 3 patients and the tumor diameters were 10, 18 and 25 mm, respectively. In the CK group, local recurrence occurred in 3 patients and the tumor diameters and total dose were 18 mm/60 Gy, 33 mm/39 Gy, and 46 mm/36 Gy, respectively. The tumor diameters of 2 of the 3 patients in the CK group were greater than the mean of all patients in the group, and the mean total dose was slightly lower than that for the whole group. Intrahepatic distant recurrence occurred in 9 patients in the RFA group, but there was no significant difference in the tumor diameter in these cases compared to others in the group (P = 0.87) (Table 2). Intrahepatic distant recurrence occurred in 7 patients in the CK group, and the incidence was significantly higher in cases with a large tumor diameter (P = 0.045) and low total dose (P = 0.036) (Table 3). In the RFA group, RFA was performed for all local recurrence (3 cases), and for intrahepatic distant recurrence (9 cases), RFA and transarterial chemoembolization (TACE) were performed for 7 and 1 cases, respectively and untreated case was 1. In the CK group, for local recurrences (3 cases), CyberKnife® treatment and TACE were performed for 2 and 1 cases, and for intrahepatic distant recurrence (7 cases), CyberKnife® treatment and TACE were performed for 3 each and untreated case was 1.
| RFA | |||
| Distant recurrence (-) | Distant recurrence (+) | P value | |
| n | 29 | 9 | |
| Tumor size (mm) | 17.4 ± 6.2 | 17.8 ± 6.0 | 0.87 |
| CyberKnife® | |||
| Distant recurrence (-) | Distant recurrence (+) | P value | |
| n | 28 | 7 | |
| Tumor size (mm) | 26.6 ± 11.1 | 36.3 ± 10.6 | 0.045 |
| Total dose (Gy) | 50.6 ± 7.8 | 43.6 ± 6.7 | 0.036 |
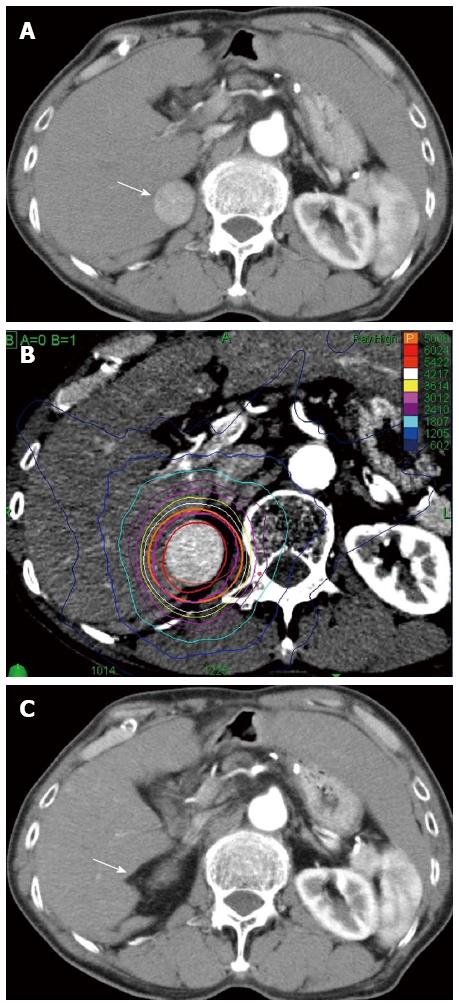
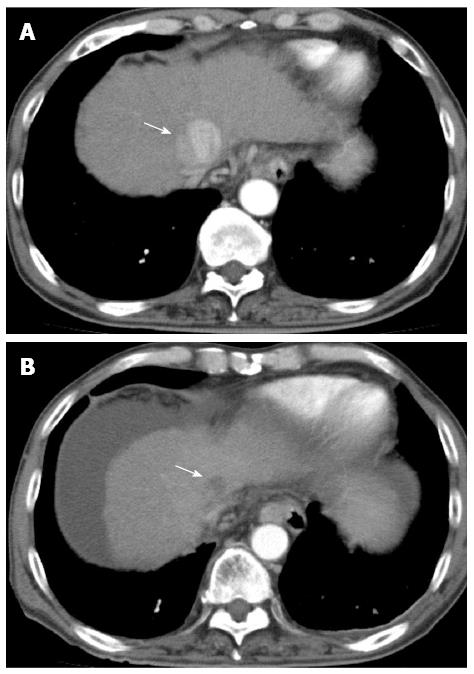
There were no early adverse events in either group. There were also no late adverse event in the RFA group, but 4 of the 35 patients (11.4%) in the CK group had late adverse events. All 4 patients had ascites, and two of these patients were liver disease-related fatal cases, and the Child-Pugh score in both patients was higher at 12 mo after treatment compared to that before treatment (Figure 4). In the CK group, the Child-Pugh score at 12 mo after treatment was significantly higher than that in the RFA group (P = 0.003) and significantly higher than the score before treatment (P = 0.034) (Figure 5).
