Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10208
Peer-review started: March 27, 2015
First decision: May 18, 2015
Revised: June 1, 2015
Accepted: July 15, 2015
Article in press: July 15, 2015
Published online: September 21, 2015
Processing time: 177 Days and 17.8 Hours
AIM: To investigate changes in oxidative stress in Crohn’s disease (CD) before and after anti-tumor necrosis factor (TNF)-α treatment.
METHODS: A total of 42 patients with active CD, who were scheduled to be treated by anti-TNF-α antibodies, were enrolled. Serum levels of diacron-reactive oxygen metabolites (d-ROM), biological antioxidant potential (BAP), and modified ratio of oxidative stress and antioxidant capacity (m-OA) were measured using the Free Radical Analytical System before and 8 wk after induction of therapy with infliximab or adalimumab. The values for oxidative stress were correlated with disease activity and clinical response as determined by the CD activity index (CDAI) at 8 and 54 wk after the therapy.
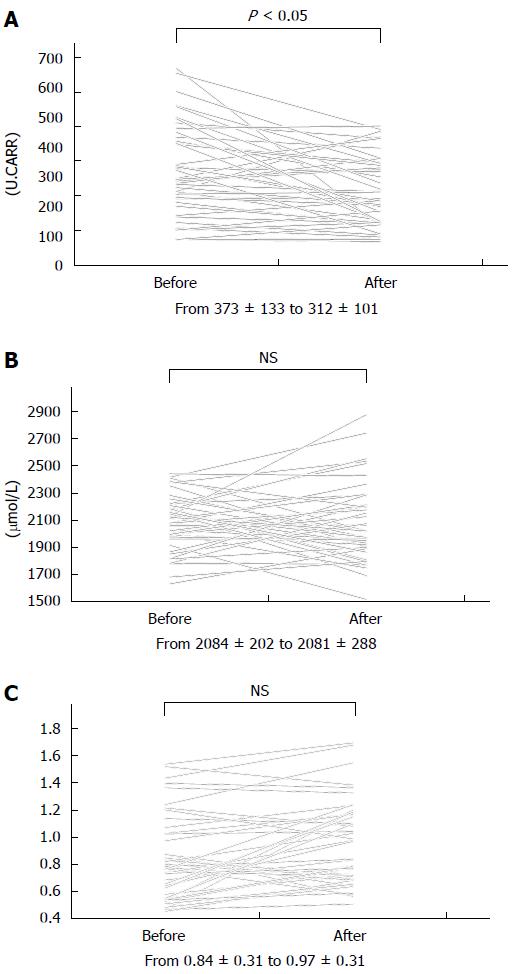
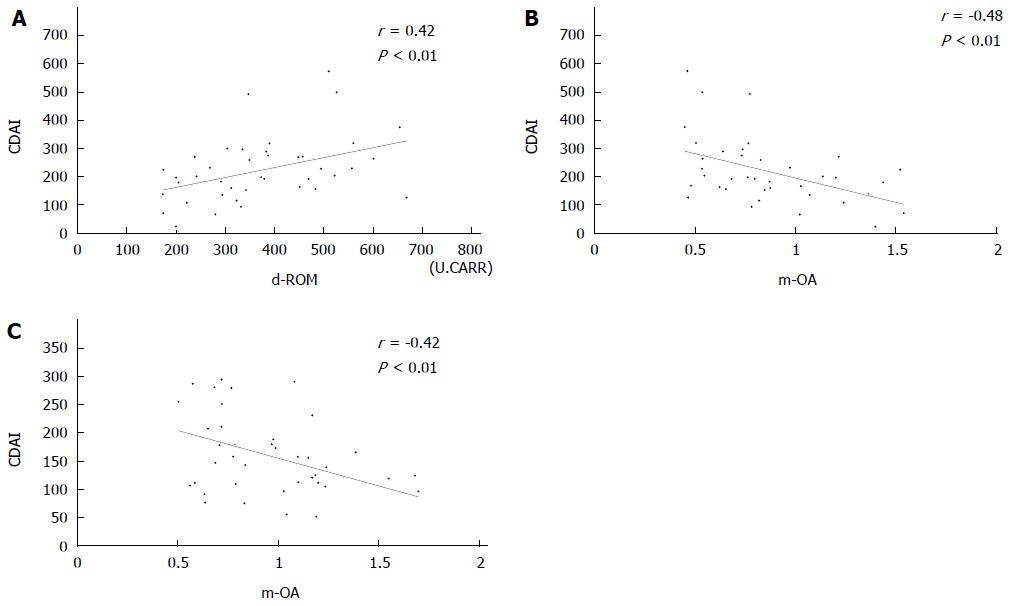
RESULTS: Prior to treatment, d-ROM showed significant correlations with CDAI (r = 0.42, P < 0.01). There was a significant negative correlation between m-OA and CDAI before and after treatment (r = -0.48 vs r = -0.42, P < 0.01). CDAI and d-ROM had decreased significantly by 8 wk after treatment (CDAI; 223.3 ± 113.2 vs 158.3 ± 73.4, P < 0.01, d-ROM; 373 ± 133 vs 312 ± 101, P < 0.05). However, neither BAP nor m-OA had changed significantly. In patients who had responded to the treatment at 8 wk, d-ROM, BAP, and m-OA levels before treatment did not differ significantly between patients with and without loss of response.
CONCLUSION: Anti-TNF-α therapy decreases oxidative stress in patients with CD, but does not alter the production of antioxidants. Dysregulation of antioxidants may be associated with the disease.
Core tip: We measured serum markers of oxidative stress (d-ROM) and antioxidant potential (BAP) prior to and 8 wk after the initial administration of infliximab or adalimumab in CD. As a consequence, d-ROM decreased significantly after treatment. However, BAP and the ratio of oxidative stress and antioxidant potential remained unchanged. Anti-tumor necrosis factor-α therapy decreases oxidative stress, but does not alter the production of antioxidants. Dysregulation of antioxidants may be associated with CD.
- Citation: Yamamoto K, Chiba T, Matsumoto T. Effect of tumor necrosis factor-α antagonists on oxidative stress in patients with Crohn’s disease. World J Gastroenterol 2015; 21(35): 10208-10214
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10208
Crohn’s disease (CD) is a chronic inflammatory condition of the intestinal tract. While the etiology is unknown, it is commonly thought that oxidative stress underlies the persistence of a chronic inflammatory process in the gut[1]. Oxidative stress is generally defined as an imbalance between pro-oxidant and anti-oxidant systems[2]. The oxidative stress consists of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Excessive ROS attack target molecules such as lipids, proteins, and nucleic acids, and as a consequence can cause protein denaturation, inactivation of enzymes, and modifications to DNA[1,3,4]. Since CD is a disease characterized by neutrophil infiltration in the intestinal wall, there is a large degree of tissue damage because of the release of ROS[1,5].
To date, the influence of tumor necrosis factor (TNF)-α, which is the most effective treatment for CD, on oxidative stress, has not been much investigated[6]. In 1999, Cesarone et al[7] described a simple procedure for measuring oxidative stress by means of quantification of serum diacron-reactive oxygen metabolites (d-ROM). d-ROM is a marker of hydroperoxide originating from ROS[8-11]. Biological antioxidant potential (BAP) is an antioxidant marker, which is representative of serum antioxidant capacity[12,13]. d-ROM and BAP can be readily measured by the Free Radical Analytical System 4 (FRAS4) (H&D srl, Parma, Italy)[13,14]. Furthermore, a modified ratio of oxidative stress to antioxidant capacity (m-OA), calculated from d-ROM and BAP, is believed to indicate the degree of resistance to oxidative stress.
In the present study, we measured d-ROM and BAP in CD patients before and after induction of remission by anti-TNF-α therapy to investigate whether oxidative stress is associated with disease activity and efficacy of anti-TNF-α antibodies. We also studied whether oxidative stress is associated with the long-term efficacy of the treatment.
This was a pilot study of 42 patients with active CD, who were scheduled to be treated with anti-TNF-α antibodies. The demographics of the patients appear in Table 1. There were 30 males and 12 females with a mean age of 32 years. Eleven patients had ileal disease, 26 had ileo-colonic disease, and 5 had colonic disease. The duration of the disease ranged from 0 to 16 years with a mean of 6 years. Eight patients had a prior history of intestinal resection. Ten patients were treated with infliximab (IFX), while the remaining 32 patients were treated with adalimumab (ADA).
| Characteristics | All patients (n = 42) |
| Sex | |
| Male | 30 (71) |
| Female | 12 (29) |
| Age (yr), median | 31.9 (17-57) |
| Disease duration (yr), median | 6.2 (0-16) |
| Disease type | |
| Ileal | 11 (26) |
| Ileo-colonic | 26 (62) |
| Colonic | 5 (12) |
| Patients with concomitant medication | |
| 5-aminosalicylic acid | 41 (98) |
| Prednisone | 9 (21) |
| Azathioprine | 7 (17) |
| Enteral nutrition | 24 (57) |
| Previous segmental resection | 8 (19) |
| Infliximab | 10 (24) |
| Adalimumab | 32 (76) |
IFX was administered at a dose of 5 mg/kg intravenously at 0, 2 and 6 wk as induction therapy. Subsequently, IFX at a dose of 5 mg/kg every 8 wk was administered as maintenance therapy. ADA was administered subcutaneously at a dose of 160 mg at wk 0 and 80 mg at wk 2, followed by scheduled maintenance therapy at a dose of 40 mg every other week. Treatment responses were determined by physical examination and the CD activity index (CDAI) before and 8 wk after the initial administration of anti-TNF-α antibodies. Peripheral blood samples were also collected before and 8 wk after starting treatment. White blood cell count (WBC) and serum levels of C-reactive protein (CRP) and albumin were measured. CDAI was assessed at 8 wk and every 4 wk thereafter up to 54 wk after initial administration of anti-TNF-α.
Patients were assessed for response to treatment as defined by a decrease in the CDAI score of 70 points or more from the baseline value, and at least a 25% reduction in the total score after 8 wk of treatment. In patients who showed a clinical response to induction therapy, those who fulfilled any one of the following criteria were regarded as having a loss of response (LOR): (1) an increase in CDAI of at least 70 points from the score at 8 wk, with a total score of at least 175; (2) an increase in CDAI of 35% or more from the baseline value; or (3) the introduction of a new treatment for active CD[15].
Oxidative stress was measured as serum d-ROM, BAP and m-OA. The stress was assessed before and 8 wk after the initial administration of anti-TNF-α. Blood samples were stored on ice after collection, and centrifuged to separate the serum. The serum was then stored at -80 °C until analysis.
Immediately prior to measurement, the samples were defrosted at room temperature and vortexed. To measure d-ROM levels, the FRAS4 system was used. For the measurement of d-ROM, 20 μL serum and 1 mL buffered solution (R2 reagent of the kit, pH 4.8) were mixed in a cuvette, and then 10 μL chromogenic substrate (R1 reagent) was added. After mixing and centrifugation for 60 s, the cuvette was incubated in a thermostatic block for 5 min at 37 °C. Then, the absorbance at 505 nm was recorded. Measurements were expressed as Carr U. Reference values measured by the manufacturer were indicated as being from 250 to 300 Carr U. Values greater than 300 Carr U suggest oxidative stress[7-9].
To measure BAP levels, the BAP test was performed using the same analyzer. In brief, 50 μL R2 reagent (ferric chloride) was added to a cuvette containing R1 reagent (thiocyanate derivative). The absorbance was measured and the reagent blank value subtracted. Then, 10 μL serum sample was added to the cuvette. After incubation for 5 min at 37 °C, the absorbance at 505 nm was recorded. The BAP levels were expressed as μmol/L. Reference values provided by the manufacturer were greater than 2200 μmol/L. Values lower than 2200 μmol/L suggest a reduction of antioxidant capacity[8,12]. The modified ratio of oxidative stress to antioxidant capacity (m-OA) was calculated as BAP/d-ROM/7.541[16].
Data are expressed as mean ± SD, or as median (25th–75th percentiles). Values were compared between groups using the paired t-test or Student t-test, as appropriate. Correlation coefficients were assessed by linear regression analysis. P < 0.05 was considered to be statistically significant.
After 8 wk, 32 (76%) of the patients showed a response to treatment. At 54 wk, 22 patients (52%) were in remission, while 6 patients (14%) had dropped out, and 4 (15%) had shown LOR. CDAI decreased significantly 8 wk after treatment initiation (223.3 ± 113.2 vs 158.3 ± 73.4, P < 0.01). Changes in serum albumin, white blood cell count (WBC), and CRP are shown in Table 2. CRP values decreased significantly, while there was no statistically significant change in serum albumin or WBC.
| Biochemical tests | Before | 8 wk | P value |
| CRP (mg/dL) | 2.4 ± 2.6 | 1.1 ± 1.6 | < 0.01 |
| Alb (g/dL) | 3.4 ± 0.75 | 3.7 ± 0.84 | NS |
| WBC (per μL) | 7339 ± 2484 | 7278 ± 2532 | NS |
Figure 1 illustrates changes in d-ROM, BAP, and m-OA before and 8 wk after induction of therapy. After 8 wk, d-ROM had decreased significantly (373 ± 133 vs 312 ± 101, P < 0.05) (Figure 1A). The decrease was statistically significant in each type of regional involvement (ileal, ileocolonic, or colonic disease) (data not shown). However, neither BAP nor m-OA changed significantly after 8 wk (Figure 1B, 1C).
Table 3 shows the correlations between oxidative markers and clinical parameters. Before treatment, d-ROM showed statistically significant correlations with CRP (r = 0.64) and CDAI (r = 0.42) (Figure 2A). The correlation between d-ROM and CRP was significant even after induction therapy (r = 0.53). d-ROM showed no significant correlation with the other parameters either before or after treatment. We failed to find any significant correlation of BAP with CRP, CDAI, WBC, or serum albumin.
| Oxidative markers | Clinical parameters | r | P value |
| d-ROM | CDAI | ||
| Before | 0.42 | < 0.01 | |
| After | 0.33 | 0.03 | |
| CRP | |||
| Before | 0.64 | < 0.01 | |
| After | 0.53 | < 0.01 | |
| WBC | |||
| Before | 0.12 | 0.47 | |
| After | 0.03 | 0.84 | |
| Alb | |||
| Before | -0.33 | 0.03 | |
| After | -0.38 | 0.01 | |
| BAP | CDAI | ||
| Before | -0.17 | 0.28 | |
| After | -0.18 | 0.24 | |
| CRP | |||
| Before | 0.03 | 0.85 | |
| After | 0.12 | 0.44 | |
| WBC | |||
| Before | -0.04 | 0.79 | |
| After | 0.01 | 0.95 | |
| Alb | |||
| Before | 0.24 | 0.13 | |
| After | 0.14 | 0.38 | |
| m-OA | CDAI | ||
| Before | -0.48 | < 0.01 | |
| After | -0.42 | < 0.01 | |
| CRP | |||
| Before | -0.63 | < 0.01 | |
| After | -0.45 | < 0.01 | |
| WBC | |||
| Before | -0.20 | 0.21 | |
| After | -0.01 | 0.73 | |
| Alb | |||
| Before | 0.45 | < 0.01 | |
| After | 0.47 | < 0.01 |
There were statistically significant negative correlations between m-OA and CDAI both before (r = -0.48) and after (r = -0.42) treatment (Figure 2B, 2C). There were negative correlations between m-OA and CRP before (r = -0.63) and after (r = -0.45) treatment. Significant correlations were also observed between d-ROM and CRP. Furthermore, we found a positive correlation between m-OA and serum albumin before (r = 0.45) and after (r = 0.47) treatment.
We then compared d-ROM, BAP, and m-OA between responders and non-responders after 8 wk of treatment. d-ROM, BAP, and m-OA were not significantly different between the 2 groups of patients either before or after treatment. When we compared d-ROM, BAP, and m-OA before and after the therapy between patients with and without LOR, we found no significant differences, suggesting that these parameters of oxidative stress are not predictive of LOR.
Increases in oxidative stress have been demonstrated in the serum and intestinal mucosa of patients with CD[17-20]. More recently, Kupčova et al[21] described a reduction of oxidative stress in patients with CD after administration of anti-TNF-α antibody. In the present investigation, we found that serum oxidative stress decreased in patients with CD after anti-TNF-α treatment. We also showed that the ratio of antioxidant activity vs oxidative stress calculated as m-OA was negatively associated with the activity of CD even after anti-TNF-α treatment. These findings strongly suggest that a decrease in antioxidant activity is characteristic of CD, and that m-OA may be one of the specific biomarkers for patients with the condition.
d-ROM and BAP levels have been used to assess oxidative status, and their significance as clinical markers has been described in several fields. The 2 parameters have been studied in particular in cardiovascular disease, central nervous system disease, and lifestyle-related illnesses, and it has been shown that increases in reactive oxygen metabolites are associated with an increased risk of vascular endothelial damage[22-26]. Furthermore, Sugiura et al[22] measured serum d-ROM levels in patients at high risk of cardiovascular disease and found that the d-ROM level coupled with serum high-sensitivity CRP was predictive of cardiovascular events. In our patients with CD, we found that d-ROM, serum CRP, and CDAI decreased significantly during induction therapy. Although we have not presented the data, there was no difference in the decrease in d-ROM between patients treated with IFX and those with ADA. Thus it seems likely that anti-TNF-α therapy suppresses the production of oxidative stress, thereby resulting in clinical benefit. In consideration of the biologic activity of anti-TNF-α, a decrease in the activity of circulating neutrophils seems to have made an important contribution to the decrease in oxidative stress measured as d-ROM.
Unlike d-ROM, we failed to show any changes in BAP, which represents antioxidant capacity, in our patients with CD even after anti-TNF-α treatment. While we did not include healthy controls in this investigation, the values of BAP in our patients were generally low, with more than half of the patients having values less than 2200 μmol/L. In previous studies, a reduction in serum antioxidant capacity was found in patients with inflammatory bowel disease (IBD)[17,27,28]. On the other hand, evidence for enhancement of antioxidant capacity was also found in biopsy specimens from IBD patients[19]. However, since the latter investigation included both patients with ulcerative colitis and with CD, it is uncertain that the antioxidant capacity was actually increased in CD. From our present results, it seems likely that the impairment of antioxidant capacity is specific to CD. This may be a consequence of a genetically determined disturbance in autophagy, which does not seem to be increased by any therapeutic strategy[29].
Normally, cells handle oxidative stress by mechanisms that include the production of antioxidant agents. These compounds have a limited capacity to buffer against an increase in oxidative stress and prevent its toxic consequences. There are several naturally occurring antioxidant defenses in the human intestine that serve to protect cell membranes from lipid peroxidation, protein oxidation, and enzyme inactivation[18]. In this regard, m-OA has been considered to be a candidate serum marker for the balance between oxidative stress and antioxidant capacity. In the present investigation, m-OA showed the strongest and negative correlations with CDAI and CRP. Furthermore, the correlations were significant even after induction of anti-TNF-α antibody treatment. These observations strongly suggest that m-OA is a marker of persistent chronic inflammation of the intestine, and may be one of the surrogate biomarkers for patients with CD. While we could not show any significant utility of m-OA for predicting the short-term response to anti-TNF-α treatment or LOR in our CD patients, the potential role of the marker warrants further study.
There are some limitations to this study. First, as we used frozen samples of serum, we may have underestimated the concentrations of d-ROM, BAP, and m-OA. Second, as we did not enroll healthy controls, we could not determine the reference values of the concentrations of the markers for oxidative stress, especially BAP, in the Japanese population. Third, since our investigation was a pilot study with a relatively small number of patients, we could not make strong conclusions regarding the roles of d-ROM, BAP, and m-OA in the prediction of the response to treatment or the long-term clinical course of CD. This was especially the case for the lack of data regarding serum concentrations of and serum antibodies to IFX and ADA. Furthermore, we could not apply practical intestinal damage, namely mucosal healing, as a parameter of disease activity[30]. These issues are under investigation in another prospective study.
In conclusion, we have shown that anti-TNF-α therapy decreases oxidative stress in patients with CD without affecting the production of antioxidants. In addition, m-OA, a marker for the balance between oxidative stress and antioxidant capacity, showed significant correlations with other parameters of disease activity in CD. Therefore, we propose that impairment of antioxidant activity may be associated with intractability of CD.
While the etiology of Crohn’s disease (CD) is unknown, it is commonly thought that oxidative stress underlies the persistence of a chronic inflammatory process in the gut.
The authors investigated whether oxidative stress is associated with disease activity and efficacy of anti- tumor necrosis factor (TNF)-α antibodies in patients with CD.
Serum oxidative stress decreased after anti-TNF-α treatment, while antioxidant capacity remained unchanged. The ratio of antioxidant activity vs oxidative stress (m-OA) negatively correlated with disease activity prior to and after anti-TNF-α treatment. Dysregulation of antioxidants may be associated with CD.
The ratio of antioxidant activity vs oxidative stress measured by m-OA may be one of the specific biomarkers for patients with CD.
This paper is interesting to know a relationship between oxidative stress and CD. The authors have necessary additional investigation in order to adjust for certain confounder in the correlation with oxidative markers.
P- Reviewer: Mosli MH S- Editor: Yu J L- Editor: Cant MR E- Editor: Liu XM
| 1. | Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J Gastroenterol. 2013;19:6540-6547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 716] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 3. | Tanida S, Mizoshita T, Mizushima T, Sasaki M, Shimura T, Kamiya T, Kataoka H, Joh T. Involvement of oxidative stress and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in inflammatory bowel disease. J Clin Biochem Nutr. 2011;48:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Naito Y, Takano H, Yoshikawa T. Oxidative stress-related molecules as a therapeutic target for inflammatory and allergic diseases. Curr Drug Targets Inflamm Allergy. 2005;4:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:605-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127-130. [PubMed] |
| 8. | Komatsu F, Kudoh H, Kagawa Y. Evaluation of oxidative stress and effectiveness of low-dose glucocorticoid therapy on exacerbation of chronic obstructive pulmonary disease. J Gerontol A Biol Sci Med Sci. 2007;62:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Trotti R, Carratelli M, Barbieri M, Micieli G, Bosone D, Rondanelli M, Bo P. Oxidative stress and a thrombophilic condition in alcoholics without severe liver disease. Haematologica. 2001;86:85-91. [PubMed] |
| 10. | Dani C, Martelli E, Bertini G, Pezzati M, Filippi L, Rossetti M, Rizzuti G, Rubaltelli FF. Plasma bilirubin level and oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F119-F123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Carratelli M, Porcaro L, Ruscica M, De Simone E, Bertelli AA, Corsi MM. Reactive oxygen metabolites and prooxidant status in children with Down’s syndrome. Int J Clin Pharmacol Res. 2001;21:79-84. [PubMed] |
| 12. | Gerardi G, Usberti M, Martini G, Albertini A, Sugherini L, Pompella A, Di LD. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med. 2002;40:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Dohi K, Satoh K, Ohtaki H, Shioda S, Miyake Y, Shindo M, Aruga T. Elevated plasma levels of bilirubin in patients with neurotrauma reflect its pathophysiological role in free radical scavenging. In Vivo. 2005;19:855-860. [PubMed] |
| 14. | Alberti A, Bolognini L, Macciantelli D, M Caratelli. The radical cation of N, N-diethyl-para-phenylendiamine: a possible indicator of oxidative stress in biological samples. Res Chem Intermed. 2000;26:253-267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 16. | Nagata K, Hasegawa H, Hirokado Y, Kiyama K, Otsuki C. Lifestyle related diseases and the oxidative stress regulation system. Jpn J Psychosom Med. 2008;48:177-183. |
| 17. | Krzystek-Korpacka M, Neubauer K, Berdowska I, Zielinski B, Paradowski L, Gamian A. Impaired erythrocyte antioxidant defense in active inflammatory bowel disease: impact of anemia and treatment. Inflamm Bowel Dis. 2010;16:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 340] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Bouzid D, Gargouri B, Mansour RB, Amouri A, Tahri N, Lassoued S, Masmoudi H. Oxidative stress markers in intestinal mucosa of Tunisian inflammatory bowel disease patients. Saudi J Gastroenterol. 2013;19:131-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood). 2012;237:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 21. | Kupčova V, Tureckỳ L, Uhlíkova E. The role of oxidative stress in anti-tumor necrosis factor antibody treatment in Crohn’s disease. Curr Med Chem. 2012;19:5226-5231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Sugiura T, Dohi Y, Takase H, Yamashita S, Tanaka S, Kimura G. Increased reactive oxygen metabolites is associated with cardiovascular risk factors and vascular endothelial damage in middle-aged Japanese subjects. Vasc Health Risk Manag. 2011;7:475-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, Shintani S, Walsh K, Ouchi N, Murohara T. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Shimano M, Shibata R, Inden Y, Yoshida N, Uchikawa T, Tsuji Y, Murohara T. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm. 2009;6:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Yamanaka G, Kawashima H, Suganami Y, Watanabe C, Watanabe Y, Miyajima T, Takekuma K, Oguchi S, Hoshika A. Diagnostic and predictive value of CSF d-ROM level in influenza virus-associated encephalopathy. J Neurol Sci. 2006;243:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34:1041-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | D’Odorico A, Bortolan S, Cardin R, D’Inca’ R, Martines D, Ferronato A, Sturniolo GC. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Boehm D, Krzystek-Korpacka M, Neubauer K, Matusiewicz M, Berdowska I, Zielinski B, Paradowski L, Gamian A. Paraoxonase-1 status in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1450] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 30. | Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 327] [Article Influence: 10.5] [Reference Citation Analysis (0)] |