INTRODUCTION
Despite the availability of potent vaccines, hepatitis B virus (HBV) infection remains a major public health problem worldwide with approximately 350 million individuals chronically infected[1-3]. Twenty percent of these chronically infected patients progress to life-threatening complications[4]. Acute-on-chronic liver failure (ACLF) is a critical complication of chronic hepatitis B (CHB) and can develop at any stage in the progression of CHB[5]. In China, it is estimated that approximately 70% of liver failure is caused by HBV infections, and, therefore, the prevalence of HBV-ACLF will likely increase in the coming decades.
HBV-ACLF has a poor prognosis, with an in-hospital mortality rate > 70% if emergency liver transplantation (LT) is not available. In the real-life setting, a very small number of HBV-ACLF patients undergo LT because of the shortage of liver donors. Although antiviral therapy can improve long-term survival and reduce the recurrence risk of ACLF, it does not improve short-term survival even when HBV DNA replication is controlled[6]. In this situation, it is believed that stopping or slowing the progression of CHB to ACLF at an early stage may be the most effective way of reducing the morbidity and mortality of patients with HBV-ACLF.
There is evidence suggesting that severe acute exacerbation of CHB is an inevitable stage in the development of ACLF, which is associated with many factors[7-9]. It can occur either over the natural course of the disease or following intensive chemotherapy or immunosuppressive therapy, and it easily progresses to ACLF if the abrupt flare is severe. In recent years, early warning and identification of severe acute exacerbation of CHB has attracted much attention, as effective control of the factors that trigger and worsen the disease is crucially important to slow down or reverse the progression of acute exacerbation of CHB to ACLF.
It is worth mentioning that due to the high cost of treatment and overly pessimistic view of the prognosis, many patients with HBV-ACLF choose to forego or discontinue treatment, especially in China. If an accurate assessment of the outcome of individual patients was possible, clinicians would encourage more active treatments for eligible patients with potentially better prognosis, and a considerable proportion of CHB patients may recover from HBV-ACLF.
In this review, we highlight recent developments in indicators and models for early warning and clinical outcome prediction of HBV-ACLF, which may help optimize current prevention and comprehensive treatments for ACLF in CHB patients.
CLINICAL MANIFESTATIONS FOR EARLY WARNING OF HBV-ACLF
Prior to the onset of typical abnormal laboratory findings, many patients present with certain clinical manifestations that may indicate the occurrence of ACLF in those with severe acute exacerbation of CHB[10]. In addition to the progressive symptoms of fatigue and weakness, it is also important to be aware of new and persistent nausea, vomiting, and hiccup in clinical practice, as these symptoms are related to the presence of endotoxemia. Although serum levels of total bilirubin (TBil) do not reach the diagnostic criteria for ACLF (10 times the upper limit of normal), rapid worsening jaundice also indicates a high probability of ACLF in CHB patients.
During CHB deterioration, manifestations such as aggravated bloating and hypoactive or missing bowel sounds are extremely common prior to or during the development of ACLF and are always associated with ascites, spontaneous bacterial peritonitis, or endotoxemia-induced toxic intestinal paralysis. For CHB patients with severe liver dysfunction, clinical manifestations such as skin petechiae and gingival bleeding are important signs of coagulation disorders. In addition, foul smelling gas and stools may indicate hepatic encephalopathy. Early diagnosis and treatment of coagulation disorders and hepatic encephalopathy may help to prevent or delay the development of ACLF.
However, it should be noted that the above-mentioned clinical manifestations are not specific to HBV-ACLF. To achieve more accurate identification of early warning, integrated monitoring and analysis of these clinical manifestations are warranted.
HOST PARAMETERS FOR EARLY WARNING AND PROGNOSIS PREDICTION IN HBV-ACLF
Biochemical indicators
It is well-known that liver dysfunction can lead to abnormal synthesis of important biologic signal molecules, metabolic disturbances, and alterations in serum biochemical indicators. Significantly elevated levels of alanine aminotransferase (ALT), TBil, and prothrombin activity (PTA) often suggest serious liver damage and are often used to diagnose HBV-ACLF[11]. However, there is no significant change in these indicators at the early stages of progression from acute exacerbation of CHB to ACLF. Therefore, when only relying on the levels of these conventional biochemical indicators, it is difficult to achieve an early and accurate diagnosis of HBV-ACLF.
Evidence has shown that lipopolysaccharide (LPS)-induced endotoxemia can cause secondary hepatic injury, which is associated with the secretion of tumor necrosis factor α (TNFα) and the production of inflammatory cytokines[12,13]. In patients with ACLF, the highest serum levels of LPS are observed in the peak phase of TBil, and dynamic changes in LPS are correlated with disease severity[14]. Thus, for those patients with a high risk of endotoxemia, it is necessary to monitor serum LPS levels at admission and during treatment, as they will achieve maximum clinical benefit from timely and effective control of endotoxemia. Hyperammonemia is common in HBV-ACLF patients with hepatic encephalopathy and can cause problems with bilirubin metabolism by interfering with energy synthesis[15]. Kumar et al[16] recently reported that patients with persistent, mild hyperammonemia (≥ 85 μmol/L for 3 d) were more likely to develop complications and had higher mortality than those with serial ammonia levels < 85 μmol/L. Therefore, the evaluation of arterial ammonia at an early stage would be useful for risk stratification of disease progression.
Prealbumin is a protein produced by the liver, and it has a much shorter half-life (about 1.9 d) than that of albumin. A decline in prealbumin is more sensitive than albumin and more specific than ALT in reflecting early liver dysfunction. Huang et al[17] reported that there was also a positive correlation between the decline in prealbumin concentration and the severity of liver damage. Thus, monitoring the dynamic changes in serum prealbumin may provide early clues for the acute exacerbation of CHB to ACLB. Studies also reported that serum alpha fetoprotein (AFP) levels are increased in CHB patients with impaired liver functions[18,19], and compared to non-survivors with HBV-ACLF, survivors exhibited a higher increase in serum AFP level. However, a recent study showed that an early peak in AFP level prior to an elevation of prothrombin time may indicate a high risk of death[18]. Therefore, to predict the prognosis of HBV-ACLF, a comprehensive dynamic analysis of elevated AFP may be necessary.
It has been reported that serum metabolite and peptide profiling can vary during the progression of CHB to liver failure and that these dynamic changes may be used to distinguish different stages of the disease[20,21]. Recently, apolipoprotein (Apo) and lipid abnormalities were found to be associated with chronic liver failure[22]. In addition, serum levels of high-density lipoprotein and apolipoprotein A-I (ApoA-I) were inversely correlated with liver reserve and disease severity in cirrhotic patients with severe sepsis[23]. The low level of ApoA-I was also associated with a marked impairment in effective arterial volume, multiple organ dysfunction, and a poor prognosis[23]. Although the roles of ApoB, ApoE, and ApoA5 in ACLF have also been studied, the corresponding data are limited and future studies are needed.
In addition to the strong association between dysregulated iron homeostasis and both multi-organ failure and early mortality in ACLF[24], serum ferritin (SF) at admission was shown to be significantly higher in HBV-ACLF patients than in CHB and healthy controls, and elevated concentrations of SF were associated with increased severity of liver disease and 3 month mortality due to HBV-ACLF[25]. However, the predictive power of SF for mortality in HBV-ACLF patients was low [area under the curve (AUC) value: 0.640 ± 0.061]; and combining SF with the model of end-stage liver disease (MELD) score increased the power for predicting mortality (AUC value: 0.911 ± 0.035). Recently, sphingolipids were reported to be involved in the progression of liver disorders and to reflect the severity of hepatic injury in CHB[26]. The difference in sphingolipid profiles between CHB and HBV-ACLF patients was more significant than that between healthy controls and CHB, which indicated that serum sphingolipid levels were more likely to be associated with the progression of HBV-ACLF than CHB. For example, the serum levels of dhCeramides (dhCer) (d18:0/24:0) were significantly lower in patients who died compared with survivors. The decline in dhCer (d18:0/24:0) also showed a similar prognosis in terms of 3 mo mortality to that of the MELD score, thus, dhCer (d18:0/24:0) may be a useful prognostic biomarker for the early prediction of HBV-ACLF[27]. Additionally, the serum levels of thyroid stimulating hormone (TSH) have been reported to be a significant factor for predicting mortality in ACLF patients, and the cumulative survival rate decreased significantly when the serum TSH level was less than 0.38 IU/mL[28]. Therefore, serum TSH level may be a useful indicator for assessing severity and prognosis in ACLF patients.
It is known that kidney injury plays an important role in the prognosis of HBV-ACLF. Rapid deterioration of estimated glomerular filtration rate was recently reported to be associated with on-treatment mortality of CHB patients experiencing acute exacerbation[29]. In addition, Cystatin C (CysC) has been reported as a biomarker for predicting acute kidney injury in patients with ACLF[30]. By combining serum CysC and TBil, Wan et al[31] constructed a Prognostic Index (PI) to predict the 3 month mortality of HBV-ACLF patients [PI = 0.933 × CysC (mg/L) + 0.075 × TBil (mg/dL)]. Their findings showed that a PI < 3.91 may indicate an extremely good prognosis in patients with normal levels of serum creatinine (survival rate: 94.3% for PI < 3.9% vs 17.4% for PI = 3.91).
Immune cells and related cytokines
An abnormal immune reaction and an imbalance in the production of proinflammatory and anti-inflammatory cytokines contribute to the outcome of acute exacerbation of CHB and prognosis of HBV-ACLF[32]. An abnormal immune reaction is mediated by the complex interactions between liver cells and host immune cells. Th17 cells are a subset of T helper cells that produce interleukin 17 (IL-17), thus promoting the activation of dendritic cells (DCs) and monocytes and enhancing the capacity to produce proinflammatory cytokines, including IL-1β, IL-6, TNFα, and IL-23[33]. It has now been confirmed that the overexpression of these proinflammatory cytokines plays an important role in liver damage progression[12,34] and that the activation of STAT3 upon IL-6 stimulation may contribute to the enhanced Th17 response (IL-17 production) in the deterioration of liver damage[35].
Some studies have reported that the frequency of peripheral Th17 cells and serum concentrations of IL-17 are gradually increased with immune inflammation aggravation in asymptomatic HBV carriers (AsC), CHB to ACLF, and overactive Th17 cells may be associated with immune tolerance breakthrough and severe exacerbation of CHB[33,36]. In addition, the frequency of peripheral Th17 cells in advanced-stage ACLF was significantly higher than that in early-stage ACLF, and surviving ACLF patients had an initially lower frequency of Th17 cells and IL-17 levels than non-survivors[36,37]. In ACLF patients, a positive correlation between peripheral Th17 cell frequency and both prothrombin activity and MELD score was observed[36]. Thus, monitoring of Th17 cell frequency and IL-17 levels helps to assess the exacerbation of liver damage during chronic HBV infection[33], and high Th17 cell frequency and serum IL-17 concentration in ACLF patients may indicate a poor prognosis.
It is well-known that regulatory T (Treg) cells can suppress the expansion and interferon gamma (IFNγ) secretion of autologous peripheral blood mononuclear cells (PBMCs) when stimulated with HBV antigen. Recently, Th17/Treg cell imbalance in disease progression was also demonstrated. For example, in the remission stage of ACLF, Th17 cells increase and Treg cells decrease, creating an imbalance that is negatively correlated with disease progression[38]. Thus, the ratio of Th17/Treg cells may be a good prognostic marker in predicting ACLF progression[37], and restoring the Th17/Treg cell ratio could maintain the immune system at a steady state in ACLF patients.
DCs are specific antigen-presenting cells (APCs) and are abundant in the liver. The number and function of DCs are closely associated with the prognosis of HBV-ACLF patients treated with different therapies. A high number of myeloid DCs at baseline and the recovery of myeloid DCs numbers at the end of treatment may represent a prognostic marker for a favorable response to corticosteroids and granulocyte colony-stimulating factor (G-CSF) in patients with HBV-ACLF[39,40].
It is well-known that the number of monocytes and macrophages is greatly increased in the circulation and liver of patients with ACLF[41]. Activated monocytes/macrophages release a large number of proinflammatory and anti-inflammatory cytokines, and the level of proinflammatory cytokine production is closely related to disease severity in patients with liver failure[12]. The procoagulant molecule, human fibrinogen-like protein 2/fibroleukin (hfgl2), is an inflammatory mediator produced by activated macrophages and can directly cleave prothrombin into activated thrombin, resulting in intravascular fibrin deposition in the liver[42]. Importantly, there is a positive correlation between hfgl2 expression and the severity of liver damage in CHB patients[43]. Compared to 7.7% of CHB patients with mild liver damage expressing hfgl2, 91.3% of HBV-ACLF patients had hfgl2 expression[43], and high levels of virus-induced hfgl2 were observed in liver sections from patients with HBV-ACLF[44]. Thus, hfgl2 may play a pivotal role in initiating acute severe hepatitis in those with chronic hepatitis, and hfgl2 expression in peripheral blood may also be used as a biomarker to monitor the acute exacerbation of CHB and to assess the severity of ACLF.
In addition to the secretion of inflammatory cytokines, monocytes/macrophages can induce adaptive immune responses through their antigen-presenting functions. The expression of human leukocyte antigen (HLA) class II molecules, especially HLA-DR (a heterodimeric cell surface glycoprotein) is particularly important for monocyte activation. Antoniades et al[45] recently reported a strong relationship between monocyte HLA-DR expression and the indices of disease severity, mediators of inflammation, and outcome. A level of HLA-DR ≤ 15% had 96% sensitivity, 100% specificity, and 98% accuracy in predicting poor prognosis in patients with liver failure[45]. Xing et al[46] also reported similar findings, suggesting that monocyte HLA-DR expression in patients who died was significantly lower than that in patients who survived in the early and late stages of ACLF. Therefore, the functional status of PBMC and HLA-DR expression may be used to monitor disease severity and predict clinical outcome in patients with ACLF.
Chemokines
The CC chemokines, monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-3α (MIP-3α), may be involved in the pathogenesis of ACLF. Compared to healthy controls and patients with chronic hepatic failure, serum concentrations of MCP-1 and MIP-3α were significantly enhanced in ACLF patients[47]. Interestingly, Leifeld et al[48] investigated the time course of CC-chemokine release in concanavalin A and LPS-induced liver failure mouse models and found that intrahepatic MCP-1 and MIP-3α upregulation occurred prior to hepatic infiltration and liver damage. In addition, elevations in MCP-1 and MIP-3α serum concentrations reflected the degree of liver inflammation[49]. Therefore, serum concentrations of MCP-1 and MIP-3α may be used as early warning indicators of ACLF.
In endotoxemic liver injury in mice, CXC chemokines are instrumental in regulating endotoxin-induced transmigration and extravascular tissue accumulation of leukocytes, and interference with MIP-2 and neutrophil chemoattractant functions protect against septic liver damage[50]. In massive hepatectomy induced acute liver damage, the CXC chemokine CXCL1 was significantly increased[51]. Additionally, the serum level of CXCL10 was significantly related to the degree of liver inflammation or damage in CHB patients, and it may play an important role in trafficking inflammatory cells to the local focus in the liver and induce disease progression[15]. It is worth mentioning that the polymorphism G-201A is present in the promoter of CXCL10 gene and can alter the binding affinity of nuclear protein and regulate CXCL10 expression. Therefore, G-201A may be involved in the genetic variation underlying the susceptibility of individuals to acute exacerbation of CHB[52].
Host hemodynamic derangements
Recently, Garg et al[53] reported that the presence of a high hepatic venous pressure gradient (HVPG) is an independent baseline predictor of mortality in HBV-ACLF patients, as the raised portal pressure predisposes patients to a high risk of variceal bleeding. During the dynamic follow-up of surviving patients, these authors observed a reduction in HVPG; and this reduction correlated with clinical and biochemical recovery and a reduction in Child-Turcotte-Pugh (CTP) score. It is worth mentioning that hyponatremia is also common in patients with ACLF. For example, compared to 12.3% of patients without ACLF in the CANONIC study, 24.3% of patients with ACLF had hyponatremia at inclusion[54]. In addition, the presence of hyponatremia influences the outcome of patients with ACLF. The 3 mo transplant-free survival in ACLF patients with hyponatremia is only 35.8% but is as high as 58.7% in patients without hyponatremia[54]. Thus, hepatic and systemic hemodynamic derangements may predict early mortality and recovery in patients with ACLF.
VIRAL FACTORS IN THE EARLY WARNING OF HBV-ACLF
It is well-known that ACLF patients have distinct quasi-species characteristics with higher complexity and diversity within the basal core promoter (BCP)/precore (PC) region of HBV[55]. Both single mutations, including T1753C, A1762T, G1764A, A1846T, C1913A/G, G1896A, and G1899A, and double mutations, including A1762T/G1764A and G1896A/G1899A, are more frequently detected in HBV-ACLF patients than in CHB patients[56,57]. At present, there is evidence suggesting that CHB patients infected with BCP/PC mutations are more susceptible to ACLF, and HBV-ACLF patients with BCP/PC mutations also have a higher risk of mortality than those infected with BCP/PC wild-type virus[58-60].
It should be noted that either the absolute frequency of genotype B or the ratio of genotype B to C is significantly higher in HBV-ACLF patients than in CHB patients[58,61]. In genotype B patients, the A1762T/G1764A, A1846T, and G1896A mutations are also significantly more prevalent in patients with ACLF than in patients with CHB, and genotype B patients with G1896A and A1762T/G1764A have a higher tendency to develop HBV-ACLF than patients with genotype C[62]. To a certain extent, HBV genotyping and detection of BCP/PC mutations may have implications for the prediction of HBV-ACLF in clinical practice.
Studies have also shown that severe HBV reactivation can lead to disease flare and even liver failure, which often occurs following interrupted/discontinued antiviral therapy, intensive chemotherapy, or immunosuppressive therapy. For those who are at high risk of HBV reactivation, dynamic monitoring of serum HBV DNA would help in the early warning of possible HBV-ACLF occurrence, and long-term control of viral replication is necessary and important to prevent the occurrence of ACLF. Additionally, evidence suggests that effective control of HBV DNA replication would help to reduce the long-term mortality and recurrence rates of HBV-ACLF[63].
SCORING SYSTEMS FOR PROGNOSIS PREDICTION IN HBV-ACLF
In the past decades, a number of scoring systems have been used for outcome prediction in end-stage liver diseases, including CTP, MELD, and their derivative scores, King’s College Hospital (KCH) criteria, sequential organ failure assessment (SOFA), acute physiology, and chronic health evaluation (APACHE), Clichy criteria, and artificial neural network (ANN). However, not all of these scores are well validated in HBV-ACLF patients. Consequently, many new mathematical models have recently been developed using multiple regression and have shown certain advantages.
The CTP score was originally devised for the assessment of liver disease severity to predict the outcome of patients with cirrhosis, in whom surgical treatment for portal hypertension was planned. The CTP score mainly applies to cirrhotic patients, with subjective judgments on hepatic encephalopathy and ascites and limited discriminant ability. Additionally, the CTP score does not distinguish the clinical significance between mild-to-moderate and severe abnormal laboratory parameters, and patients with different disease severity have the same CTP score[64]. Thus, the CTP score has poor accuracy in predicting the prognosis of ACLF patients. Similarly, the KCH and Clichy criteria are also rarely used to predict the prognosis of patients with ACLF. Instead, they are more appropriate for assessing the severity of patients with acute liver failure and the urgency for LT.
In order to overcome these limitations of the CTP, the MELD score was developed, and its advantages are that it can accurately reflect hyperbilirubinemia, coagulation disorders, and renal impairment. Many reports have confirmed that the MELD score is a good mortality predictor in ACLF patients awaiting LT. However, there is some controversy regarding its efficacy in predicting post-transplantation outcomes[65]. For example, ACLF patients with a high MELD score (≥ 30) have a good post-transplantation outcome compared with those patients with a score < 30 after LT[66-68]. Therefore, the MELD score may not be a good prognostic scoring system for HBV-ACLF patients undergoing LT.
The MELD-based MELD-Na and iMELD scores were also investigated in clinical practice[69], but there was no significant improvement in prediction accuracy of the prognosis of HBV-ACLF. This is because important factors (i.e., hepatic encephalopathy, hepatorenal syndrome, and upper gastrointestinal bleeding) that can affect the prognosis of patients were not taken into consideration in these scores[70-72]. In addition, diuretic therapy or artificial liver treatment can easily lead to a fluctuation in serum Na, bilirubin, and creatinine, and the latter could significantly affect the reliability of MELD-associated scores. Noticeably, MELD-based scores are still mainly used in cirrhotic patients. For non-cirrhotic HBV-ACLF patients, their prediction efficacy is relatively poor. In a previous study, we evaluated the efficacy of the MELD and MELD-Na scores in predicting short-term prognosis of HBV-ACLF patients, and the results showed that both the MELD (AUC: 0.758 vs 0.840) and MELD-Na (AUC: 0.776 vs 0.849) scores appeared to have a weaker predictive effect in non-cirrhotic HBV-ACLF patients than in cirrhotic HBV-ACLF patients[73]. Therefore, for non-cirrhotic HBV-ACLF patients, care should be taken when MELD-based scores are used to predict possible outcomes.
For a long time, the SOFA score and APACHE II/III scores were reported to be good indicators of prognosis in critically ill patients. Except for initial scores of more than 11 (mortality rate > 90%), a decreasing SOFA score during the first 48 h predicts a mortality rate of at least 50% in intensive care unit patients[74]. In patients with alcohol-related ACLF, the APACHE II score seems superior to SOFA, CTP, and MELD in predicting short-term mortality[75]. However, the use of SOFA and APACHE II/III scores in patients with HBV-ACLF is rare. Thus, it is unclear whether these scores could effectively predict the outcome of patients with HBV-ACLF.
In recent years, many new mathematical models have been established to assess the short-term prognosis of HBV-ACLF patients[76]. For example, the logistic regression model established by Zheng et al[70] has greater accuracy than MELD and CTP in predicting the prognosis of HBV-ACLF patients, regardless of the presence or absence of cirrhosis. The predictive validity of the APLH-Q score established by cox proportional hazard regression analysis seems significantly better than that of the previously reported LSM and MELD[77]. In addition, Ning et al have also established a Tongji prognostic predictor model (TPPM) by integrating biochemical parameters, coagulation parameters, indicators of hepatitis virology, and complications. The TPPM has better sensitivity and specificity in predicting 3 mo mortality in HBV-ACLF patients than the MELD score. However, these mathematical models were used in single-center retrospective studies with a relatively small sample size and need to be validated in other well-designed prospective studies.
CONCLUSION
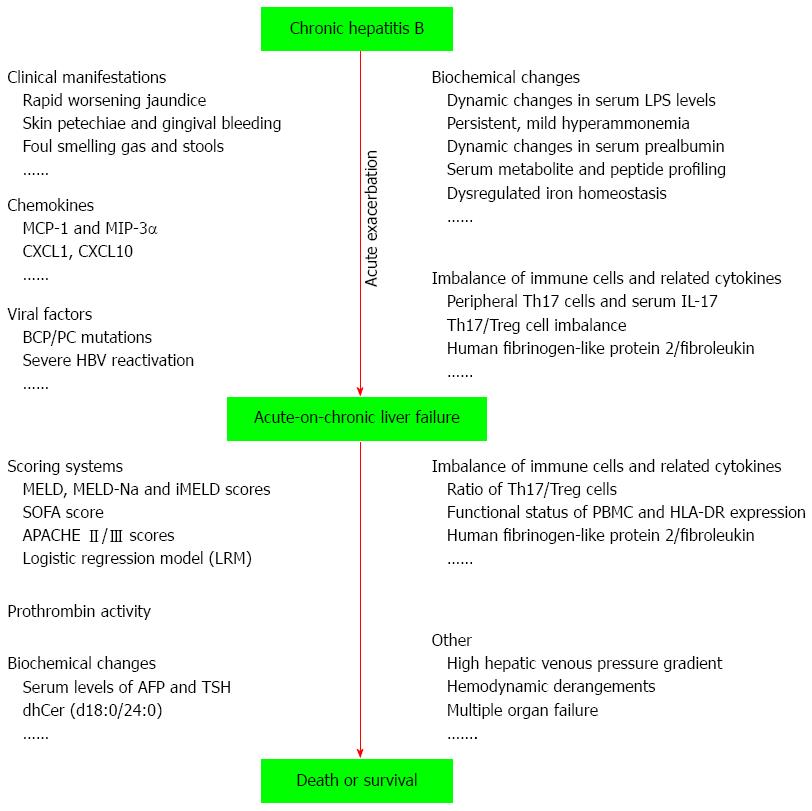
ACLF is a devastating disease with a very high short-term mortality in CHB patients, and acute exacerbation of CHB is an inevitable stage in the development of HBV-ACLF. Here, we reviewed the indicators and models for early warning of the progression of acute exacerbation of CHB to ACLF and the prediction of the outcome of patients with HBV-ACLF (Figure 1). Careful observation of dynamic changes in certain clinical manifestations and laboratory variables (such as host biochemical variables, immune cells, cytokines, chemokines, virus genotypes, and mutants) can provide important references to help physicians determine the timely diagnosis and effective treatment of HBV-ACLF. However, due to the complexity of HBV-ACLF development, a considerable number of those indicators and models have either methodological or reporting limitations. For example, their accuracy in early warning or outcome prediction of HBV-ACLF may vary significantly between cirrhotic and non-cirrhotic patients. Thus, ideal early warning and prognostic prediction systems, which pay more attention to various underlying diseases and complications, are needed. In addition, their accuracy and validity should be verified in well-designed prospective studies with a large sample size.
Figure 1 Overview of indicators and models for early warning of the progression of acute exacerbation of chronic hepatitis B to acute-on-chronic liver failure and prediction of the outcome of patients with hepatitis B virus-acute-on-chronic liver failure.
LPS: Lipopolysaccharide; MCP-1: Monocyte chemoattractant protein-1; MIP-3α: Macrophage inflammatory protein-3α; MELD: Model of end-stage liver disease; BCP: Basal core promoter; PC: Precore; HBV: Hepatitis B virus; SOFA: Sequential organ failure assessment; APACHE: Acute physiology and chronic health evaluation; AFP: Alpha-fetoprotein; TSH: Thyroid stimulating hormone.
P- Reviewer: Pokorska-Spiewak M S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S