Chronic hepatitis and liver cirrhosis are present in the background liver as an underlying disease in many HCC cases. In addition to tumor factors such as the tumor diameter and number of tumors, background factors including liver function are important in deciding on a treatment method. Several stage classification systems are used to evaluate hepatic functional reserve, including the Barcelona Clinic Liver Cancer Staging System[16,17], Okuda Staging System[18], and Cancer of the Liver Italian Program Scoring System[19]. In Japan, the therapeutic policy is mainly decided using the Evidence-based Clinical Practice Guidelines for HCC of the JSH[3], although with some differences among institutions. For early HCC with liver damage A or B and ≤ 3 tumors and a tumor diameter ≤ 3 cm, liver resection and percutaneous ablation therapy are recommended. Liver resection is the primary curative treatment for HCC, with a current 5-year survival rate of about 70%, especially for small HCCs < 5 cm in diameter, due to improved surgical techniques and postoperative management[20]. However, liver resection is only an option in 10% to 30% of patients at diagnosis for various clinical reasons[21]. Moreover, intrahepatic distant recurrence occurs after liver resection in many cases due to multicentric carcinogenesis[22], a characteristic of HCC, and some patients do not want liver resection.
For these reasons, many institutions perform RFA as the first choice for treatment of early HCC. RFA is the most common ablation modality worldwide with 80%-95% complete tumor necrosis and a 33%-57% 5-year survival rate in patients with small HCC[23,24]. A recent retrospective study found that overall survival (OS) and disease-free survival with RFA were significantly better than those with liver resection for central HCC ≤ 2 cm in diameter[25]. With advances in RF systems and techniques, the therapeutic effect of RFA is now comparable to that of liver resection. However, even though the tumor diameter and number of tumors meet the indication, RFA is not applicable in cases with the tumor in a deep region, directly under the liver dome, or near a thick blood vessel or bile duct, or if the tumor cannot be visualized by US including CEUS. CyberKnife® may be a good indication for such cases.
CyberKnife® for cancers of the trunk was approved in Japan relatively recently, and the therapeutic effect and adverse events in application to these cancers, including HCC, remain unclear. For this reason, we compared the utility of CyberKnife® treatment of HCC with that using RFA in the same period in the same institutions. The subjects were limited to patients with an initial solitary HCC. The background factors in the groups were mostly similar, but there were significantly more elderly patients and larger tumor diameters in the CK group. Since CyberKnife® can detect and respond to a respiratory fluctuation-induced slight movement of the target lesion, it may be a good indication for elderly patients for whom breath-holding during RFA puncture is difficult. In addition, only a one-day hospital stay is required to place a gold fiducial marker for the irradiation target, and irradiation is applied for 3-5 d at an outpatient clinic, which reduces medical costs and causes less interference with daily life. Because of this low invasiveness, there has been an increase in elderly patients requesting CyberKnife®, which may have been reflected in our results. The tumor diameter was ≤ 3 cm in the RFA group due to the guidelines for RFA, but a tumor of diameter ≤ 5 cm may be included in the indication of CyberKnife®.
The local control rate did not differ significantly between the two groups. Several previous studies have examined CyberKnife® for liver tumors, including liver metastasis and cholangiocellular carcinoma (CCC). Bibaut et al[12] found 1- and 2-year local control rates of 89.8% in a study on CyberKnife® applied to 96 nodes in 75 HCC patients; and Janoray et al[13], Choi et al[26], and Cardenes et al[27] reported 1-year local control rates of 84% in 21 HCC patients, 71.9% in 32 HCC patients, and 100% in 25 HCC patients, respectively. In contrast, Yoon et al[28] found 1- and 3-year local control rates of 94.8% and 92.1%, respectively, in 103 HCC nodes treated with conventional SBRT, indicating that the outcome of CyberKnife® is almost equivalent to conventional SBRT. The 1-year local control rate was 97.1% in the CK group in the current study.
The tumor diameter exceeded 30 mm (33 and 46 mm) in 2 of the 3 cases with local recurrence in the CK group, and the total doses were 39 and 36 Gy, respectively, which were lower than those in the other patients. It has occasionally been reported that there is no correlation between development of local recurrence and total dose in use of conventional SBRT[29]. Using CyberKnife®, Janoray et al[13] found a 1-year local control rate of 100% in cases treated with a total dose of 60 Gy, and Dewas et al[11] showed that the local control rate was significantly higher in cases with a total dose of 45 Gy compared to < 45 Gy in 99 patients with liver metastasis, 48 with HCC, and 6 with CCC. The reasons for the differences in the association of local recurrence with total dose between conventional SBRT and CyberKnife® are unclear, but it cannot be ruled out that total dose is related to local recurrence after CyberKnife® treatment.
Large tumors, such as those with a diameter of ≥ 30 mm or volume ≥ 32 mL, are significantly more likely to show local recurrence after conventional SBRT[28,30]. In this study, the tumor diameter was >30 mm in two cases with local recurrence in the CK group, which suggests that the diameter is also an important factor in local control in treatment with CyberKnife®. Currently, CyberKnife® is indicated for tumors with a diameter ≥ 30 mm at our hospital and related institutions, but it is uncertain if this tumor diameter cut-off is appropriate. Moreover, local recurrence was also noted in a case with a tumor diameter of 18 mm. Not only the tumor diameter but also the degree of differentiation of HCC, tumor marker[31] and serum ferritin[32] may be involved in local control, and this also applies to RFA. It will be necessary to investigate many cases to establish the indication for CyberKnife® treatment, including the tumor diameter and degree of differentiation.
There was no significant difference in the intrahepatic distant recurrence control rate between the RFA and CK groups. Bibaut et al[12] found an incidence of intrahepatic distant recurrence of 24% (18/75 cases) over a median observation periods of 10 mo (30-49 mo). Our study had a similar incidence of 20% (7/35 cases) over a median observation periods of 379 d. There was no significant correlation between the tumor diameter and intrahepatic distant recurrence in the RFA group, but the incidence was significantly higher in cases with a large tumor treated with low total dose in the CK group. Risk factors for intrahepatic distant recurrence after treatment of HCC with SBRT have not been examined in detail, but it is well known that intrahepatic metastasis undetectable by imaging can be present in large HCC. Thus, there is a general risk of intrahepatic distant recurrence in cases of HCC with a large tumor diameter, and the required total dose should be fully investigated before CyberKnife® treatment.
The observation period was short, but there was no significant difference in OS between the RFA and CK groups. The Japanese Nationwide Survey reported 3-year OS rates of HCC patients treated with RFA of 82%-88% and 66%-82% in patients with tumor diameters of ≤ 20 mm and 21-50 mm, respectively[33]. The 1- and 2-year OS rates were 78.5% and 50.4%, respectively, in Bibaut et al[12], and Janoray et al[13] found a 1-year OS of 89% in 56 patients with liver tumors, including liver metastasis. These results show that CyberKnife® achieved OS equivalent to that in other standard local treatment for liver tumors, including HCC. The 1-year OS rate was 95.2% in our study, which is more favorable than those in previous reports. This may have been due to limiting the subjects to those with an initial solitary tumor.
There were two liver disease-related fatal cases in the CK group. Both patients had grade 3 or more severe late adverse events (CTCAE ver.4.0) and the Child-Pugh score increased at 12 mo after treatment compared to that before treatment. In previous reports, grade 3 or more severe hepatotoxicity occurred at rates of 0-25.8% within 3 mo after conventional SBRT[28], and Sanuki et al[29] found a rate of grade 3 or more severe early adverse events of 13% after SBRT. In contrast, Bibaut et al[12] found grade 1-2 adverse events, such as hepatic pain, nausea, and asthenia, in 15%-17% of cases after CyberKnife® treatment, but no radiation-induced liver disease (RILD)[34] in any patient. In our study, grade 1-2 nausea and malaise occurred in several patients, but the incidences of grade 3 or more severe early and late adverse events were lower than those after conventional SBRT.
In previous studies of SBRT for HCC[27,35], the total dose was significantly correlated with adverse events. The incidence of hepatotoxicity was high in cases with Child-Pugh classification B, and liver cirrhosis progressed with repeated SBRT. Compared to conventional SBRT, the beam direction can be freely set because it can be moved using a robot arm, and the number of beams and the total dose centrality are high with CyberKnife®, which may reduce adverse effects. However, RILD may develop following CyberKnife® treatment, and the Child-Pugh classification changed from A to B in 16.1% of 31 HCC patients[26]. In our study, the Child-Pugh score was significantly higher at 12 mo after treatment in the CK group compared to the RFA group, and in the CK group death occurred in two cases in which the Child-Pugh score was significantly higher than that before treatment. The incidence of serious complications induced by CyberKnife® may be lower than with conventional SBRT, but such complications may affect the outcome. Thus, the hepatic functional reserve should be fully evaluated before CyberKnife® treatment and adverse events should be carefully monitored, as for conventional SBRT.
The limitations of this study include the small number of cases, the different criteria in the both groups, the heterogeneous in two aspects that precisely have an impact in both the relapse and the survival, the short observation period, and the retrospective design. The ideal design for comparison between different techniques is a randomized controlled trial or at least a propensity score analysis. Moreover, less than 2 years of follow up are even less reliable with regard to survival, local and intrahepatic distant recurrence control rate. However, the results suggest that the therapeutic effect of CyberKnife® for HCC was equivalent to that of RFA as a pilot study.
In some cases, RFA is difficult because of the tumor location, and liver resection and the breath-holding during RFA puncture may be a risk in elderly patients with HCC, the number of whom is likely to increase. Including the cost of hospitalization, the costs of RFA and CyberKnife® were 380000 and 630000 yen, respectively and the cost performance of CyberKnife® is higher in approximately 2 times than RFA treatment in Japan. However, CyberKnife® is a low-invasive procedure that is synchronous with respiration and requires no breath holding. Based on the hepatic functional reserve, this procedure may be a good indication for cases with difficulty with RFA.
In conclution, there were many elderly patients and large tumors in the CK group, but the therapeutic outcome was equivalent to that in the RFA group. Tumors with a diameter of ≤ 30 mm are a good indication for CyberKnife®. Relatively favorable outcomes were achieved in cases with a tumor diameter of 30-50 mm, but a further investigation is needed before these can be included in the indication, in consideration of the patient background. The occurrence of adverse events is a concern, but CyberKnife® treatment is likely to become an important option for local treatment of early HCC.
Radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) is performed at many institutions because it can coagulate a wide area in one session, and local control is high. Recently, CyberKnife® became the adaptation in the cancer of the trunk, including HCC. Tumors in the trunk move with respiration, but CyberKnife® detects minute body movements and fine-tunes the irradiation angle using a seeker. Therefore, this approach has potential as a novel local treatment for HCC due to its low invasiveness and reduced burden on the patient.
