Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9430
Peer-review started: March 11, 2015
First decision: April 13, 2015
Revised: April 22, 2015
Accepted: June 16, 2015
Article in press: June 16, 2015
Published online: August 21, 2015
Processing time: 165 Days and 21.4 Hours
AIM: To investigate the relationship between Helicobacter pylori (H. pylori) and mucin expression in gastric mucosa.
METHODS: English Medical literature searches were conducted for gastric mucin expression in H. pylori infected people vs uninfected people. Searches were performed up to December 31th 2014, using MEDLINE, PubMed, EMBASE, Scopus, and CENTRAL. Studies comparing mucin expression in the gastric mucosa in patients positive and negative for H. pylori infection, were included. Meta-analysis was performed by using Comprehensive meta-analysis software (Version 3, Biostat Inc., Englewood, NJ, United States). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated compared mucin expression in individual studies by using the random effects model. Heterogeneity between studies was evaluated using the Cochran Q-test, and it was considered to be present if the Q-test P value was less than 0.10. I2 statistic was used to measure the proportion of inconsistency in individual studies, with I2 > 50% representing substantial heterogeneity. We also calculated a potential publication bias.
RESULTS: Eleven studies, which represent 53 sub-studies of 15 different kinds of mucin expression, were selected according to the inclusion criteria. Every kind of mucin has been considered as one study. When a specific mucin has been studied in more than one paper, we combined the results in a nested meta-analysis of this particular mucin: MUC2, MUC6, STn, Paradoxical con A, Tn, T, Type 1 chain mucin, LeA, SLeA, LeB, AB-PAS, MUC1, and MUC5AC. The odds ratio of mucin expression in random analysis was 2.33, 95%CI: 1.230-4.411, P = 0.009, higher expression in H. pylori infected patients. Odds ratio for mucin expression in H. pylori positive patients was higher for MUC6 (9.244, 95%CI: 1.567-54.515, P = 0.014), and significantly lower for MUC5AC (0.447, 95%CI: 0.211-0.949, P = 0.036). Thus, H. pylori infection may increase MUC6 expression and decrease MUC5AC expression by 924% and 52%, respectively.
CONCLUSION: H. pylori inhibits MUC5AC expression in the gastric epithelium, and facilitates colonization. In contrast, increased MUC6 expression may help inhibiting colonization, using MUC6 antibiotics properties.
Core tip: In this meta-analysis we looked at studies that investigated the relationship between Helicobacter pylori (H. pylori) and mucin expression in the human gastric mucosa. English Medical literature searches were conducted for studies comparing mucin expression in the gastric mucosa in patients positive and negative for H. pylori infection. Meta-analysis was performed, and pooled odds ratios were calculated compared mucin expression in individual studies. Eleven studies, which represent 53 sub studies of 15 different kinds of mucin, were found. H. pylori inhibited MUC5AC expression and facilitated colonization. In contrast, increased MUC6 expression may help inhibiting colonization, using MUC6 antibiotics properties.
-
Citation: Niv Y.
Helicobacter pylori and gastric mucin expression: A systematic review and meta-analysis. World J Gastroenterol 2015; 21(31): 9430-9436 - URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9430.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9430
Mucins are the main component of the mucus layer attached to the gastric mucosa. These high molecular weight glycoproteins give the mucus unstirred layer the quality of viscosity and protect the mucosa form bacterial invasion or damage of toxic material, pepsin and acid. Mucin and Helicobacter pylori (H. pylori) have a complicated relationship. On the one hand this specific bacterium adopted to live in the mucin environment, enable moving in the viscous material by liquefying the surrounding mucin using urease and higher pH, and on the other hand mucin has antibiotic effect against the bug that control its proliferation and aggressiveness[1-3].
There are 3 main mucin types expressed in the gastric mucosa: MUC1, a membrane-bound mucin, and MUC5AC and MUC6 that are secreted mucins. MUC5AC is expressed mainly in the superficial epithelium and MUC6 in the glands[4]. Mucins are heavily glycosylated with sugar side-chains, and relatively stable to the active action of peptidases such as pepsin.
In this meta-analysis we looked at studies that investigated the relationship between H. pylori and mucin expression in the gastric mucosa. The possible hypothesis that the bug suppresses mucin synthesis, secretion and expression is controversial and some small studies gave confusing results. Mucin secretion could prevent aggressive behavior of H. pylori by inhibition of the bug proliferation and movement, but also supplies a preferred environment for the bug survival, protected from acid and pepsin.
We collected all the relevant studies that looked at mucin expression in the gastric mucosa of H. pylori infected patients in comparison with healthy controls.
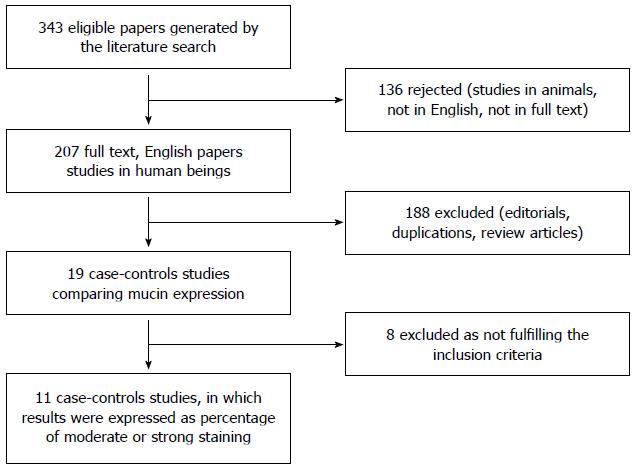
English Medical literature searches were conducted for gastric mucin expression in H. pylori infected vs uninfected people. Searches were performed through December 31th 2014, using MEDLINE, PubMed, EMBASE, Scopus, and CENTRAL. Search terms were: “Helicobacter pylori” OR “H. pylori” OR “Helicobacter” AND “mucin”. Hand searches of articles were performed after the initial search, and included article bibliography. Only fully published human studies in English were included (Figure 1).
Case-control studies comparing mucin expression in the gastric mucosa in patients positive and in those negative for H. pylori infection, were included. H. pylori infection should be diagnosed with at least one of the following method: histology, urease test, 13C-urea breath test, stool antigen test or H. pylori DNA. We selected only studies that used standard immunohistochemistry with antibodies against mucin proteins, and only those that expressed results by percentage of moderate or strong positive staining. Thus, studies where results were expressed with mean ± SD of staining scores were excluded, since meta-analysis could not be performed. We looked at all sorts of mucins that have been studied, in most of the cases more than one in a single study. In some of the studies mucins were separately measured at the superficial epithelium and the deep glands.
Mucin gene expression in the gastric mucosa compared quantitatively between the groups: patients with and without H. pylori infection. In the first run we considered every study where more than one mucin compared as a composite of several studies, and calculated all the sub-studies together. Then, in nested calculations, we isolated comparisons of different mucins, combined the sub-studies of different papers.
Meta-analysis was performed by using Comprehensive meta-analysis software (Version 3, Biostat Inc., Englewood, NJ, United States). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated compared mucin expression in individual studies by using the random effects model.
Heterogeneity between studies was evaluated using the Cochran Q-test, and it was considered to be present if the Q-test P value was less than 0.10. I2 statistic was used to measure the proportion of inconsistency in individual studies, with I2 > 50% representing substantial heterogeneity. We also calculated a potential publication bias.
All together we found 19 studies in human beings that measured gastric mucin staining intensity, using immunohistochemistry, and compared the staining score between positive and negative H. pylori patients (Figure 1)[5-21]. Eight studies were excluded for using average score and standard deviation for comparison of H. pylori positive and negative states, thus, meta-analysis could not be performed. From the results of these papers we could not retrieve the relative strength of each study as we could for studies where results were expressed as percentage of moderate or strong staining. We were left with 11 studies that fulfilled the inclusion criteria, published between 1997 to 2012 from 10 countries, 1 from United States, 1 from Argentina, 4 from Europe (Italy, Portugal, England and Turkey) and 5 from Asia (2 Japan, 1 Hong Kong, 1 China, 1 Israel).
Each study looked at 1 to 7 different kinds of mucin. All together 15 kinds of mucin were studies: MUC1, MUC2, MUC3, MUC5AC, MUC6, Paradoxical con A (PcA), Tn Ag (Tn), Sialyl Tn Ag (STn), T Ag (T), Type 1 chain mucin (T1), Lewis A Ag (LeA), Sialyl Lewis A Ag (SLeA), Lewis B Ag (LeB), T Ag after treatment with neuroaminidase (TN), AB-PAS positive (Figure 2).
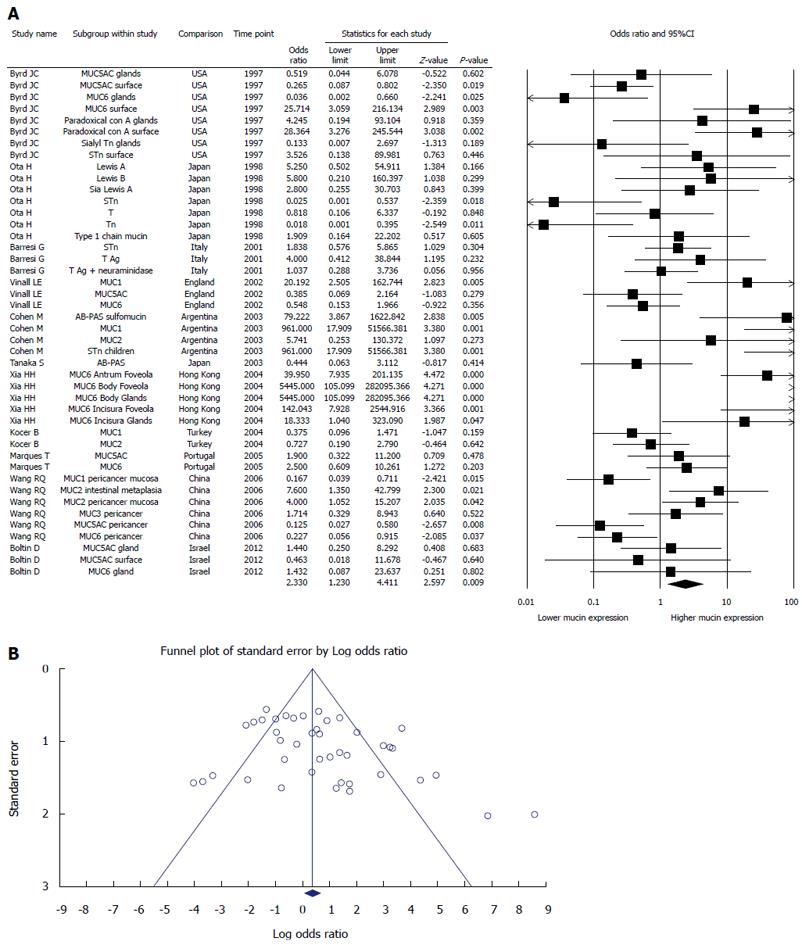
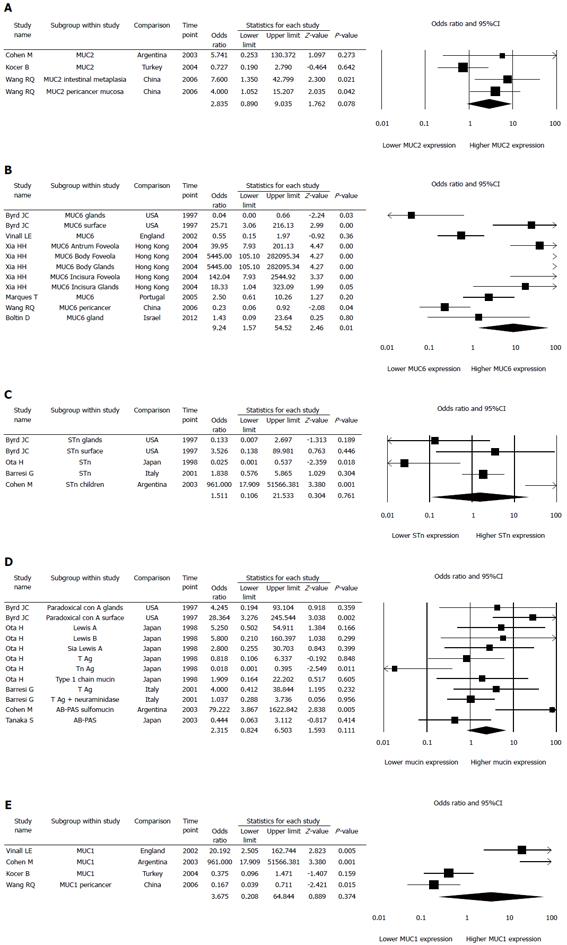
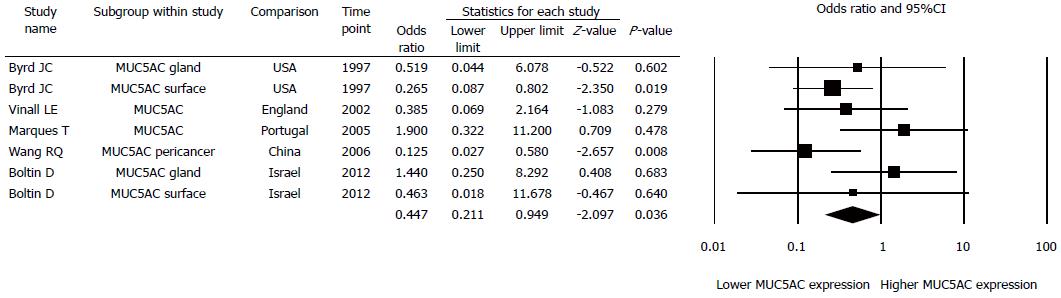
Every kind of mucin has been considered as one study, thus we had 53 studies investigated 15 different mucins (Figure 2A). When a specific mucin has been studied in more than one paper, we combined the results in a nested meta-analysis of this particular mucin: MUC2 (3 papers, 4 studies) (Figure 3A), MUC6 (6 papers, 11 studies) (Figure 3B), STn (4 papers, 5 studies) (Figure 3C), Paradoxical con A, Tn, T, Type 1 chain mucin, LeA, SLeA, LeB, AB-PAS (5 papers, 12 studies) (Figure 3D), MUC1 (4 papers, 4 studies) (Figure 3E), and MUC5AC (5 papers, 7 studies) (Figure 4).
Eleven papers represent 53 sub-studies of 15 different kinds of mucin expression (Figure 2A). The odds ratio of mucin expression in random analysis was 2.33, 95%CI: 1.230-4.411, P = 0.009, higher in H. pylori infected patients. Funnel plot denies a significant publication bias (Figure 2B). There was significant heterogeneity in the included studies: Q = 190.6, df (Q) = 43, I2 = 77.4, P < 0.0001.
Odds ratio for mucin expression in H. pylori positive patients was higher for MUC2 (2.835, 95%CI: 0.890-9.035, P = 0.078; Figure 3A), MUC6 (9.244, 95%CI: 1.567-54.515, P = 0.014; Figure 3B), STn (1.511, 95%CI: 0.106-21.533, P = 0.761; Figure 3C), PcA, Tn, T, T1, LeA, SLeA, LeB, TN, and AB-PAS taken together (2.315, 95%CI: 0.824-6.503, P = 0.111, Figure 3D), and for MUC1 (3.675, 95%CI: 0.208-64.844, P = 0.374; Figure 3E). Odds ratio for mucin expression in H. pylori positive patients was lower only for MUC5AC (0.447, 95%CI: 0.211-0.949, P = 0.036; Figure 4). Thus, H. pylori infection increased MUC6 expression and decreased MUC5AC expression by 924% and 52%, respectively.
Higher mucin expression in the gastric epithelium of H. pylori positive patients than in healthy controls was demonstrated. This observation has a limited importance since mucins synthesis in the gastric epithelium is a complex of many processes, and involved different kinds of secreted and membrane-bound mucins. But, nested evaluation of gastric specific mucins, MUC5AC and MUC6 revealed a very interesting observation. The main mucin that protect the gastric surface epithelium is MUC5AC, which also responsible for H. pylori adhesion to LeA and LeB antigens[1]. We observed a decrease in MUC5AC expression in H. pylori positive patients, explained by the inhibition of galactosyltransferase[2]. The decrease in MUC5AC expression may facilitate H. pylori swimming and attaching the epithelium, become closer to the mucosa, and facilitate its nutrition support. On the other hand MUC6 expression increased in H. pylori positive patients. MUC6 has an antibiotic effect on H. pylori, and may be part of the stomach defensive mechanisms against the bug[3]. Only these changes in MUC5AC and MUC6 achieved statistical significance when patients positive and negative for H. pylori infection were compared.
Interestingly, there is increase in MUC1 expression in H. pylori positive patients (not significant). MUC1 is the main membrane-bound mucin in the gastrointestinal epithelium, and in addition to direct protection against bacteria and toxic material functions as a receptor, with cross talk ability with intracellular, cytoplasmatic proteins such as β-catenin, glycogen synthase kinase, APC and E-cadherin. Increase in MUC1 expression may facilitate the activation of the Wnt pathway and nuclear NF-κB. The effect of H. pylori infection on MUC1 should be further explored, since this may be a way for the bug to exert its carcinogenesis potential toward gastric adenocarcinoma or MALT lymphoma.
Other mucins studies did not present a stable direction for mucin expression when H. pylori positive and negative patients were compared. Nested collections of these studies for MUC2, STn, Tn, T, T1, TN, PcA, AB-PAS and MUC1 could not reach statistical significance (Figure 2A, 2C-E).
Our paper has several limitations. First, our meta-analysis is based on studies that used different immunohistochemical methods, antibodies against many kinds of mucins that manufactured by different companies. Second, we could only use studies comparing proportion of positive expression, and to exclude 9 papers that compared average scores. Third, the study performed in different populations of patients as well as different H. pylori species, about which we have no data. Thus, caution should be taken in interpreting the results.
In conclusion, H. pylori may inhibit MUC5AC expression by the human gastric epithelium, and thus facilitate colonization. In contrast, increased MUC6 expression may help inhibiting colonization using MUC6 antibiotics properties.
Three main mucin types are expressed in the gastric mucosa: MUC1, a membrane-bound mucin, MUC5AC and MUC6, which are secreted mucins. MUC5AC is expressed mainly at the superficial epithelium and MUC6 in the glands. Change in mucin expression was described in Helicobacter pylori (H. pylori) infection, which may contribute to the bug infectivity and harm for the integrity of gastric mucosa.
Mucins are high molecular weight glycoproteins which give the mucus unstirred layer of the stomach the quality of viscosity, and protect the mucosa form bacterial invasion. Mucin and H. pylori have a complicated relationship. On the one hand Helicobacter pylori is adopted to live in the mucin environment, enable moving in the viscous material by liquefying the surrounding mucin using urease and higher pH, and on the other hand mucin has antibiotic effect against the bug that control its proliferation and aggressiveness.
In this meta-analysis, the authors looked at studies that investigated the relationship between H. pylori and mucin expression in the gastric mucosa. The possible hypothesis that the bug suppresses mucin synthesis, secretion and expression is controversial and some small studies gave confusing results. Mucin secretion could prevent aggressive behavior of H. pylori by inhibition of the bug proliferation and movement, but also supplies a preferred environment for the bug survival, protected from acid and pepsin.
The study results suggest that manipulation of mucin secretion by the gastric mucosa may contribute to better eradication therapy of H. pylori.
H. pylori infection may cause gastritis, duodenitis, gastric ulcer, duodenal ulcer, gastric adenocarcinoma or lymphoma. The mucous layer, composed of mucins, are the barrier from bacterial invasion and have an important role in the body defense mechanisms.
Author presented a well-constructed meta-analysis of studies assessing the effect of Helicobacter pylori on gastric mucin secretion. This study is makes sense of the contradictory results in the literature. The discussion is excellent and elegantly provides a physiological basis for the results observed.
P- Reviewer: Boltin D, Ierardi E S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Niv Y. H. pylori/NSAID--negative peptic ulcer--the mucin theory. Med Hypotheses. 2010;75:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Tanaka S, Mizuno M, Maga T, Yoshinaga F, Tomoda J, Nasu J, Okada H, Yokota K, Oguma K, Shiratori Y. H. pylori decreases gastric mucin synthesis via inhibition of galactosyltransferase. Hepatogastroenterology. 2003;50:1739-1742. [PubMed] |
| 3. | Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 4. | Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598-1606. [PubMed] |
| 5. | Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, Graham DY, Genta RM. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Barresi G, Giuffrè G, Vitarelli E, Grosso M, Tuccari G. The immunoexpression of Tn, sialyl-Tn and T antigens in chronic active gastritis in relation to Helicobacter pylori infection. Pathology. 2001;33:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Vinall LE, King M, Novelli M, Green CA, Daniels G, Hilkens J, Sarner M, Swallow DM. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology. 2002;123:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Cohen M, Drut R, Cueto Rúa E. SIALYL-Tn antigen distribution in Helicobacter pylori chronic gastritis in children: an immunohistochemical study. Pediatr Pathol Mol Med. 2003;22:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Xia HH, Yang Y, Lam SK, Wong WM, Leung SY, Yuen ST, Elia G, Wright NA, Wong BC. Aberrant epithelial expression of trefoil family factor 2 and mucin 6 in Helicobacter pylori infected gastric antrum, incisura, and body and its association with antralisation. J Clin Pathol. 2004;57:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kocer B, Ulas M, Ustundag Y, Erdogan S, Karabeyoglu M, Yldrm O, Unal B, Cengiz O, Soran A. A confirmatory report for the close interaction of Helicobacter pylori with gastric epithelial MUC5AC expression. J Clin Gastroenterol. 2004;38:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Marques T, David L, Reis C, Nogueira A. Topographic expression of MUC5AC and MUC6 in the gastric mucosa infected by Helicobacter pylori and in associated diseases. Pathol Res Pract. 2005;201:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Wang RQ, Fang DC. Effects of Helicobacter pylori infection on mucin expression in gastric carcinoma and pericancerous tissues. J Gastroenterol Hepatol. 2006;21:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Boltin D, Halpern M, Levi Z, Vilkin A, Morgenstern S, Ho SB, Niv Y. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J Gastroenterol. 2012;18:4597-4603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Kawano S, Tsujii M, Nagano K, Ogihara T, Tanimura H, Hayashi N, Ito T, Sato N, Kamada T, Tamura K. Different effect of Helicobacter pylori on the human gastric antral and body mucosal intracellular mucin. Scand J Gastroenterol. 1990;25:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Morgenstern S, Koren R, Moss SF, Fraser G, Okon E, Niv Y. Does Helicobacter pylori affect gastric mucin expression? Relationship between gastric antral mucin expression and H. pylori colonization. Eur J Gastroenterol Hepatol. 2001;13:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Morgenstern S, Koren R, Fraser G, Okon E, Niv Y. Gastric corpus mucin expression after partial gastrectomy, in relation to colonization with Helicobacter pylori. J Clin Gastroenterol. 2001;32:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Fichman S, Niv Y. Histological changes in the gastric mucosa after Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2004;16:1183-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Vilkin A, Levi Z, Morgenstern S, Shmuely H, Gal E, Hadad B, Hardi B, Niv Y. Higher gastric mucin secretion and lower gastric acid output in first-degree relatives of gastric cancer patients. J Clin Gastroenterol. 2008;42:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Kang HM, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Lee HS, Jung HC, Song IS. Effects of Helicobacter pylori Infection on gastric mucin expression. J Clin Gastroenterol. 2008;42:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Niv Y, Boltin D, Halpern M, Cohen M, Levi Z, Vilkin A, Morgenstern S, Manugian V, St Lawrence E, Gagneux P. Membrane-bound mucins and mucin terminal glycans expression in idiopathic or Helicobacter pylori, NSAID associated peptic ulcers. World J Gastroenterol. 2014;20:14913-14920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |