Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10126
Peer-review started: January 16, 2015
First decision: March 10, 2015
Revised: March 25, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: September 21, 2015
Processing time: 245 Days and 22.6 Hours
AIM: To study the effects of QHF-cisplatin on H22 hepatocellular carcinoma (HCC) and their mechanisms of action.
METHODS: Sixty BALB/c mice were randomly divided into a model group (n = 48) and a normal control group (n = 12). An HCC xenograft tumor was created by injecting H22 cells directly into the liver parenchyma of the mice. The 48 BALB/c mice in the model group were randomly divided into four groups: QHF, DDP (cisplatin), QHF plus DDP, and model control. The inhibitory effects of these drugs on tumor growth were evaluated by calculating the rate of tumor growth inhibition. The mice were examined by observing their general condition, body weight and survival time. Changes in tumor tissue were observed under an optical microscope. Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and α-fetoprotein (AFP) levels in serum were measured. Hepatocyte growth factor (HGF), c-mesenchymal-epithelial transition (c-Met) factor, phosphorylated (p)-c-Met, p38, p-p38, extracellular signal-regulated kinase (ERK), p-ERK and vascular endothelial growth factor (VEGF) levels were evaluated in tumor and liver tissues using western blotting.
RESULTS: Compared with the DDP group, a lower incidence of toxic reactions and a higher survival time were observed in the QHF plus DDP group. Tumor weight was significantly lower in the QHF, DDP and QHF plus DDP groups than in the model control group (0.24 ± 0.07, 0.18 ± 0.03 and 0.14 ± 0.01 g vs 0.38 ± 0.05 g, respectively), and the differences were statistically significant (P < 0.01). The rate of tumor growth inhibition in the QHF, DDP and QHF plus DDP groups was 38.7%, 52.6% and 63.5%, respectively. AST, ALT and AFP levels in serum were significantly lower in the QHF, DDP and QHF plus DDP groups compared to the model control group (P < 0.05). Similarly, HGF, p-c-Met, p-p38, p-ERK and VEGF levels in tumor tissue were significantly lower in the QHF, DDP and QHF plus DDP groups (P < 0.05).
CONCLUSION: QHF and DDP have an antiangiogenic effect on H22 HCC in mice. QHF inhibits tumor growth via blocking the HGF/c-Met signaling pathway, inhibiting p38, ERK and VEGF signaling.
Core tip: QHF and cisplatin (DDP) have an antiangiogenic effect on H22 hepatocellular carcinoma (HCC) in mice. QHF in combination with low-dose DDP has a synergistic antiangiogenic effect and can improve survival and reduce the incidence of toxic reactions in mice with H22 HCC. Moreover, QHF can significantly decrease the expression of hepatocyte growth factor and phosphorylated-c-mesenchymal-epithelial transition factor in liver tumor tissue.
- Citation: Chen T, Yuan SJ, Wang J, Hu W. Mechanism of QHF-cisplatin against hepatocellular carcinoma in a mouse model. World J Gastroenterol 2015; 21(35): 10126-10136
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10126
Traditional Chinese medicine (TCM) has unique, but little known advantages in the treatment of liver cancer[1]. TCM researchers have found that the treatment protocol of Qingrejiedu (clears away heat and toxins), Huoxuehuayu (promotes blood flow to remove stasis) and Fuzhengguben (strengthens healthy qi and root) (QHF) can improve the function of the immune system and its local microenvironment. The protocol also inhibits tumor growth and therefore prolongs survival time in cancer patients[2]. Tumor microenvironment, virus infection, and cell factors are closely related to liver cancer. Chronic inflammation of the liver, persistent infection by the hepatitis virus, cell factors involved in solid tumor development, and the generation of growth factors, constitute a complex microenvironment[3,4]. The liver microenvironment consists of tumor cells, cytokines, growth factors, mesenchymal cells and liver cancer development-related genes and proteins[5]. The development of liver cancer involves abnormal molecular signaling pathways, mainly the growth factor signaling pathways [vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and hepatocyte growth factor (HGF)], mitogen-activated protein kinase (MAPK) signaling pathway, phosphatidylinositol-3-kinase (inositol)/AKT (PI3K/AKT/mammalian) and WNT/β-catenin. From a therapeutic point of view, modification of these signaling cascades may help to reverse, delay or prevent the process of carcinogenesis[2,6].
In a preliminary study, we found that the TCM formula, QHF, had a significant therapeutic effect in liver cancer[7]. The inhibition of hepatocellular carcinoma (HCC) invasion and metastasis is associated with the MAPK signaling pathway, by inhibiting extracellular signal-regulated kinase (ERK), activating C-jun N-terminal kinase (JNK) and p38. However, the mechanisms involved in the regulation of MAPK upstream and downstream molecular targets are unclear. Some researchers found that the MAPK signaling pathway is activated by HGF through a paracrine signal loop which interacts with c-Met[8]. Thus, on the basis of these findings, we propose the following hypothesis: the anti-hepatoma effect of the TCM, QHF, is mediated by the HGF/c-Met and MAPK signaling pathways.
H22 tumor cells were purchased from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and were cultured by the Immune Research Center at our university. SPF BALB/c mice of either sex weighing 18-20 g were provided by the Laboratory Animal Center of Huazhong University of Science and Technology, Tongji Medical School, China (certificate number SCXK (E) 2011-0061). The animals were raised in laboratory conditions (20 °C ± 2 °C, 55%-65% humidity, 12 h/12 h light/dark, and ad libitum access to food and water).
Cinobufacini injection was purchased from Anhui Toad Biochemical Co. Ltd. (Anhui, China); 20 (R) ginsenoside Rg3 standard, Shanghai Source Leaves Biotechnology Co. Ltd. (Shanghai, China); lentinan standard, Nanjing Zelang Plant Extract Technology Co. (Nanjing, China); cisplatin injection was from Qilu Pharmaceutical Co. Ltd. (Jinan, China); pentobarbital sodium, Merck (Shanghai, China); RIPA lysis buffer, protease inhibitors (PMSF), phosphorylated protease inhibitor, horseradish-peroxidase-labeled goat anti-mouse IgG (H + L), horseradish-peroxidase-labeled goat anti-rabbit IgG (H + L), ERK/MAPK antibody, and p38/MAPK antibody were purchased from Blue Skies Biotechnology Research Institute; p-ERK/MAPK and p-p38/MAPK antibodies were obtained from Cell Signaling; angiogenic factor (VEGF) antibody, HGF receptor (c-Met) antibody, and p-c-Met antibody were gifts from Shanghai Biological Engineering Co. Ltd., China; developer, ECL chemiluminescence reagent, and the BCA Protein Assay kit were purchased from Wuhan Google Biotechnology Co. Ltd., China; aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) test kits were purchased from Nanjing Jiancheng Bioengineering Institute, China; The mouse α-fetoprotein (AFP) ELISA kit was purchased from Shanghai Jin Ma Co. Ltd., China; and hematoxylin was purchased from Beijing Bo Orson Biological Technology Co. Ltd., China.
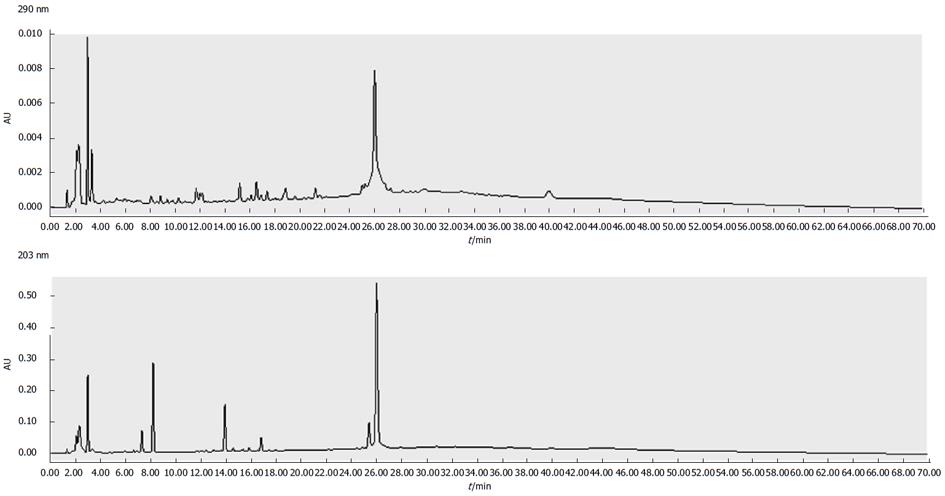
(1) QHF formula preparation was made according to compound cinobufotalin: Rg3: Panax notoginseng saponins: Lentinan, formulated in the proportion of 57: 1: 0.4: 7. Cinobufotalin 800 mg/kg, Ginsenoside Rg3 14 mg/kg, Notoginseng 5.5 mg/kg and Lentinan 100 mg/kg, a formula created by Professor Chen Tao[9]. All drugs were refrigerated at 4 °C (Figure 1); (2) The cisplatin solution was diluted in normal saline (NS) to a final concentration of 1 mg/mL and stored in the dark at 4 °C; (3) PBS solution: KCl 0.2 g, NaCl 8.0 g, KH2PO4 0.24 g, and Na2HPO4·12 H2O 3.58 g in 1 L double distilled water, adjusted to pH 7.2 and autoclaved to ensure it was sterile; (4) Electrophoresis buffer: glycine 14.4 g, Tris 3.03 g, 10% SDS 10 mL in 1 L double distilled water; (5) Transfer buffer: glycine 14.4 g and Tris 3.03 g in 600 mL double distilled water. Once dissolved, 200 mL methanol was added to the solution up to 1 L; (6) TBST solution: Tris 2.42 g, NaCl 8.8 g, Tween-20 500 μL in double distilled water to 1 L, with the pH adjusted to 7.4.
Under aseptic conditions, H22 hepatoma tumor-bearing mice were sacrificed by cervical dislocation 7 d after intraperitoneal inoculation of H22 tumor cells. The tumor cells in milk-white ascites were collected aseptically and washed by centrifugation in D-Hanks solution (800 r/min × 5 min, twice). The trypan blue dye exclusion assay was carried out to detect cell viability, which was ≥ 95%. H22 cells were diluted with NS and the cell concentration was adjusted to 5 × 108/mL and then placed on ice until use.
Sixty male or female BALB/c mice were randomly divided into a model group of 48 and a normal control group of 12. Mice in the model group were anesthetized using 1% sodium pentobarbital (40 mg/kg, intraperitoneally) and fixed in the supine position on an experimental board, which had been disinfected with iodine. The linea alba was incised to reveal the abdominal cavity. The rib cage was then gently pressed to expose the liver and the liver lobe closest to the surface was injected with tumor cells. The syringe needle pierced the liver about 1 cm and the cell suspension was slowly injected into the liver lobe (0.03 mL containing about 3 × 106 H22 hepatoma cells). The liver was returned to the abdominal cavity and the abdomen was sutured layer by layer. Similarly, mice in the normal control group were injected with NS using strict aseptic techniques.
A total of 60 BALB/c mice were randomly divided into a model group of 48, consisting of a model control group, QHF formula group, DDP (cisplatin) group, and combination group (QHF + DDP) of 12 mice each, and a normal control group of 12 mice. During the first 3 d, mice in the normal control group were injected intraperitoneally with NS. The HCC xenograft tumor model groups received DDP (intraperitoneal), QHF formula (intragastric), QHF (intragastric) combined with DDP (intraperitoneal) and NS (intraperitoneal), at a dose of 10 mL/kg for 15 d. Tissue specimen preparation was carried out, and the rate of tumor inhibition and the levels of AST, ALT and AFP in serum were assessed.
Following the last day of drug administration, tumor tissue samples were removed from the mice killed by cervical dislocation after eyeball blood was collected. Under sterile conditions, the tumors were weighed. The rate of tumor inhibition was calculated using the following formula: Inhibition rate (%) = [tumor weight of NS group (g) - tumor weight of treatment group (g)]/tumor weight of NS group (g) × 100%. After weighing, the tumor mass was divided into two parts: one part was frozen at -80 °C for further analysis and the other part was fixed in 4% paraformaldehyde at 4 °C for 4 h, stained with hematoxylin and eosin, embedded in paraffin, and slices prepared for microscopic examination of changes in mouse liver tissue morphology.
Blood obtained from the eyeballs was immediately placed in Eppendorf tubes containing heparin and maintained at 4 °C. The tubes were centrifuged at 3000 rev/min for 10 min. The upper serum was used in a microplate assay of serum AST and ALT levels.
AFP was detected by ELISA as follows: dilution of the standards, blank plates, standard plates and sample plates were set up, respectively. An accurate enzyme sample of 50 μL was added to the standard plates. To each sample plate, 40 μL diluent plus 10 μL sample was added. The final sample was diluted five times and the samples were mixed by gentle shaking. Following closure of the plate membrane, the samples were incubated at 37 °C for 30 min in an air bath, washed five times, and each sample was patted dry. Enzyme standard reagent of 50 μL was added to each plate, except for the blank plates, the plates were incubated and then washed. For color development, 50 μL chromogenic agent A plus 50 μL chromogenic agent B were added and the samples blended by oscillation for 10 min at 37 °C in the absence of light. To terminate the reaction, 50 μL stop solution was added to each well. The termination liquid was examined within 15 min at OD450.
A western blot assay was used to detect the expression of HGF, c-Met, p38/MAPK, ERK/MAPK and VEGF in liver tumor and liver tissue.
Lysates were collected a few minutes after adding PMSF and phosphorylase inhibitors. Stock solutions of PMSF and phosphorylase inhibitors were prepared at a final concentration of 1 mmol/L. A 30-mg sample of frozen tissue was taken and 300 μL protein lysate was added. The sample was placed in a precooled homogenizer and milled until fully cleaved. The sample was then placed on ice for 5 min, centrifuged at 12000 rev/min for 10 min and the supernatant was placed in a new 0.5-mL Eppendorf tube. A 5-μL aliquot of the isolated protein sample was used to determine the protein content, and the remainder was added to 5× the concentration of SDS-PAGE protein sample buffer, and the tube was placed in boiling water for 5 min to denature the proteins and stored at -80 °C for subsequent analysis.
The BCA protein assay kit instructions were carefully followed and a standard curve was constructed. The working BCA solution was diluted with PBS solution, after dilution of the protein sample by 1:10, and 20 μL diluents were added to 96-well plates containing 200 μL BCA solution. The plates were kept at 37 °C for 30 min and OD562 was measured. The protein concentration in the sample was calculated using the standard curve.
The 12% separating gel and 5% stacking gel configurations are shown in Table 1. After stacking, the glass plates were placed in the electrophoresis tank, together with electrophoresis buffer, a pulling comb and the sample. Electrophoresis was carried out at 70 V to the bromophenol blue concentrate layer, for about 40 min, and then the voltage was increased to 110 V. When the bromophenol blue migrated to the lower edge of the separation gel, electrophoresis was stopped (about 2 h).
| Component | 12% separating gel | 5% stacking gel |
| 7.5 mL | 3 mL | |
| H2O | 2.47 mL | 2.1 mL |
| 1.5 mol/L Tris (pH 8.8) | 1.88 mL | - |
| 1.0 mol/L Tris (pH 6.8) | - | 0.375 mL |
| 30% Ace-Bis (29:1) | 3 mL | 0.5 mL |
| 10% SDS | 75 μL | 30 μL |
| 10% APS | 75 μL | 30 μL |
| TEMED | 3 μL | 3 μL |
The transfer buffer was precooled to 4 °C. After electrophoresis, the gel was removed, the stacking gel excised, and the gel and filter paper soaked in transfer buffer. The nitrocellulose membrane was immersed in methanol and after 30 s and placed in the transfer buffer. The filter paper, nitrocellulose membranes, gels, and filters were then placed in an electricity transfer tank set at the correct polarity, ice was added, and a current of 250 mA applied to the transfer film for 90 min.
The PVDF membrane was blocked by TBST solution containing 5% skimmed milk at room temperature and decolored in a shaker for 2 h. Membranes were washed with TBST three times, for 10 min each, in a shaker. Primary antibody (ERK, p38, p-ERK, p-p38) with an anti-dilution solution at 1:800 dilution was then added to the PVDF membrane containing the target protein. Samples were maintained at 4 °C and placed on a shaker overnight. The membranes were washed with TBST, and washed again three times (vide supra) and a horseradish-peroxidase-labeled secondary antibody (with TBST at 1:2500 dilution) was added at room temperature, shaken for 2 h and the membranes were again washed three times.
The ECL fluorescence detection reagents A and B were mixed in equal volumes in a dark room, and then dripped onto the PVDF membrane. An X-ray film was placed on the PVDF membrane and the exposure time adjusted in accordance with the luminous intensity. The X-ray film was then placed in developing liquid and the negatives rinsed in tap water. They were then placed in fixing solution until the strip was clear, rinsed in tap water and left to dry.
The relative protein content was determined using Gelpro32 software analysis, with an integrated OD of the target protein bands/β-actin protein bands.
The statistical methods used in this study were reviewed by Qing Deng from the Medical Science College of China Three Gorges University who is a teacher of Statistics. All data were analyzed using SPSS version 13.0. Measurement data are expressed as the mean ± SD. Differences between groups were analyzed by one-way analysis of variance. Homogeneous variance in each group was measured with the Student-Newman-Keuls test, i.e., a Q test) or Bonferroni multiple sample mean pairwise comparison. When the variance in each group was missing, Tamhane’s T2 method was used to compare the experimental data between the two groups. The differences were considered statistically significant when P < 0.05.
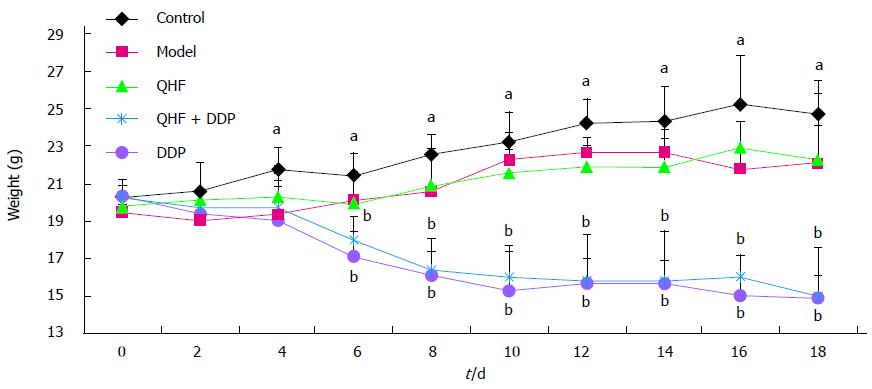
Mice were administered drugs before and after a change in body weight. During the first 4 d after inoculation, that is, the second day after drug administration, the mouse weight in the normal control group increased linearly. The weight of mice in the model control group increased for 10 d after inoculation (i.e., the first 8 d of administration). For the first 4 d, weight did not change significantly and then there was a downward trend. Changes in the body weight of mice in the QHF group were not obvious, but showed a tendency to increase. In contrast, in the QHF plus DDP group and the DDP alone group, the weight of mice significantly decreased, with the greatest weight loss in the DDP group (Figure 2).
The tumor tissue was peeled from the liver, weighed and the rate of growth inhibition calculated. In the QHF, QHF plus DDP and DDP groups, the inhibition rates were 38.7%, 63.5% and 52.6%, respectively. The tumor mass in each treatment group was significantly smaller than that in the model control group (P < 0.01). Group pairwise comparisons revealed that the tumor mass in the QHF plus DDP group was significantly smaller than that in the QHF group (P < 0.01); compared with the DDP group, tumor mass in the QHF plus DDP group was not significantly different.
Serum AFP levels in the model control group were higher than those in the normal control group (P < 0.01); compared with the model control group, the AFP levels in the treatment groups were lower (P < 0.05); and there were no statistical differences between the treatment groups. In addition, levels of serum ALT and AST in the model control group were significantly increased compared with the normal control group (P < 0.01). These results showed that liver function in the model control group was abnormal. In the DDP group, serum AST was significantly lower than that in the model control group (P < 0.05). In the treatment groups, serum AST activity appeared to decrease, but was not significantly different when compared with the model control group. In the QHF group, serum ALT activity was significantly lower than that in the model control group (P < 0.01). In the QHF plus DDP and DDP groups, serum ALT activity appeared to be lower than that in the model control group, but the difference was not significant (Table 2).
| Groups | Tumor mass (g) | Inhibition rate | AFP (U/L) | AST (U/L) | ALT (U/L) |
| Control | / | / | 8.2991 ± 0.5020 | 39.012 ± 2.8909 | 31.1377 ± 3.7956 |
| Model | 0.3836 ± 0.0520 | / | 10.1266 ± 0.9538f | 57.7102 ± 4.2786f | 56.8068 ± 2.3614f |
| QHF | 0.2353 ± 0.0709b | 38.70% | 9.3479 ± 0.9329af | 49.0341 ± 1.7405e | 29.3448 ± 3.7215b |
| QHF + DDP | 0.1401 ± 0.0145bd | 63.50% | 9.4775 ± 0.3631af | 48.0122 ± 4.751b | 50.4108 ± 3.8763f |
| DDP | 0.1819 ± 0.0269b | 52.60% | 9.3836 ± 0.7724af | 40.4485 ± 2.0745a | 56.0173 ± 3.1589f |
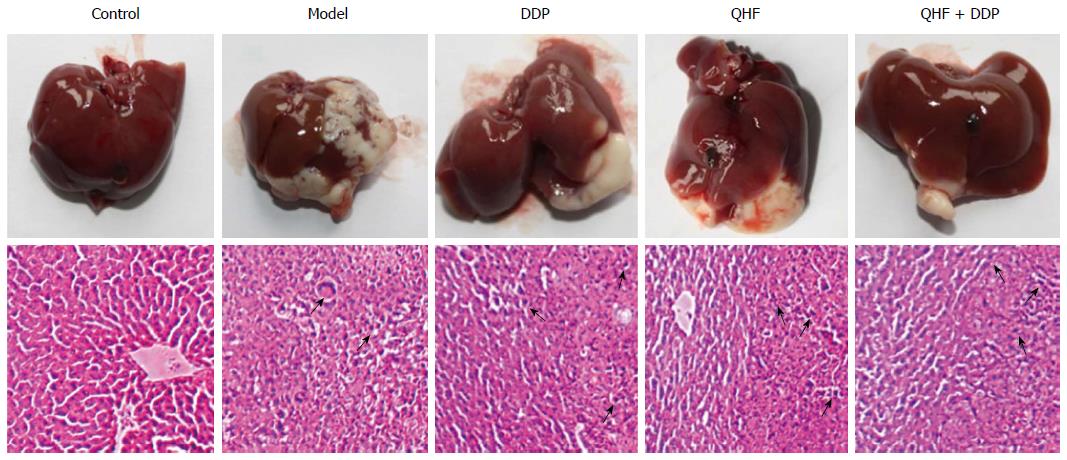
With the naked eye, the liver was bright red in color in the normal control group, but was dark red in the model group. In the model group, tumor was present not only in the left mid-lobe which was inoculated with tumor cells, but also on the surface of the other liver lobes, and extended over a much larger area. In the QHF, QHF plus DDP and DDP groups, the tumor coverage area on the liver surface was significantly reduced compared with the model control group. Most of the tumor appeared in the left mid-lobe which had been inoculated with tumor cells; in the other lobes a smaller tumor/no tumor was present. Hematoxylin and eosin staining revealed that liver tissue in the normal control group was in order, but in the model group liver cells had a disordered arrangement at the site of tumor invasion, with incomplete cell morphology, deeper nuclear staining and many areas of necrosis. Figure 3 shows the effects of Chinese herbal compound QHF, QHF plus DDP and DDP compared with the model control group. Liver cell morphology was disordered; and hepatocyte morphology, tumor invasion sites and deeper nuclear staining could be seen. The necrotic area was smaller than that in the model control group.
The results of western blotting used to detect liver tumors and the expression of HGF, c-Met, p-c-Met, p38, p-p38, ERK, p-ERK and VEGF proteins were as follows:
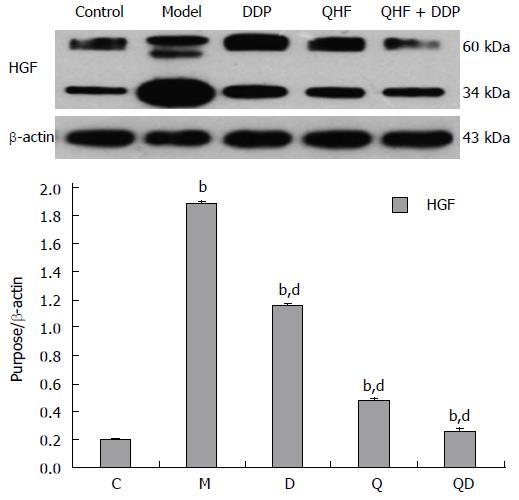
Detection of HGF. Mice in the model group had significantly increased HGF expression in liver and tumor tissues compared with the normal control group (P < 0.01). Compared with the model control group, the treatment groups had lower levels of HGF expression in liver and tumor tissues (P < 0.01). In the QHF plus DDP group, the effect was even more pronounced. These results clearly showed that QHF inhibited the expression of HGF (Figure 4).
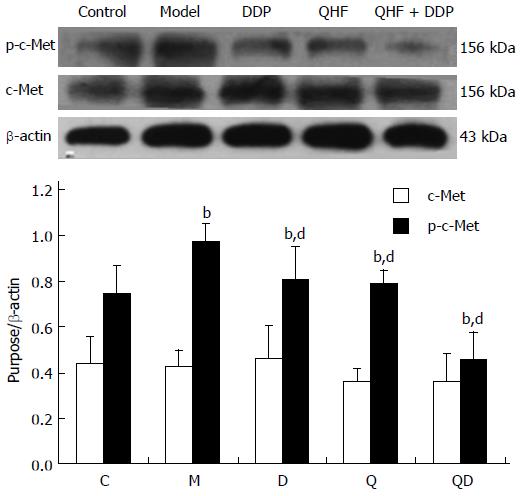
Detection of c-Met. Mice in the model group had significantly increased p-c-Met expression in liver and tumor tissues compared with the normal control group (P < 0.01). Compared with the model control group, the treatment groups had lower p-c-Met expression in liver and tumor tissues (P < 0.01). The effects of QHF plus DDP produced an even more obvious effect, however, the expression of c-Met total protein was unchanged in the treatment groups (Figure 5).
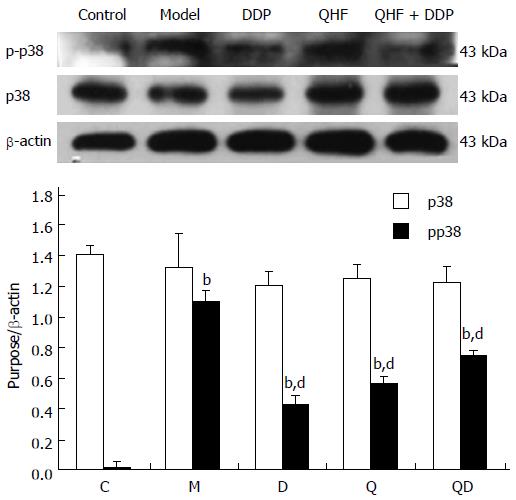
Detection of p38. Compared with the normal control group, mice in the model group showed significantly increased p-p38 expression in liver and tumor tissues (P < 0.01). Compared with the model control group, the treatment groups exhibited lower p-p38 expression in liver and tumor tissues (P < 0.01), with the effect in the DDP group being more apparent. The expression of p38 total protein was not obviously altered among the groups. Thus, these results show that QHF inhibited the p-38 signaling pathway (Figure 6).
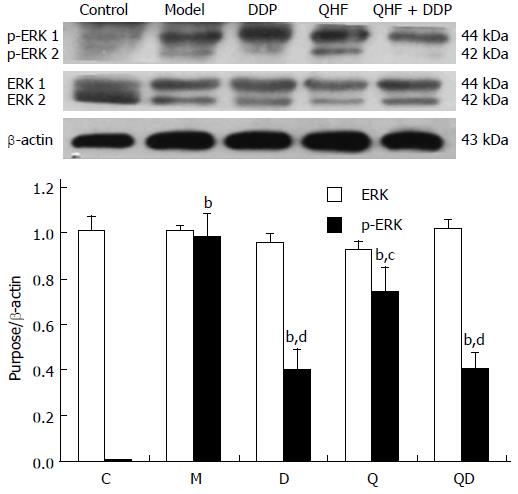
Detection of ERK. Compared with the normal control group, the expression of p-ERK in liver and tumor tissues was significantly increased in the model group (P < 0.01). Compared with the model control group, the treatment groups had lower p-ERK expression in liver and tumor tissues (P < 0.05). The effect in the QHF plus DDP group was even more obvious, however, there was no change in the expression of ERK total protein. These results showed that QHF inhibited the ERK signaling pathway (Figure 7).
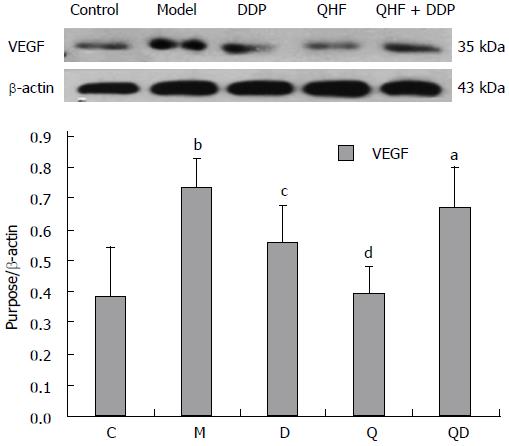
Detection of VEGF. Compared with the normal control group, the expression of VEGF in liver and tumor tissues was significantly increased in the model group (P < 0.01). Compared with the model control group, QHF significantly lowered the expression of VEGF in liver and tumor tissues (P < 0.01). Compared with the model control group, the expression of VEGF in the DDP group and the QHF plus DDP group was not statistically significant. These results showed that QHF inhibited the expression of VEGF (Figure 8).
The current treatments for HCC include liver surgery, interventional chemotherapy, and biological therapy. The purpose of treatment is to reduce the tumor size or ideally kill the tumor cells. However, these treatments have only a short-term beneficial influence, but not long-term effects, with recurrence occurring following radiotherapy after only 1-2 years[10]. Moreover, these well-intentioned interventions inevitably damage the body’s normal function, suppress the immune system, and may even promote the spread of cancer, thus leading to failure in the desired curative effect[11]. Numerous clinicians have reported that TCM improves symptoms in cancer patients, improves quality of life, and prolongs survival time[12]. Thus, TCM plays an important role compared with other treatments, especially for the many inoperable cases and in patients who cannot tolerate radiotherapy or conventional chemotherapy[13]. It is especially valuable following recurrence and metastasis after surgery or radiotherapy in advanced cancer patients. In patients treated with TCM, some severe symptoms are significantly improved and survival time is prolonged. Moreover, Chinese herbal compounds with multi-components and multi-target characteristics may be more suitable for comprehensive tumor treatment, because they aim to improve the overall therapeutic efficacy in cancer patients[14].
The Chinese herbal QHF formula is directed at classical liver cancer “stasis”, “poison” and “virtual” pathogenic characteristics and aims at detoxification, promoting blood circulation and strengthening the multiple effective anti-HCC components. Using an ingredients prescription screened uniform design method, the tumor inhibition rate was 55.9% and the life extension rate in the patients was 38.1%, arguably better than the actions of each single herb[15]. QHF compound increased the proportion of tumor cells in the G0/G1 phase, decreased the S phase, and induced tumor cell apoptosis (apoptotic rate 33.9%). It also reduced tumor tissue microvessel density by inhibiting the formation of tumor blood vessels, and significantly lowered VEGF, EGFR and matrix metalloproteinase (MMP)-2 expression to control tumor metastasis and recurrence. QHF combined with DDP can antagonize DDP-induced leukopenia, thymus toxicity, spleen atrophy and other toxic effects, with an inhibition rate up to 82.5% and life extension rate of 87.0%[16,17].
In recent years, it has been reported that there is a close relationship between HGF/c-Met and liver tumors, with pro-HGF secreted by stromal cells and mesenchymal cells after proteolytic activation into HGF[18]. HGF through a paracrine-signaling loop interacts with c-Met, inducing phosphorylation of the cell surface specific receptor c-Met and activation of a variety of intracellular signal transduction pathways to produce many biological effects. By activating mitogen-activated protein kinase (MAPK) and the phosphatidylinositol enzyme (PI3K) cascade, epithelial-mesenchymal transition can promote tumor survival. c-Met can also directly activate p38, JNK and NF-κB, which act together in cell cycle regulation, resistance to phagocytosis, enhance cell movement, promote angiogenesis and tumor growth[19]. Associated HGF, c-Met and VEGF pathways are also involved in promoting tumor survival. HGF/c-Met using the paracrine pathway can increase the induction of other angiogenic factors, such as the secretion of MMP-1, MMP-2, MMP-3, MMP-9 and VEGF, thereby initiating angiogenesis. This sequence of events is intimately involved in tumor metastasis, neovascularization and the promotion of tumor cell growth and invasion[18,20]. MAPK signaling, by activating ERK, JNK and p38 kinases, plays an important role in the repair of cell morphology and dynamic reactions[21]. The ERK/MAPK signaling pathway (also known as the Raf/MEK/ERK pathway) regulates cell proliferation and differentiation, angiogenesis and coexistence[22]. More importantly, overexpression or activation of the components of this pathway contributes to tumor occurrence, tumor formation and metastasis of solid tumors. ERK/MAPK signaling downstream of various growth factors indicates that this pathway is of vital importance in the activation of HCC[23-26].
The present study shows that c-Met protein is overexpressed in tumor and liver tissues, especially at the site of tumor invasion and metastasis. There was also a higher degree of differentiation in liver tumor tissues, which is one of the main indicators of liver cancer in clinical pathology. HGF is a ligand of c-Met, and its encoded proteins have important physiological roles in survival, growth and migration of different cells and tissues[27]. Moreover, c-Met and its ligand binding can cause its own phosphorylation, thereby activating downstream JAK, PI3K/AKT and ERK/MAPK signaling pathways, inducing cell apoptosis, proliferation and migration and tumor metastasis[28-30]. If we can inhibit HGF or prevent significant c-Met protein phosphorylation, and thus inhibit the activation of downstream signaling pathways, this should limit the occurrence and development of tumors.
In the present study, it was found that the TCM, QHF, can reduce liver tumor size and the surrounding liver tissue expression of c-Met protein and phosphorylated c-Met protein, and particularly reduce the expression of the c-Met ligand, HGF protein. The expression of HGF in tumor and liver tissues in the QHF group was reduced more than that in the DDP group. No difference in tumors and peripheral liver tissue regulation of phosphorylated c-Met protein was found between the QHF and the DDP groups. It is noteworthy that the combined treatment group had the most obvious effect on phosphorylated c-Met protein and HGF. Clearly, QHF in combination with DDP had an additional effect in regulating the expression of c-Met and its ligand HGF. Furthermore, the c-Met downstream activation of p38/MAPK, ERK/MAPK protein detection revealed that QHF inhibits the c-Met downstream ERK/MAPK, p38/MAPK signaling pathway. Compared with the model control group, the amount of p-ERK1/2 was reduced in the treatment group, and phosphorylation of ERK1/2 protein was reduced more in the DDP group than in the QHF group. There was no significant difference between the combination and DDP groups, which indicates that QHF in combination with DDP had no additive effects in terms of regulating the expression of ERK protein. Compared with the model control group, the amount of p-p38 in the treatment groups was significantly reduced. QHF in combination with DDP in terms of regulating the expression of p38 protein had no additive effect. In the QHF group, there was a reduction in the expression of the angiogenic factor, VEGF, in tumor and liver tissues, and downregulation of VEGF protein, indicating that QHF in combination with DDP in terms of regulating the expression of VEGF protein had no additive effect. Accordingly, QHF may function via inhibiting the HGF/c-Met signaling pathway, thereby inhibiting the downstream ERK/MAPK and p38/MAPK signaling pathway. Inhibition of VEGF expression may also play a major role[31]. It is now known that QHF regulates HGF/c-Met, but whether HGF/c-Met downstream of p38/MAPK, the ERK/MAPK signaling pathway and the regulation of VEGF play a direct role requires further research.
The current treatments for hepatocellular carcinoma (HCC) include liver surgery, interventional chemotherapy, and biological therapy, with the purpose of reducing the tumor size or ideally killing the tumor cells. These treatments have a good short-term influence, but no long-term effects. Traditional Chinese medicine (TCM) has unique, but little known advantages in the treatment of liver cancer. TCM can improve the function of the immune system and local tumor microenvironment, inhibit tumor growth, and therefore prolong survival time in cancer patients. The development of liver cancer involves abnormal molecular signaling pathways, mainly the growth factor signaling pathways (vascular endothelial growth factor, platelet-derived growth factor, epidermal growth factor and hepatocyte growth factor (HGF)], mitogen-activated protein kinase (MAPK) signaling pathway, phosphatidylinositol 3-kinase/AKT (PI3K/AKT/mammalian) and WNT/β-catenin. From a therapeutic point of view, modification of these signaling cascades may help to reverse, delay or prevent the process of carcinogenesis.
The anti-invasion and metastasis of HCC is associated with an effect on the MAPK signaling pathway, by inhibiting extracellular signal-regulated kinase (ERK), activation of C-jun N-terminal kinase (JNK), and p38. However, the mechanisms involved in the regulation of MAPK upstream and downstream molecular targets remain unclear. Some researchers have found that the MAPK signaling pathway is activated by HGF through a paracrine signal loop that interacts with c-Met. In recent years, it has been found that HGF/c-Met and liver tumors have a close relationship.
Numerous clinicians have reported that TCM improves symptoms in cancer patients, improves quality of life, and prolongs survival times. It is especially valuable following recurrence and metastasis after surgery or radiotherapy in advanced cancer patients. The research found that QHF reduced liver tumor size and the surrounding liver tissue expression of c-Met protein and phosphorylated c-Met protein, and particularly reduced the expression of the c-Met ligand, HGF protein.
In recent years, it has been found that HGF/c-Met and liver tumors have a close relationship, with pro-HGF secreted by stromal cells and mesenchymal cells after proteolytic activation into HGF. If HGF can prevent significant c-Met protein phosphorylation and thus inhibit the activation of downstream signaling pathways, this should limit the occurrence and development of tumors.
QHF formula: it is prepared based on compound cinobufotalin: Rg3: Panax notoginseng saponins: Lentinan, formulated in the proportion of 57:1:0.4:7. The formula consisting of Cinobufotalin 800 mg/kg, Ginsenoside Rg3 14 mg/kg, Notoginseng 5.5 mg/kg and Lentinan 100 mg/kg, was created by Prof. Chen Tao.
The present study confirms the curative effect of QHF on HCC and the mechanism of HCG/c-Met signaling pathway. This manuscript is well written and documented. It is suitable and worth publishing.
P- Reviewer: Chong DQQ, Ke X S- Editor: Yu J L- Editor: Kerr C E- Editor: Ma S
| 1. | Wang M, Qin KJ, Yang MQ, Wang LN. The Research Progress of Traditional Chinese Medical Diagnosis and Treatment for Primary Hepatocellular Carcinoma. Zhongyiyao Daobao. 2015;21:49-51. |
| 2. | Blumenschein GR, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol. 2012;30:3287-3296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Skardal A, Devarasetty M, Rodman C, Atala A, Soker S. Liver-Tumor Hybrid Organoids for Modeling Tumor Growth and Drug Response In Vitro. Ann Biomed Eng. 2015;Epub ahead of print. [PubMed] |
| 4. | Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Song le H, Binh VQ, Duy DN, Kun JF, Bock TC, Kremsner PG, Luty AJ. Serum cytokine profiles associated with clinical presentation in Vietnamese infected with hepatitis B virus. J Clin Virol. 2003;28:93-103. [PubMed] |
| 6. | Venepalli NK, Goff L. Targeting the HGF-cMET Axis in Hepatocellular Carcinoma. Int J Hepatol. 2013;2013:341636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Tao C, Dan L, Ling F, Peng G. In vivo and in vitro effects of QHF combined with chemotherapy on hepatocellular carcinoma. J Biomed Res. 2010;24:161-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Chen T, inventor . Chinese medicine effective component optimization formula of against hepatocellular carcinoma. State Intellectual Property Office of The P.R.C ZL. 2006;1 0135871.X. 2006 Oct 16. |
| 9. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1060] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 10. | Dmytriw AA, Morzycki W, Green PJ. Prevention of alopecia in medical and interventional chemotherapy patients. J Cutan Med Surg. 2015;19:11-16. [PubMed] |
| 11. | Lee YW, Chen TL, Shih YR, Tsai CL, Chang CC, Liang HH, Tseng SH, Chien SC, Wang CC. Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer: a population-based study. Cancer. 2014;120:1338-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Nie D, You QS, Luan JW, Li Y, Li XL, Guo RT, Zhang LP, Wu J. [Long-term results of personalized treatment in 72 breast cancer patients who failed chemotherapy]. Zhonghua Zhongliu Zazhi. 2013;35:941-945. [PubMed] |
| 13. | Konkimalla VB, Efferth T. Anti-cancer natural product library from traditional chinese medicine. Comb Chem High Throughput Screen. 2008;11:7-15. [PubMed] |
| 14. | Chen T, Li D, Fu YL, Hu W. Screening of QHF formula for effective ingredients from Chinese herbs and its anti-hepatic cell cancer effect in combination with chemotherapy. Chin Med J (Engl). 2008;121:363-368. [PubMed] |
| 15. | Chen T, Fu Y, Gong Z, Li D, Hu Y. Studies on the Anti-angiogenic Mechanism of the Formula of Chinese Medicine Active Ingredients Combined with Small Dose Cisplatin in Mice of Hepatocellular Carcinoma. Zhongguo Shiyan Fangjixue Zazhi. 2010;16:137-140. |
| 16. | Chen T, Fu Y, Gong Z, Li R, Hu Y. QHF formula in combination with low-dose cisplatin inhibits angiogenesis in H22 hepatocellular carcinoma in mice. Shijie Huaren Xiaohua Zazhi. 2010;18:137-140. |
| 17. | Appleman LJ. MET signaling pathway: a rational target for cancer therapy. J Clin Oncol. 2011;29:4837-4838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Garner OB, Bush KT, Nigam KB, Yamaguchi Y, Xu D, Esko JD, Nigam SK. Stage-dependent regulation of mammary ductal branching by heparan sulfate and HGF-cMet signaling. Dev Biol. 2011;355:394-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161-1176. [PubMed] |
| 20. | Brantley-Finley C, Lyle CS, Du L, Goodwin ME, Hall T, Szwedo D, Kaushal GP, Chambers TC. The JNK, ERK and p53 pathways play distinct roles in apoptosis mediated by the antitumor agents vinblastine, doxorubicin, and etoposide. Biochem Pharmacol. 2003;66:459-469. [PubMed] |
| 21. | Booth A, Trudeau T, Gomez C, Lucia MS, Gutierrez-Hartmann A. Persistent ERK/MAPK activation promotes lactotrope differentiation and diminishes tumorigenic phenotype. Mol Endocrinol. 2014;28:1999-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 23. | Deng W, Sui H, Wang Q, He N, Duan C, Han L, Li Q, Lu M, Lv S. A Chinese herbal formula, Yi-Qi-Fu-Sheng, inhibits migration/invasion of colorectal cancer by down-regulating MMP-2/9 via inhibiting the activation of ERK/MAPK signaling pathways. BMC Complement Altern Med. 2013;13:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196-1212. [PubMed] |
| 25. | Zhu H, Zhou C, Bai Y, Wang X. The research progress of anticancer Chinese herbal active ingredients. Shizhen Guoyi Guoyao. 2002;1:682-684. |
| 26. | Su H, Liu F, Hao T, Hong F. Hepatocyte growth factor/hepatocyte growth factor receptor pathway research status in tumors. Ai Zheng. 2013;35:321-324. |
| 27. | Chen W, Wu J, Shi H, Wang Z, Zhang G, Cao Y, Jiang C, Ding Y. Hepatic stellate cell coculture enables sorafenib resistance in Huh7 cells through HGF/c-Met/Akt and Jak2/Stat3 pathways. Biomed Res Int. 2014;2014:764981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Du W, Uslar L, Sevala S, Shah K. Targeting c-Met receptor overcomes TRAIL-resistance in brain tumors. PLoS One. 2014;9:e95490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, Frederick DT, Barzily-Rokni M, Straussman R, Haq R. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014;4:816-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 30. | Phillip CJ, Zaman S, Shentu S, Balakrishnan K, Zhang J, Baladandayuthapani V, Taverna P, Redkar S, Wang M, Stellrecht CM. Targeting MET kinase with the small-molecule inhibitor amuvatinib induces cytotoxicity in primary myeloma cells and cell lines. J Hematol Oncol. 2013;6:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Su H, Liu F. HGF/c-MET pathways in cancer research progress. Shiyong Aizheng Zazhi. 2010;28:98-100. |