The authors compared therapeutic outcomes and adverse events in patients with initial solitary HCC treated with RFA or CyberKnife®, and retrospectively investigated the efficacy of CyberKnife® for HCC treatment. In this study, there were many elderly patients and large tumors in the CyberKnife® group, but the therapeutic outcome was equivalent to that in the RFA group.
CyberKnife® detects minute body movements and fine-tunes the irradiation angle using a seeker. Therefore, this approach has potential as a novel local treatment for HCC due to its low invasiveness and reduced burden on the patient.
The occurrence of adverse events is a concern, but CyberKnife® treatment is likely to become an important option for local treatment of early HCC.
CyberKnife® stereotactic body radiotherapy is image-guided robotic radiosurgery using a radiation delivery platform that can detect and correct for intrafraction tumor motion, as well as adapt to the patient’s breathing pattern by moving the linear accelerator in concert.
The main strength of the paper by Shiozawa et al is its novelty as studies directly comparing RFA and CyberKnife® for HCC are lacking.
P- Reviewer: Abdel-Wahab M, Facciorusso A, Varona MA S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 2. | Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219-1222. [PubMed] |
| 5. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222. [PubMed] |
| 6. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [PubMed] |
| 7. | Yuan Z, Tian L, Wang P, Song Y, Dong Y, Zhuang H. Comparative research on the efficacy of CyberKnife® and surgical excision for Stage I hepatocellular carcinoma. Onco Targets Ther. 2013;6:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Que JY, Lin LC, Lin KL, Lin CH, Lin YW, Yang CC. The efficacy of stereotactic body radiation therapy on huge hepatocellular carcinoma unsuitable for other local modalities. Radiat Oncol. 2014;9:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Shiozawa K, Watanabe M, Wakui N, Ikehara T, Iida K, Sumino Y. Analysis of patients with tumor seeding after percutaneous radiofrequency ablation of hepatocellular carcinoma. Mol Med Rep. 2008;1:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Shiozawa K, Watanabe M, Wakui N, Ikehara T, Iida K, Sumino Y. Risk factors for the local recurrence of hepatocellular carcinoma after single-session percutaneous radiofrequency ablation with a single electrode insertion. Mol Med Rep. 2009;2:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Dewas S, Bibault JE, Mirabel X, Fumagalli I, Kramar A, Jarraya H, Lacornerie T, Dewas-Vautravers C, Lartigau E. Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol. 2012;7:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Bibault JE, Dewas S, Vautravers-Dewas C, Hollebecque A, Jarraya H, Lacornerie T, Lartigau E, Mirabel X. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8:e77472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Janoray G, Chapet S, Ruffier-Loubière A, Bernadou G, Pointreau Y, Calais G. Robotic stereotactic body radiation therapy for tumors of the liver: radiation-induced liver disease, incidence and predictive factors. Cancer Radiother. 2014;18:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 15. | Osaki Y, Ikeda K, Izumi N, Yamashita S, Kumada H, Hatta S, Okita K. Clinical effectiveness of bipolar radiofrequency ablation for small liver cancers. J Gastroenterol. 2013;48:874-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] |
| 18. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 19. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] |
| 20. | Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Nakachi K, Suzuki E, Yoshino M. Hepatectomy for hepatocellular carcinoma patients who meet the Milan criteria. Hepatogastroenterology. 2008;55:621-626. [PubMed] |
| 21. | Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14:3301-3309. [PubMed] |
| 22. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [PubMed] |
| 23. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [PubMed] |
| 24. | Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547-1555. [PubMed] |
| 25. | Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, Yoon SK, Chai GY, Kang KM. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Yoon SM, Lim YS, Park MJ, Kim SY, Cho B, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Sanuki N, Takeda A, Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3100-3111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (4)] |
| 30. | Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg. 2015;39:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Facciorusso A, Del Prete V, Antonino M, Neve V, Crucinio N, Di Leo A, Carr BI, Barone M. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29:1905-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Reed GB, Cox AJ. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol. 1966;48:597-611. [PubMed] |
| 35. | Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, Iwabuchi S, Kunieda E. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |