Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9297
Peer-review started: February 5, 2015
First decision: April 24, 2015
Revised: May 12, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: August 21, 2015
Processing time: 198 Days and 9.7 Hours
Pancreatic ductal adenocarcinoma (PDAC) is an incurable lethal disease whose incidence rate is growing. There is no effective screening for detection of early stage tumors and, in most cases, PDAC is diagnosed at advanced disease stages, when radical pancreatic resection is not possible. The aggressive nature of pancreatic tumor cells lies in the complex genetic mechanisms behind their uncontrolled capability to grow and metastasize, which involve essential adaptive changes in cellular metabolism, signaling, adhesion and immunoediting. In addition, PDAC cells promote a dense functional stroma that facilitates tumor resistance to chemotherapy and radiation. During the last two decades, gemcitabine has been the reference for the systemic treatment of PDAC. However, recently, a regimen combining fluorouracil, irinotecan, oxaliplatin, and leucovorin (FOLFIRINOX) and another combining albumin-bound paclitaxel with gemcitabine have shown clear therapeutic advantage in advanced PDAC, with survival outcomes of 11.3 and 8.5 mo on phase III trials, respectively, over single-agent gemcitabine. With the pending issue of their higher toxicities, these regimens set the reference for ongoing and future clinical studies in advanced PDAC. In addition, the efficacy of oral fluoropyrimidine (S-1) has been well documented in Asiatic PDAC patients. The development of therapeutic approaches other than cytotoxic drugs has proven difficult in the past, with only one drug (erlotinib) approved to date. Besides, a number of agents targeting signaling pathways in tumor or stroma cells are being investigated. Likewise, immunotherapies that target PDAC in various ways are the subject of a number of clinical trials. The search for reliable biomarkers with diagnostic and prognostic value using genomics and mass spectrometry methods may facilitate monitoring and refinement of therapies. This review focuses on current understanding of the pathogenesis of PDAC and the latest developments in the treatment of advanced PDAC.
Core tip: Pancreatic adenocarcinoma is a life threatening, fast evolving disease for which there is no cure. Recently, new chemotherapy regimens have shown significant improvement in survival in patients with advanced disease, opening a way for further progress. New therapeutic strategies based on targeted inhibitors or immunotherapy approaches, in particular antibody and adoptive T cell therapies, are getting growing attention as they are proving beneficial in pre-clinical and early phase clinical studies in combination with chemotherapy. Progress in understanding pancreatic tumor genetics, epigenetics and metabolism is providing new biomarkers that may be of value in early detection and progression assessments.
- Citation: Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol 2015; 21(31): 9297-9316
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9297
Over 95% of pancreatic cancers develop in the exocrine pancreas. Of these, about 95% are adenocarcinomas originating in the ducts of the pancreas. Pancreatic ductal adenocarcinoma (PDAC) is, together with renal cancer, the twelfth most frequent cancer worldwide, representing the eighth and ninth leading cause of death by cancer in men and women, respectively. In the year 2012 about 338000 new cases of PDAC were reported in the world[1]. The incidence varies across countries, ranging from 1 to 10 cases per 100000 (age standardized rate)[1]. According to the United States National Cancer Institute, the 5-year relative survival rate is about 25% for localized PDAC (stages I and IIA), 9.9% for cases with regional lymph node involvement (stages IIB and III) and 2.3% for metastasized PDAC (stage IV)[2]. However, only 9% of cases are diagnosed at the local stages, while 27% are detected at the regional and 53% at the distant stages, with 11% of cases unstaged[2]. Hence, the overall 5-year survival rate is in the range of 6%-10%, what makes PDAC the most lethal cancer type. Approximately 70% of deaths follow widespread metastasis while the rest have limited metastasis but extensive primary tumors. Currently, there is no effective screening for detection of early stage tumors.
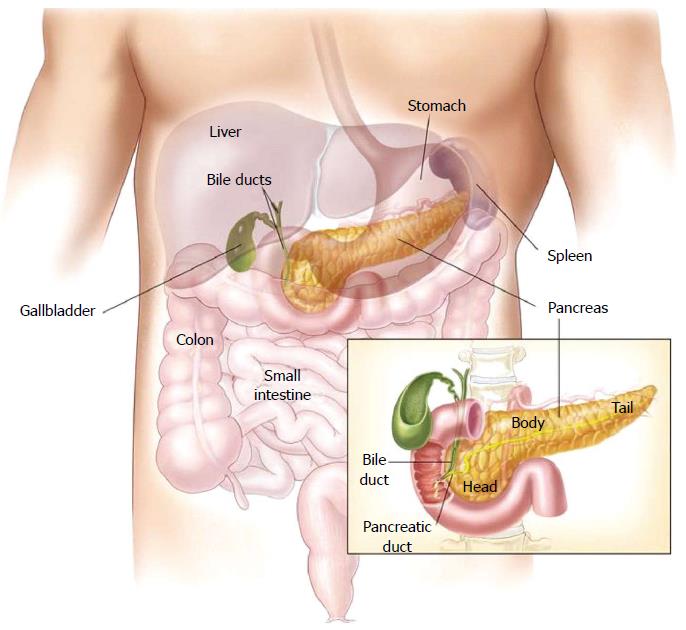
The pancreas is a 12-15-cm (6 inches) long, lobulated, retroperitoneal gland, which lies transversally behind the stomach, across the lumbar spine (L1-L2) (Figure 1), in close contact with the duodenum where the bile and pancreatic ducts drain through the major papilla (ampulla of Vater) and the pancreatic accessory duct through the minor papilla[3,4]. The wider end of the pancreas, close to the duodenum, is referred to as the head, the middle portion is called the body and the rest, called tail, extends to the hilum of the spleen. The exocrine pancreas produces the digestive enzymes and represents more than 95% of the pancreatic mass. The endocrine part (the islets of Langerhans) comprises only 1%-2% of pancreatic mass. A ductal system drains the enzymes produced by the acinar cells in a bicarbonate-rich medium secreted by the ductal cells.
At the histopathologic level, PDAC develops in a stepwise progression from low grade to high grade dysplastic lesions known as pancreatic intraepithelial neoplasia (PanIN) types 1, 2 and 3. In addition, intraductal papillary mucinous neoplasms (IPMN) are considered precursor to invasive pancreatic cancer. These tumors transform the stroma into a dense connective tissue called desmoplastic reaction[5,6], a microenvironment consisting of extracellular matrix proteins (hyaluronic acid, type I and III collagens and fibronectin), fibroblasts, pancreatic stellate cells (myofibroblast-like cells with tissue maintenance function), inflammatory immune cells, endothelial cells, pericytes and nerve fibers. PDAC cells stimulate the stroma and induce the desmoplastic reaction, whereas stroma cells release factors that stimulate tumor cell proliferation, escape from immune surveillance, invasiveness and resistance to therapy[7]. However, several lines of evidence coming from studies in mouse models suggest that stromal cells may also detain tumor growth directly or indirectly[8-10].
Molecular pathology and genomics studies have shown accumulating genetic changes, usually mutations in oncogenes and tumor suppressor genes[11]. Over 90% of PanIN of all grades and 40%-65% of IPMN carry KRAS mutations[12,13], being KRAS G12D the most common mutation[14,15]. KRAS activation is essential for pancreatic cancer cell survival[16]. Activated mutant KRAS signals primarily through the PI3K, p38, JNK and FAK signaling pathways. It also involves the epidermal growth factor receptor (EGFR), BCL-XL and the nuclear factor κB[17-20].
KRAS is thought to play a key role in reprogramming the metabolism of hypoxic pancreatic adenocarcinoma cells through activation of glucose uptake and glycolysis to yield pyruvate, which instead of being processed via the tricarboxylic acid cycle is converted into lactic acid[21]. Excess of lactic acid released by hypoxic cells causes local acidosis, which facilitates extracellular matrix breakdown and hence tumor invasiveness[22]. In addition, the neighboring normoxic cancer cells use the released lactate to fulfill the increased metabolic needs due to their higher proliferation rates. Indeed, these cells show increased expression of MCT1, a proton-linked monocarboxylate transporter that catalyzes the rapid transport of lactate, pyruvate and other monocarboxylates across the plasma membrane[23]. Moreover, KRAS activates glutamine metabolism to yield glutamate and α-ketoglutarate, thus enhancing citrate synthesis and the tricarboxylic acid cycle, i.e., a glucose-independent metabolic pathway; generates NADPH, a cofactor in anabolic reactions and an antioxidant[24], and promotes de novo lipogenesis through the isocitrate dehydrogenase (IDH1 and 2)[25,26].
Besides KRAS activation, mutations inactivating tumor suppressor genes accumulate during progression from PanIN1 to PanIN3. Mutational inactivation of p53 is detected in 60%-70% of PDAC, and mutations in CDKN2A (involved in G1 cell cycle arrest) and in members of the TGF-β signaling pathway (most frequently SMAD4, TGF-β1 and TGF-β2) in about 50% of cases[27]. In 10%-15% of cases, exome sequencing has revealed loss-of-function mutations in genes involved in nucleosome remodeling (ARID1A, ARID1B, SMARCA1), responses to DNA damage (ATM, BRCA2) and histone methylation (MLL2, MLL3, KDM6A). It has been estimated that genetic predisposition is present in 5%-10% of PDAC cases (familial PDAC) and several susceptibility genes have been identified. For example, inherited mutations in the gene STK11 cause the Peutz-Jeghers syndrome, and these patients have 130-fold increased risk of PDAC; germline mutations in the p16/CDKN2A gene cause the familial atypical multiple mole melanoma (FAMMM) syndrome, which is associated with a 13 to 37-fold increased risk of PDAC; mutations in BCRA2 cause familial breast cancer and increase the risk of PDAC 3.5-fold (reviewed by Hruban et al[28]). In addition, as a consequence of genetic changes, cytology studies have shown frequent chromosomal alterations in PDAC such as deletions and rearrangements leading to aneuploidy. For instance, the gene CLPTM1L, which is overexpressed in PDAC as compared with normal pancreatic tissue and has been identified by GWAS (Genome-Wide Association Studies) among the PDAC susceptibility alleles on chromosome 5p15.33, has been shown to interfere with normal cytokinesis and induce aneuploidy in vitro[29]. Furthermore, an extensive multistage GWAS of 7683 patients diagnosed with pancreatic cancer and 14397 control individuals identified multiple loci associated with pancreatic cancer, which harbor genes associated with cancer, such as LINK-PIN, BCAR1/CTRB1/CTRB2, PDX1, ZNRF1, TERT and PVT1[30]. These studies illustrate a more accurate concept of genetic risk of PDAC.
Inflammatory cells (mainly lymphocytes, plasma cells, macrophages and mast cells) are components of the desmoplastic reaction that forms the microenvironment of PDAC. However, rather than fighting the tumor these cells seem to promote an inflammatory microenvironment that helps tumor cells escape from immune surveillance via paracrine cross-talk mechanisms[31]. Indeed, studies have shown that chronic pancreatitis increases the risk of developing pancreatic adenocarcinoma, specially in smokers[32], and that subjects with hereditary pancreatitis caused by mutations in the gene PRSS1 have a significantly increased relative and absolute risk of developing PDAC[33]. Escape from antitumor immunity seems to be linked to KRAS activation, since it has been shown that already in early PanIN stages KRAS G12D induces production and release of GM-CSF[34], which attracts Gr1+CD11b+ myeloid suppressor cells to the tumor stroma[34] as well as immunosuppressive regulatory T cells[35,36]. Furthermore, the serum levels of some proinflammatory cytokines, such as IL-6, IL-8, IL-10 and IL-1 receptor antagonist (IL-1RA) are increased in PDAC patients and correlate with tumor aggressiveness[37]. IL-6 signals through the signal transducer and activator of transcription 3 (STAT3), which plays an essential role in the development of most PDAC cases[38]. Studies in mice have shown that tumor infiltrating macrophages release IL-6 in the stroma activating STAT3 and promoting progression from PanIN to PDAC[39]. In yet another mouse model of human PDAC it was shown that a subset of stromal fibroblasts expressing fibroblast activation protein (FAP) release the chemokine CXC-motif ligand 12 (CXCL12), which may facilitate immunosuppression by impeding the contact of T cells with the adenocarcinoma cells. T cell accumulation nearby tumor cells could be restored using an antagonist of CXCR4[40], a cognate receptor for CXCL12, which acted synergistically with the anti-programmed cell dead 1 ligand 1 (PD-L1) antibody[41] to induce tumor regression in a mouse model of PDAC[42].
To date there is no effective preventive screening for detection of early stage tumors. Further, there is no consensus on the suitability of current biomarkers to predict tumor progression or recurrence. Carbohydrate antigen 19-9 (CA19-9), a sialylated Lewis antigen identified in a colon cancer cell line[43], is the biomarker currently used in the surveillance of PDAC. Its expression depends on the Lewis blood antigens; therefore, 5%-10% of Caucasian patients that are Lewis negative (a-/b-) do not express CA19-9. Because its expression is not limited to PDAC, CA19-9 cannot be used as screening marker. However, it has been used as prognostic marker after surgical resection[44,45], and some studies suggest that it may be used to predict resectability and outcome after adjuvant chemotherapy[46,47]. Looking for biomarkers linked with survival or response, in the RTOG 9704 study (Table 1), an adjuvant therapy trial comparing 5-FU with gemcitabine chemotherapy administered before and after 5-FU-based chemoradiation in patients with resected PDAC, a probe panel of antibodies was used to detect and quantify 42 proteins. As already shown in previous studies, lower levels of CEA (carcinoembryonic antigen) and CA19-9 were associated with improved overall survival in all patients, whereas low levels of matrix metalloproteinases (MMP)-7 were linked to improved overall survival in the adjuvant gemcitabine arm, but not in the 5-FU arm, suggesting that PDAC patients with low MMP-7 expression levels benefit from gemcitabine rather than 5-FU adjuvant therapy[48].
| Study name | No. of patients enrolled | Regimen | Survival rate | P value | Ref. |
| EORTC | 207 | 5-FU + radiotherapy | 34% (2 yr) | 0.099 | [72] |
| Observation | 26% (2 yr) | ||||
| ESPAC-1 | 289 | 5-FU + radiotherapy | 10% (5 yr) | - | [73] |
| Observation or 5-FU only | 20% (5 yr) | ||||
| RTOG 9704 | 451 | Gemcitabine + radiotherapy | 18% (5 yr) | 0.120 | [147] |
| 5-FU + radiotherapy | 22% (5 yr) |
The protein SMAD4 has been also suggested as prognostic marker. Immunohistochemistry characterization of the expression of SMAD4 in a series of 249 tumors from PDAC patients who underwent pancreaticoduodenectomy showed that cases with SMAD4 expression had significantly longer survival: median survival of 19.2 mo compared with 14.7 mo in patients whose tumors did not express SMAD4 (P = 0.03)[49]. Moreover, analysis of SMAD4 (DPC4) expression in a series of rapid autopsies in patients with documented PDAC showed loss of SMAD4 expression in 41 of 65 tumors analyzed, indicating a rate of inactivation of 63%[50]. Interestingly, loss of SMAD4 expression was seen in only 2 (22%) of 9 locally advanced carcinomas, but in 16 (73%) of 22 metastatic carcinomas (P = 0.007)[50]. In a prospective phase II trial enrolling 69 patients with locally advanced carcinoma who were treated with gemcitabine/oxaliplatin and cetuximab followed by chemoradiotherapy plus cetuximab, 11 (73.3%) of 15 patients with intact SMAD4 expression had a local dominant pattern of progression, whereas 10 (71.4%) of 14 patients with SMAD4 loss had distant dominant pattern of spread (P = 0.016)[51], indicating that SMAD4 loss significantly correlated with distant rather than local dominant pattern of spread. In yet another study, retrospective analysis of 471 who had resected PDAC showed that loss of SMAD4 expression did not correlate with recurrence but was predictive for adjuvant chemotherapy benefit (P = 0.002)[52]. This study also showed that high expression of the CXCR4 chemokine receptor was significantly associated with worse outcome (P < 0.0001) as well as with metastatic recurrence (P < 0.001)[52].
Another biomarker that was expected to have prognostic relevance is the human equilibrative nucleoside transporter-1 (hENT1). Gemcitabine requires hENT1 to cross the cell membrane. Therefore, low expression of hENT1 might result in gemcitabine resistance in PDAC. This hypothesis was tested in a randomized clinical trial comparing gemcitabine with gemcitabine elaidate (CO-101), an unsaturated fatty acid ester derivative of gemcitabine, which was designed to enter the cells by diffusion independently of hENT1[53]. This study enrolled 367 patients. The expression of hENT1 was measured in 253, of whom 232 (64.8%) were classified as low-hENT1. It was found no difference in median overall survival between the low and high hENT1 subgroups, indicating that hENT1 status is not relevant and cannot be used to predict gemcitabine outcome.
A recent study has shown that elevated levels of branched-chain amino acids (BCAA) in plasma are associated with an increased risk (> 2.0) of developing PDAC[54]. The highest association was observed in subjects whose samples were collected 2-5 years before PDAC diagnosis, suggesting that early-stage subclinical disease was already present. Such increase in plasma BCAA seems due to breakdown of tissue proteins that would occur early in the development of PDAC[54]. Interestingly, in a mouse model of PDAC, elevated BCAA levels were detected also in mice with early stage pancreatic tumors harboring mutant KRAS, but not in mice with KRAS-driven tumors in other tissues.
The predictive value of other biomarkers, such as ULBP2, a ligand of the natural killer activating receptor NKG2D, and the macrophage inhibitory cytokine-1 (MIC-1) are currently being evaluated in case-control studies[55]. Non-mutated, overexpressed proteins, such as CLPTM1L[29] and DKK-1[56], if validated in terms of prognostic value could serve also as biomarkers. Furthermore, the prognostic value of detection of circulating tumor cells is being evaluated in a prospective study with 79 patients with locally advanced PDAC[57].
A widely used staging system for PDAC is that of the American Joint Committee of Cancer and the Union for International Cancer Control, which is based on the TNM classification[58]. From the management perspective PDAC is divided into three categories: (1) localized surgically resectable tumors; (2) unresectable locally advanced tumors; and (3) metastatic tumors. In between the first and second groups are tumors called borderline resectable, which need to be carefully evaluated as candidates for surgery according to the involvement of adjacent organs and vessels (celiac artery, hepatic artery, portal vein, superior mesenteric artery and vein)[59]. The most frequent location of PDAC is the head of the pancreas (60%-70%), which requires pancreaticoduodenectomy (Whipple procedure). Extensive surgery does not provide better outcomes[60,61]. Tumors of the tail of the pancreas are nowadays resected laparoscopically. Overall, only 15%-20% of newly diagnosed patients are eligible for surgical resection. The five-year survival rates after pancreaticoduodenectomy range from 25%-30% for lymph node-negative and 10% for node-positive cases[62].
In PDAC stage I the tumor is restricted to the pancreas and does not involve neither adjacent organs or vessels nor regional lymph nodes. The treatment of choice is surgical removal of all recognizable tumor tissue. However, tumor recurrence occurs in 60%-70% of stage I patients due to micrometastases during or after surgical resection. Systemic therapy (chemotherapy, chemoradiation) administered after surgery (called adjuvant therapy) improves survival rates and, eventually, the chances of cure. In some cases chemotherapy, chemoradiation or combination of therapies is applied before surgery (the so-called neoadjuvant therapy) to shrink the tumor and prevent post-surgical micrometastases and relapse.
Adjuvant therapy in localized resectable PDAC: A number of randomized trials enrolling patients with T1-4 N0-1 M0 PDAC have demonstrated that, following surgical resection of the tumor mass, adjuvant chemotherapy for 6 mo either with gemcitabine or 5-fluoruracil (5-FU) increases overall survival significantly compared with observation (Table 2, see also Goodman et al[63], 2014). These studies showed an improvement in the 5-year survival rate from approximately 10% (observation) to approximately 20% (adjuvant therapy) with no significant difference between gemcitabine and 5-FU (Table 2). However, the patients treated with gemcitabine experienced significantly less toxicity and had improved clinical benefit. More recently, in a multicenter, randomized phase III study in Japan (JASPAC 01) gemcitabine was compared with S-1 in the adjuvant treatment of patients after curative resection[64]. S-1 is an oral fluoropyrimidine prodrug (tegafur) that is converted by the enzyme dihydropyrimidine dehydrogenase (DPD) into 5-FU[65] (see also Stage IV below), The study enrolled 385 patients with ECOG performance status 0-1 and with compensated organ functions; of these, 378 were included in the final analysis. The 2-year overall survival rates were 53% for gemcitabine and 70% for S-1 (P < 0.0001). Also, the quality of life was significantly higher in the S-1 arm (P < 0.0001). The results of the JASPAC-01 study suggest that S-1 may be an effective alternative to the adjuvant chemotherapy with gemcitabine in resected PDAC. However, these studies have been conducted in Asian patients, mostly Japanese and it is uncertain whether S-1 would be as effective in Western patients. Some important aspects remain to be investigated. For instance, the expression levels of cytochrome P450 A26, the enzyme that converts tegafur into 5-FU in the liver, seem to be higher in Caucasian than in Japanese subjects[66]. In addition, gastrointestinal toxicities of grades 3/4 are more common in Caucasians than in Asians[67]. An ongoing multicenter, randomized phase III study, the European Study Group for Pancreatic Cancer trial 4 (ESPAC-4), will compare the combination gemcitabine plus capecitabine (another fluoropyrimidine prodrug that is converted into 5-FU by DPD, the same enzyme that converts tegafur) with gemcitabine alone when used as adjuvant therapy after PDAC resection[68]. This study will serve to determine the utility of the addition of a fluoropyrimidine to gemcitabine in the post-resection adjuvant therapy in non-Asian PDAC patients. Nevertheless, previous randomized trials in Western metastatic PDAC patients have not shown superiority for such combination using gemcitabine combined with a variety of oral and bolus fluoropyrimidine regimens[69-71]. Two ongoing clinical studies (CONKO 005 and RTOG 0848) should determine the benefit of combining erlotinib (EGFR inhibitor) with gemcitabine.
| Study name | No. of patients enrolled | Regimen | Survival rate | P value | Ref. |
| CONKO-001 | 368 | Gemcitabine | 20.7% (5 yr) | 0.01 | [145] |
| Observation | 10.4% (5 yr) | ||||
| ESPAC-3 | 1088 | Gemcitabine | 23.6 mo | 0.39 | [146] |
| 5-FU + Leucovorin | 23.0 mo | ||||
| JASPAC 01 | 385 | Gemcitabine | 53% | 0.0001 | [64] |
| S-1 | 70% |
The efficacy of adjuvant chemoradiation therapy is still subject of controversy, since two European studies showed no benefit in adding radiation to the adjuvant therapy[72] or even showed detrimental effects[73]. Such studies (Table 1) have been questioned for different reasons, the EORTC because it included patients with pancreatic head carcinomas as well as periampullary tumors (with possible better prognosis), and the ESPAC-1 because of the complexity of its design. Further studies should clarify if adjuvant chemoradiation may be beneficial. Thus, a phase II-R/III randomized trial ongoing in the United States (NCT01013649), which should be completed in the year 2020. Nevertheless, the impact of chemoradiation therapy on overall survival after pancreaticoduodenectomy was evaluated in a multicenter retrospective study reviewing 955 patients (classified as T1-4; N0-1; M0) who underwent complete resection (R0-1) and showed macroscopically negative margins[74]. Of these, 623 received postoperative radiation, 575 received concurrent chemotherapy, and 462 received adjuvant chemotherapy. Median follow-up was 21.0 mo. Median overall survival was 39.9 mo for patients treated with chemoradiation compared with 24.8 mo for those not receiving chemoradiation (P < 0.001), and 27.8 mo for patients treated only with adjuvant chemotherapy (P < 0.001). In the population treated with adjuvant chemoradiation (with or without chemotherapy) 5-year overall survival was 41.2% compared with 25.7% in patients treated with adjuvant chemotherapy alone[74]. Therefore, according to this retrospective study, adjuvant chemoradiation was beneficial in terms of overall survival.
Neoadjuvant therapy in borderline resectable PDAC: The high frequency of disease recurrence and the low survival rates associated with surgical resection of pancreatic adenocarcinomas, usually attributed to residual tumor cells left at the surgical margins and to involvement of lymph nodes, led to the evaluation of neoadjuvant (preoperative) chemotherapy with or without radiotherapy. Several studies evaluating different neoadjuvant therapy protocols have evidenced the limited success of this approach as compared with the outcomes of patients with resectable tumors treated with adjuvant (postoperative) therapy[75-77]. Nevertheless, to date there are no controlled, prospective studies comparing neoadjuvant and surgery-first approaches.
Currently, there is consensus that neoadjuvant therapy does not benefit patients with resectable PDAC, and that it is beneficial in tumors with borderline resectability to improve the probability of tumor-free resection margins and in locally advanced, non-resectable tumors to reduce their extension and make them resectable[78,79]. Further, neoadjuvant chemotherapy applied to borderline resectable patients may help identify a subset of patients that would not benefit from surgery. In a study enrolling 160 borderline resectable patients selected out of 2454 PDAC cases, 125 (78%) completed neoadjuvant therapy (chemotherapy, radiotherapy or both) and restaging. Of these 66 (41%) underwent surgery. Median survival was 40 mo for the 66 patients who completed all therapy and 13 mo for the 94 patients who did not undergo surgery[80]. Consistent and objective definitions of borderline resectable and unresectable PDAC are needed for ongoing and future studies to be sufficiently powered, so that the efficacy of neoadjuvant therapy can be clearly established[79].
In the past, combinations of gemcitabine and chemoradiation with 5-FU have had limited efficacy. In a large meta-analysis including retrospective and prospective studies, Gillen et al[76] analyzed 111 trials (n = 4394) showed that in the group of initially resectable tumor patients, approximately 81% of patients receiving monotherapy underwent resection, in contrast, among those receiving combination chemotherapy the number of resections was significantly lower (approximately 66%). However, a comparison of tumor response frequencies in patients treated with mono chemotherapy (n = 44) vs combination chemotherapy (n = 48) showed complete and partial responses of 2.2% and 25.8% vs 5.3% and 34.7%, respectively[76].
Although at present there is no optimal protocol for neoadjuvant chemotherapy, there is hope in multidrug chemotherapy approaches such as nab-paclitaxel (albumin bound paclitaxel) followed by gemcitabine, or the multiagent regimen FOLFIRINOX (leucovorin, 5-FU, irinotecan, oxaliplatin), which has been shown in a retrospective study to induce conversion to resectability in > 20% of locally advanced PDAC patients[81]. Nevertheless, the toxicity associated with these regimens and the relatively elevated recurrence rate observed after R0 resection[81] make necessary more prospective studies to establish approaches that may be beneficial in the neoadjuvant setting. There are at least two ongoing phase II trials using fluorouracil, irinotecan, oxaliplatin, and leucovorin (FOLFIRINOX) plus radiation therapy in borderline resectable PDAC patients (NCT01560949 and NCT01591733).
Approximately 30%-40% of newly diagnosed PDAC cases are classified as stage III: locally advanced, non-resectable, non-metastatic, with involvement of major blood vessels and regional lymph nodes. Because of the poor response rates observed with the different therapeutic approaches, management of these patients remains controversial. A frequent option has been upfront chemotherapy with 2-3 cycles of gemcitabine followed by restaging and, in favorable cases, chemoradiation. In the meta-analysis by Gillen et al[76], 107 of the 111 selected studies applied chemoradiation to non-resectable locally advanced PDAC, in most cases (54%) with 5-FU, and less frequently with gemcitabine (22%). About 33% of patients with primarily unresectable, locally advanced PDAC turned into resectable cases and the overall survival was 20.5 mo (median), comparable to that of patients with primarily resectable tumors and in contrast to 10.5 mo in those with non-resectable tumors. Although other meta-analyses drew comparable results, the general feeling is that chemoradiation has more toxicity than gemcitabine alone and increases the rate of perioperative risk[82,83]. A recent study compared chemoradiation and chemotherapy after four months of gemcitabine (with or without erlotinib, an EGFR inhibitor) in locally advanced PDAC patients (LAP 07 study, NCT00634725). The study conclusion was that chemoradiation after induction chemotherapy is not superior to continuing chemotherapy in patients with controlled locally advanced PDAC. Median follow-up was 36 mo and overall survival was 16.5 mo for the patients randomized to continue chemotherapy compared with 15.3 mo for patients receiving chemoradiation (P = 0.83)[84].
The improvements in overall survival observed in patients with metastatic PDAC treated with FOLFIRINOX or gemcitabine/nab-paclitaxel multidrug regimens (see below) has led to investigate them in locally advanced PDAC. Their efficacy and toxicities in locally advanced PDAC remain to be determined[81,85]. In their retrospective institutional study, Faris et al[81] reported 22 patients with locally advanced PDAC who received treatment with FOLFIRINOX. Median progression free survival was 11.7 mo. Five patients (23%) underwent R0 resection following neoadjuvant FOLFIRINOX and chemoradiation. Of these, three suffered distant recurrence within six months. The high rate of recurrence and the toxicities (non-neutropenic fever, dehydration), observed in 32% of the patients, demonstrate the complexity to find an adequate therapy for locally advanced PDAC patients.
In PDAC stage IV metastasis have spread to adjacent (stage IVa) or distant organs (stage IVb), such as liver, stomach, spleen or lungs. Surgical removal is not possible, although palliative surgery may be an option. Gemcitabine has been the standard chemotherapy agent since 1997, when it was shown to improve overall survival of advanced PDAC patients compared to 5-FU, with survival rates at 12 mo of 18% and 2%, respectively[86]. Ever since, different gemcitabine-based combinations, for example with irinotecan[87], oxaliplatin[88] or bevacizumab[89], have been investigated in randomized trials in comparison with standard gemcitabine. Most of them failed to improve overall survival rates, yet at least three combinations have shown beneficial effects: gemcitabine plus erlotinib, gemcitabine plus S-1 and gemcitabine plus nab-paclitaxel. In addition, a new non-gemcitabine-based multidrug regimen (FOLFIRINOX) has revealed as a clear improvement in the therapy of metastatic PDAC.
Gemcitabine plus erlotinib: A regimen of gemcitabine plus erlotinib, a reversible tyrosine kinase inhibitor of EGFR, demonstrated certain benefit in a phase III trial (NCI PA.3) enrolling 569 patients[90]. The overall survival rate at 1-year was 23% in the gemcitabine plus erlotinib arm and 17% in the standard gemcitabine arm (P = 0.023). Median overall survival was 6.2 and 5.9 mo, respectively (P = 0.038). However, this slight improvement with the erlotinib regimen was accompanied with higher toxicities (rash, infections, diarrhea, etc.) and even 6 deaths, all in the erlotinib arm. The FDA approved this protocol in the year 2005.
Gemcitabine plus S-1: The synergistic cytotoxic effects of gemcitabine and 5-FU against pancreatic adenocarcinoma cells described previously[91,92] led to investigate the combination of gemcitabine and S-1 in clinical trials. S-1 is an oral multiagent formulation with three components: tegafur (a 5-FU prodrug), gimeracil and oteracil at 1:04:1 molar ratio[93]. Gimeracil is a reversible inhibitor of dihydropyrimidine dehydrogenase, a major 5-FU catabolizing enzyme. Oteracil inhibits the phosphoribosyltransferase that phosphorylates 5-FU, and it is intended to reduce the gastrointestinal toxicity of 5-FU. Several randomized phase III studies in gastrointestinal cancer patients have shown non-inferiority of S-1 vs standard 5-FU infusion regimens[94-96]. Several phase II studies in patients with metastatic PDAC treated with gemcitabine/S-1 combinations showed median overall survival rates ranging from 7.89 to 12.5 mo[65]. The combination of gemcitabine plus S-1 was compared with S-1 alone and gemcitabine alone in a recent randomized phase III study (GEST) enrolling 834 patients with locally advanced or metastatic PDAC in Japan and Taiwan[97]. The GEST study showed a median overall survival of 8.8, 9.7 and 10.1 mo in the gemcitabine, S-1 and gemcitabine/S-1 arms, respectively. The study did not demonstrate superiority of gemcitabine/S-1 to gemcitabine alone (P = 0.15), but showed non-inferiority (P < 0.001). However, as mentioned above, S-1 trials have been performed only in Asian patients[65,98] and, therefore, further studies are required to determine whether S-1 has the same efficacy in Western PDAC patients. Although previous studies in Western patients with metastatic PDAC did not show superiority of combinations of gemcitabine plus fluoropyrimidines administered in various forms[69-71], it cannot be excluded that S-1 does.
Gemcitabine plus nab-paclitaxel: The first clinical trial with nab-paclitaxel and gemcitabine was a multicenter open label phase I/II study enrolling 67 patients with PDAC, of whom 44 received the maximum tolerated dose. In this group the 1-year survival rate was 48%, and the median overall survival was 12.2 mo[92]. In preclinical studies, Von Hoff et al[92] showed also that, in a mouse xenograft model of PDAC, the intratumoral concentration of gemcitabine was 2.8-fold increased in the mice treated with nab-paclitaxel plus gemcitabine compared with mice treated only with gemcitabine, suggestive of a synergistic effect of these two drugs. nab-Paclitaxel is an albumin-bound formulation of paclitaxel in the form of 130 nm particles, which is administered intravenously as a colloidal suspension. The combination of nab-paclitaxel with gemcitabine in the treatment of advanced PDAC was based on the finding that these tumors overexpress the secreted protein acidic and rich in cysteine (SPARC), an albumin-binding protein, and the fact that nab-paclitaxel had shown efficacy in tumors overexpressing SPARC, such as breast[99,100], melanoma[101] and lung[102] tumors. Further, it has been shown in cultured cells and in a mouse model of PDAC that paclitaxel reduces the levels of cytidine deaminase (major gemcitabine inactivating enzyme) in tumor cells[103] what might explain the higher levels of intratumoral gemcitabine. In addition, it was shown that while nab-paclitaxel accumulation in the murine tumors was dependent on SPARC at low doses, at therapeutic doses it was SPARC independent[104]. On the basis of their previous study, Von Hoff et al[92] carried out a multicenter, open label, randomized phase III trial enrolling 861 patients with metastatic PDAC to compare nab-paclitaxel plus gemcitabine (431 patients) with standard gemcitabine (430 patients). Median overall survival was 8.5 mo in the nab-paclitaxel/gemcitabine group and 6.7 mo in the gemcitabine group (P < 0.001). The 1-year survival rate was 35% and 22%, respectively, and the 2-year survival was 9% and 4%, respectively. Adverse events of grade 3 or higher, such as myelosuppression and peripheral neuropathy were increased in the combined nab-paclitaxel plus gemcitabine arm.
FOLFIRINOX: Besides the gemcitabine-based chemotherapies, in the last years a multiagent chemotherapy regimen (FOLFIRINOX) has emerged as an effective strategy with significantly higher efficiency compared to standard single-agent gemcitabine in a randomized, multicenter phase II/III study[105]. The FOLFIRINOX regimen consists of a combination of four intravenously (iv) administered drugs: oxaliplatin 85 mg/m2 2 h infusion, followed by leucovorin (calcium folinate) 400 mg/m2 2 h infusion, irinotecan 180 mg/m2 90 min. infusion, followed by 5-FU mg/m2 bolus, followed by 5-FU 2400 mg/m2 infusion over 46 hours, every two weeks. A six-month treatment was recommended for responding patients[105]. This study included 342 patients with metastatic PDAC that had not been treated with chemotherapy. The median overall survival was 11.1 mo in the FOLFIRINOX group and 6.8 mo in the gemcitabine group (P < 0.001). Overall survival rates at 6, 12 and 18 mo were 75.9%, 48.4% and 18.6% in the FOLFIRINOX group and 57.6%, 20.6% and 6.0% in the gemcitabine group. The median progression-free survival was 6.4 mo in the FOLFIRINOX group and 3.3 mo in the gemcitabine group (P < 0.001). Definitive deterioration in the quality of life at 6 mo was observed in 31% of patients in the FOLFIRINOX and in 66% in the gemcitabine group.
A relevant concern of this therapy is its increased toxicity, which has been subject of some controversy. Several reports informed of substantial toxicities, such as grade 3 or 4 neutropenia, febrile neutropenia (cause of one treatment-related dead), thrombocytopenia, diarrhea and sensory neuropathy, which were significantly more frequent than with single-agent gemcitabine therapy[105]. In a multicenter study in 61 PDAC patients, 21 (34.4%) were hospitalized as a result of therapy and 23 (37.7%) had discontinued therapy due to adverse events[106]. In a retrospective study including 22 patients, it was reported that toxicities required hospitalization in 7 cases (32%)[81]. Nevertheless, a retrospective study reviewing toxicity and efficacy in 35 patients (16 with locally advanced and 19 with metastatic PDAC), of whom 29 received a modified FOLFIRINOX regimen (attenuation of irinotecan and 5-FU bolus) showed that such regimen improved tolerability with no significant reduction in efficacy[107]. Furthermore, in an attempt to ameliorate the toxicities, a prospective study was conducted using a modified FOLFIRINOX regimen (no bolus 5-FU and addition of hematopoietic growth factor)[108]. This study enrolled a total of 60 PDAC patients, 24 (40%) with non-metastatic and 36 (60%) with metastatic PDAC. It was found that the modified FOLFIRINOX regimen maintained efficacy, whereas the safety profile was improved with significantly less grade 3-4 toxicities. Additional studies should help improve safety and efficacy of FOLFIRINOX by further refinement of regimens.
Another important issue is related to the eligibility criteria for FOLFIRINOX therapy. In a retrospective study reviewing 100 consecutive cases of metastatic PDAC it was found that only 26 patients fulfilled the ACCORD study eligibility criteria, being the most frequent reasons for FOLFIRINOX exclusion ECOG score of 2 or greater (64%), age (≥ 76 years) (22%) and liver and/or renal dysfunction (28%)[109].
Treatment of PDAC has been restricted for the most part to chemotherapy (cytotoxic) drugs. A number of agents targeting specific proteins in tumor or stroma cells to interfere with their function have shown promise in preclinical studies over the last decade. Of these, only erlotinib (EGFR inhibitor) reached regulatory approval on the basis of some benefit shown for its combination with gemcitabine in a phase III trial (see above under Management). Reasons for the slow progress in this field might be the complex genetic mechanisms taking place in the pancreatic tumor cells, which favor resistance to cytotoxic as well as targeted agents, and the intricate tumor microenvironment that seems to protect the tumor through a discrete vessel network and a hypoxic milieu. Likewise, immunotherapy approaches may find difficulty in entering the stroma and reaching the tumor cells.
PARP inhibitors: PDAC tumors harboring germline mutations in the BRCA1 or BRCA2 genes are highly sensitive to Poly[ADP-ribose] polymerase (PARP) inhibitors[110]. Several PARP inhibitors, such as olaparib, are being tested in clinical trials. A recent multicenter phase II study enrolled 298 patients with recurrent ovarian, breast, pancreatic or prostate cancer, harboring a germline BRCA1/2 mutation, who were treated with olaparib (400 mg twice per day)[111]. The overall response rate was 26.2% (78 of 298) and in the subgroup of PDAC patients (treated previously with gemcitabine) the response rate was 21.7 (5 of 23) and stable disease for 8 or more weeks was observed in 35% of the PDAC patients. Adverse events of grade 3 or higher were reported for 35% of patients, the most frequent being anemia (17%). Nine patients died as a result of adverse events. This was a single arm study and, therefore, it is not possible to compare with other therapies. Ongoing studies will determine whether treatment with olaparib or other PARP inhibitors may be an alternative to FOLFIRINOX in patients with germline BRCA1/2 mutations.
Inhibitors of MMP: Tumor growth and metastasis involve the breakdown of tissue stroma. MMPs are a family of about 30 zinc-dependent proteinases that for the most part degrade the extracellular matrix, thereby facilitating tumor growth and metastasis. This was the rationale to investigate the efficacy of MMP inhibitors in cancer therapy. An oral MMP inhibitor, marimastat, was compared with gemcitabine in a randomized study enrolling 414 patients with unresectable pancreatic cancer[112]. The survival rate for patients receiving marimastat 25 mg was similar to that of patients receiving gemcitabine. In a parallel study, 239 patients with unresectable pancreatic cancer were randomized to receive gemcitabine plus either marimastat 10 mg b.i.d or placebo[113]. There was no significant difference in overall survival between ge mcitabine plus marimastat and gemcitabine plus placebo (P = 0.95). These studies provided little support to the utility of MMP inhibitors in therapy of advanced PDAC and, therefore, were discontinued.
Inhibitors of VEGF: Several studies demonstrated a close correlation between vascular endothelial growth factor (VEGF) expression and microvessel density (MDV) in PDAC[114,115]. In addition, VEGF appeared to have predictive value for liver metastasis and poor prognosis[115], and also for early recurrence after curative resection[114]. An oral inhibitor of VEGF receptors, axitinib, was tested in a randomized, placebo-controlled phase II study enrolling 103 patients with unresectable or metastatic PDAC. The patients were divided into two groups for treatment with gemcitabine with or without axitinib. Median overall survival was 6.9 and 5.6 mo, respectively[116]. The study was continued with a phase III trial including 632 patients[117]. However, an interim analysis concluded that the study was unsuccessful and it was terminated abruptly.
Hedgehog inhibitors: The hedgehog (Hh) pathway is thought to contribute to the growth of a number of tumors of endodermal origin, including PDAC. In a mouse model of PDAC the use of saridegib, an inhibitor of the Hh pathway, in combination with gemcitabine resulted in depletion of desmoplastic stroma, increased delivery of gemcitabine to tumor cells and a statistically significant survival improvement of tumor-bearing mice[118]. A randomized, double-blind, placebo controlled phase II trial showed worse median survival for the saridegib plus gemcitabine arm compared to the gemcitabine plus placebo arm, and the study was discontinued[119]. In a randomized, placebo controlled, phase Ib/II trial vismodegib, another Hh pathway inhibitor, administered with gemcitabine failed to improve progression-free or overall survival rates compared to gemcitabine alone[120]. At least five more trials are ongoing.
Other inhibitors: Masitinib, an inhibitor of c-kit and platelet-derived growth factor receptor (PDGFR) kinases, was tested in combination with gemcitabine in a phase II trial enrolling 22 patients with unresectable locally advanced or metastatic PDAC. The median overall survival was 8.4 and 6.8 mo for locally advanced or metastatic patients, respectively[121]. A phase III of this study comparing gemcitabine plus masitinib with gemcitabine plus placebo, showed no improvement in overall survival. Rigosertib, a phosphatidylinositol-3-kinase (PI3K) inhibitor, showed certain activity in a phase I trial and is under study in a multicenter, randomized phase II trial (NCT01360853).
Advances in the understanding of the mechanisms regulating cellular immune responses and immunosurveillance are leading to improved immunotherapy approaches applicable to cancer treatment. Immunotherapy approaches have the potential to assist the patient’s immune system to eliminate metastatic tumor cells and residual tumor after pancreatic resection. Some of these have shown success in early clinical trials in PDAC patients. A representative list of immunotherapy approaches currently under study in clinical trials is summarized in Table 3 distributed into the following major groups: monoclonal antibodies (as checkpoint immunomodulators, inhibitors of signaling pathways or as cytotoxicity inducers), adoptive T cell therapy, vaccines, cytokines and adjuvants.
| Study ID | Sponsor(s) | Therapeutic products | Phase | ClinicalTrials.gov Identifier |
| Monoclonal antibodies as checkpoint immunomodulators | ||||
| CD-ON-MEDI4736-1108 | MedImmune LLC | MEDI4736 (monoclonal antibody anti-B7 homolog1; PD-L1) | I/II | NCT01693562 |
| GO27831 | Genentech Inc. | MPDL3280A (human, Fc optimized monoclonal anti-PD-L1 antibody) | I | NCT01375842 |
| 3475-028 | Merck Sharp and Dohme Corp | Pembrolizumab (humanized monoclonal anti-PD-1 antibody) | IB | NCT02054806 |
| GP28328 | Genentech, Inc. | MPDL3280A (human, Fc optimized monoclonal anti-PD-L1 antibody) plus bevacizumab (anti-VEGF antibody) and/or chemotherapy | I | NCT01633970 |
| 11-C-0100 | Georgia Regents University Cancer Center | CT-011 (pidilizumab, humanized monoclonal antibody anti-PD-1) plus gemcitabine | II | NCT01313416 |
| CA209-032 | Bristol-Myers Squibb | Nivolumab (fully human monoclonal anti-PD-1 antibody) plus ipilimumab (anti-CTLA-4 monoclonal antibody) | I/II | NCT01928394 |
| CA223-001 | Bristol-Myers Squibb | Lirilumab (fully humanized monoclonal anti-KIR2DL1/2L3 antibody) plus nivolumab (anti-PD-1 antibody) | I | NCT01714739 |
| NU 10I02 | Northwestern University and Robert H. Lurie Cancer Center | Ipilimumab (anti-CTLA-4 monoclonal antibody) plus gemcitabine | I | NCT01473940 |
| NCI-2013-00030 | M.D. Anderson Cancer Center | Ipilimumab (anti-CTLA-4 monoclonal antibody) plus imatinib | I | NCT01738139 |
| CA224-020 | Bristol-Myers Squibb | BMS-986016 (anti-LAG-3 antibody) with or without nivolumab (anti-PD-1 antibody) | I | NCT01968109 |
| CA186-011 | Bristol-Myers Squibb | Urelumab (BMS-663513, humanized agonistic monoclonal anti-4-1BB/CD137) | I | NCT01471210 |
| B1641001 | Pfizer | PF-05082566 (4-1BB humanized agonist monoclonal antibody) plus rituximab (anti-CD20) | I | NCT01307267 |
| Monoclonal antibodies as signaling pathway inhibitors | ||||
| 59R5-002 | OncoMed Pharmaceuticals, Inc. | OMP-59R5 (anti-Notch2/3) plus chemotherapy | I/II | NCT01647828 |
| 52M51-002 | OncoMed Pharmaceuticals, Inc. and GlaxoSmithKline | OMP-52M51 (anti-Notch 1 monoclonal antibody) | I | NCT01778439 |
| MORAb-066-001 | Morphotek and SCRI Development Innovations, LLC | MORAb-066: anti-human TF (tissue factor, CD142) monoclonal antibody | I | NCT01761240 |
| NCI-2012-01702 | Morphotek and National Cancer Institute (NCI) | Ontuxizumab (MORAb-004): monoclonal antibody anti-endosialin/TEM1 (CD248) | I | NCT01748721 |
| R1400-ST-1113 | Regeneron Pharmaceuticals | REGN1400 (anti-ErbB3) with or without erlotinib or cetuximab | I | NCT01727869 |
| MM-151-01-01-01 | Merrimack Pharmaceuticals | MM-151 [oligoclonal antibody composed of three fully human monoclonal antibodies anti-EGFR (ErbB1)] alone and with irinotecan | I | NCT01520389 |
| M12-375 | AbbVie (prior sponsor, Abbott) | ABT-700 [monoclonal antibody anti-c-Met human growth factor receptor (HGFR) as monotherapy, or with chemotherapy (FOLFIR/cetuximab) or with erlotinib | I | NCT01472016 |
| UPCC-04206 | University of Pennsylvania and NCI | Gemcitabine, oxaliplatin and bevacizumab followed by 5-fluorouracil, oxaliplatin, bevacizumab and radiotherapy in patients with locally advanced pancreatic cancer | II | NCT00602602 |
| Monoclonal antibodies as cytotoxicity inducers | ||||
| Neogenix 0901 | Precision Biologics, Inc | Ensituximab (NPC-1C/NEO-102) (anti-MUC5AC-related antigen) | I/II | NCT01040000 |
| CEP-37250/KHK2804-001 | Kyowa Hakko Kirin Pharma, Inc. | CEP-37250/KHK2804 (monoclonal antibody targeting glycolipids on the surface of tumor cells) | I | NCT01447732 |
| IMMU-107-04 | Immunomedics, Inc. | IMMU-107: radioimmunoconjugate of the humanized monoclonal antibody HuPAM4 (anti-MUC1), plus a chelating agent (DOTA) and radiolabeled with Yttrium Y90 | III | NCT01956812 |
| Therapeutic vaccines | ||||
| NLG0505 | NewLink Genetics Corporation | Algenpantucel-L Immunotherapy: 2 pancreatic cancer cell lines (HAPa-1 and HAPa-2) expressing murine alpha-gal carbohydrates on cell surface molecules, in combination with standard therapy, compared with standard therapy. | III | NCT01836432 |
| 11-C-0148 | NCI | Epigenetically modified autologous tumor cells with ISCOMATRIX Adjuvant plus chemotherapy | I | NCT01341496 |
| ADU-CL-04 | Aduro BioTech, Inc. | GVAX (allogeneic GM-CSF transfected pancreatic tumor vaccine) plus CRS-207 (attenuated L. monocytogenes expressing mesothelin) | II | NCT02004262 |
| J13108 | Sidney Kimmel CCC | GVAX (allogeneic GM-CSF transfected pancreatic tumor vaccine) plus ipilimumab compared with FOLFIRINOX | II | NCT01896869 |
| JHOC-J0810 | Sidney Kimmel CCC and NCI | GVAX (allogeneic GM-CSF transfected pancreatic tumor vaccine) plus cyclophosphamide | NP | NCT00727441 |
| NCI-2012-01548 | Jonsson Comprehensive Cancer Center | NY-ESO-1 (cancer-testis antigen) reactive TCR retroviral vector transduced autologous PBL | II | NCT01697527 |
| NWBio 050012 | Northwest Biotherapeutics | DCVax-Direct (autologous activated dendritic cells) | I/II | NCT01882946 |
| ONT-10-001 | Oncothyreon Inc. | ONT-10 (liposomal MUC1 cancer vaccine) | I | NCT01556789 |
| J1179 | Sidney Kimmel Comprehensive Cancer Center | PANC 10.05 pcDNA-1/GM-Neo and PANC 6.03 pcDNA-1/GM-Neo vaccine, plus cyclophosphamide followed by SBRT (fractionated stereotactic body radiation therapy) and FOLFIRINOX chemotherapy | NP | NCT01595321 |
| 101778 | Medical University of South Carolina | Poly-ICLC (ligand for toll like receptor) and dendritic cells, plus standard chemotherapy | 0 | NCT01677962 |
| I 191511 | Roswell Park Cancer Institute | DEC-205-NY-ESO-1 (cancer-testis antigen) fusion protein vaccine | I | NCT01522820 |
| Adoptive T cell therapy | ||||
| UPCC 08212 | Abramson Cancer Center of the University of Pennsylvania | Autologous T cells transfected with chimeric anti-mesothelin immunoreceptor SS1, plus chemotherapy | I | NCT01897415 |
| UPCC 31213 | Abramson Cancer Center of the University of Pennsylvania | CART-meso (autologous T cells lentivirally transduced with chimeric anti-mesothelin immunoreceptor SS1 fused to the 4-1BB and CD3ζ signaling domains) | I | NCT02159716 |
| 12-C-0111 | NCI | Anti-mesothelin CAR plus chemotherapy and aldesleukin | I/II | NCT01583686 |
| 10-C-0166 | NCI | Young tumor infiltrating lymphocytes (TILs), plus chemotherapy and aldesleukin | II | NCT01174121 |
| 13-C-0214 | NCI | Anti-NY ESO-1 mTCR PBL (autologous white blood cells genetically modified with a retrovirus expressing the gene for anti-ESO-1), plus chemotherapy and aldesleukin | II | NCT01967823 |
| 14-C-0052 | NCI | Anti-MAGE-A3-DP4 TCR (autologous T cells genetically modified with a retrovirus expressing the gene for anti-MAGE-A3-DP0401/0402), plus chemotherapy and aldesleukin | I/II | NCT02111850 |
| 11-C-0013 | NCI | Anti-VEGFR2 CAR: Autologous CD8+ T cells genetically modified with a retrovirus expressing the gene for anti-VEGFR2, plus chemotherapy and aldesleukin | I/II | NCT01218867 |
| RWH 111-32 | Roger Williams Medical Center | EGFRBi-armed autologous activated T cells, loaded with a bispecific antibody produced by heteroconjugation of anti-CD3 and anti-EGFR monoclonal antibodies | I | NCT01081808 |
| Adjuvant immunotherapies and cytokines | ||||
| NCI-2011-03565 | Roswell Park Cancer Institute, NCI and Cleveland BioLabs Inc. | Entolimod (CBLB502, recombinant Toll-like receptor 5 agonist) | I | NCT01527136 |
| AM0010-001 | ARMO BioSciences | AM0010 (pegylated recombinant human interleukin-10) in combination with chemotherapy | I | NCT02009449 |
Monoclonal antibodies as checkpoint immunomodulators, signaling pathway inhibitors or cytotoxicity inducers: The incorporation of monoclonal antibodies to the therapeutic regimens of certain types of cancer has become established over the past two decades. The therapeutic activity of monoclonal antibodies can result from: (1) their ability to activate cellular immune responses against tumor antigens; (2) through agonist or antagonist effects on their target proteins; or (3) conjugated to cytotoxic agents, killing selectively tumor cells[122].
Activation, proliferation and differentiation of T cells in response to antigens is regulated by co-stimulatory and co-inhibitory receptors and their ligands, which modulate the signaling pathways triggered by the interaction of T cell receptors with the major histocompatibility complex (MHC)[123]. The immune system uses co-inhibitory signals to maintain self-tolerance and impair deleterious immune reactions by inducing T cell exhaustion or apoptosis. Some co-inhibitory molecules of particular relevance are programmed cell death protein-1 (PD-1), PD-1 ligands 1 and 2 (PD-L1 and 2), cytotoxic T lymphocyte antigen-4 (CTLA-4) and lymphocyte-activation gene 3 (LAG-3). It has become apparent that tumors develop mechanisms to interfere with some immune checkpoint pathways and thus escape from cytotoxic T cell responses triggered by tumor antigens[124]. Monoclonal antibodies targeting PD-1, PD-L1 and CTLA-4 (so called checkpoint blockade, reviewed by Postow et al[125]) have been shown in recent clinical trials to promote endogenous antitumor immune activity[126-128]. Table 3 summarizes ongoing clinical trials including patients with advanced PDAC to test PD-1, PD-L1, CTLA-4 and LAG-3. Some of these studies investigate the potential synergistic effects of combining immune checkpoint inhibitors among them and with other therapeutic agents (Table 3). An additional study targets 4-1BB/CD137, a member of the TNF receptor superfamily, which is a potent CD8+ T cell co-stimulatory molecule. There is compelling evidence indicating that anti-4-1BB monoclonal antibodies have antitumor properties[129].
Monoclonal antibodies targeting ERBB family members (e.g., EGFR) and VEGF (vascular endothelial growth factor) and VEGFR (VEGF receptor) have been most successful in patients with solid tumors. However, the application of some of these antibodies to patients with advanced PDAC has been disappointing. In a phase III study enrolling 745 patients with advanced or metastatic PDAC, cetuximab, a monoclonal antibody against the ligand-binding domain of EGFR, was administered combined with gemcitabine in comparison with single-agent gemcitabine. No significant difference in median overall survival was observed between both arms (6.3 mo vs 5.9 mo, respectively)[130]. Likewise, the combination of cetuximab with gemcitabine in the adjuvant treatment of 73 patients with resected (R0-R1) PDAC was reported recently not to improve overall survival[131]. A study in patients with advanced solid tumors including PDAC is currently recruiting patients for testing a combination of three anti-EGFR (ERBB1) monoclonal antibodies, and another trial with an anti-ERBB2 antibody combined with cetuximab is also open (Table 3).
Bevacizumab (anti-VEGF) was tested in 52 patients with previously untreated advanced PDAC in a single arm, multicenter phase II trial. The antibody was administered after gemcitabine treatment. Median overall survival was 8.8 mo and partial responses were observed in 21% of cases[132]. Although the results were not significantly better than those previously reported for gemcitabine, the study moved on to a phase III trial of gemcitabine/bevacizumab vs gemcitabine/placebo in 535 advanced pancreatic cancer patients. No difference in median overall survival (5.8 and 5.9 mo) was observed[89]. These results were congruent with the lack of effect reported for axitinib (see above Inhibitors).
In addition, new studies have been initiated to test monoclonal antibodies against Notch, tissue factor (TF, CD142), tumor endothelial marker 1 (TEM1, endosialin) and human growth factor receptor (HGFR), which also include PDAC patients (Table 3). Furthermore, ongoing studies will determine the benefit in advanced PDAC patients of antibodies against MUC5AC, which has been shown to inhibit TRAIL-induced apoptosis in PDAC cells[133], MUC1, overexpressed in over 60% of PDAC and inducer of drug resistance in PDAC cells[134].
Therapeutic vaccines: Therapeutic vaccines are designed to stimulate the immune system to react against tumor-specific antigens, essentially by inducing specific cytotoxic T cells. These vaccines may be made of whole cells, proteins, peptides or DNA encoding tumor antigens. Several vaccines have been tested in early clinical studies during the last years and some of them have shown discrete improvement in survival. Clinical trials with vaccines currently in progress are summarized in Table 3.
Algenpantucel-L is a vaccine designed to treat PDAC that has reached phase III. It consists of 2 irradiated allogeneic pancreatic cancer cell lines (HAPa-1 and HAPa-2) transfected to express murine α-1,3-galactosyltransferase. These cells carry α-1,3-galactosyl (α-gal) carbohydrates on cell surface glycoproteins and glycolipids, which trigger a rejection of the vaccine cell allograft, to which also contributes the fact that they are recognized by pre-existing anti-α-gal antibodies (naturally occurring against gut flora), resulting in opsonization and lysis of the vaccine cells and hence processing and presentation of tumor antigens by host antigen presenting cells. It follows a T cell response against endogenous tumor cells. This vaccine was tested in a phase II trial (multicenter, open-label) enrolling 70 patients with resected (R0-1) PDAC who received Algenpantucel-L in addition to standard adjuvant gemcitabine chemotherapy and chemoradiation[135]. Median follow-up was 21 mo, 1-year median overall survival was 86% and disease-free survival was 62% in the same period. It was concluded that the vaccine added to the adjuvant setting may improve survival and a phase III trial is ongoing (Table 3).
Another whole-cell vaccine consisting of irradiated cells stably transfected to express GM-CSF (granulocyte-macrophage colony-stimulating factor) was tested in a phase II trial in 60 patients with resected PDAC in combination with 5-FU-based chemoradiation therapy, with four additional immunizations[136]. The vaccine was well tolerated, median disease-free survival was 17.3 mo and 1-year overall survival was 85%, close to published data for resected PDAC treated with standard adjuvant therapy. The vaccine induced mesothelin-specific CD8+ T cells in HLA-A1 and HLA-A2 patients, correlating with longer disease-free survival. The GVAX-pancreas vaccine (GM-CSF-secreting allogenic pancreatic tumor cells) was tested recently in 90 patients with metastatic PDAC. GVAX was administered with low-dose cyclophosphamide (Cy/GVAX) to inhibit regulatory T cells and was combined or not with CRS-207, a live-attenuated Listeria monocytogenes expressing mesothelin, in a prime/boost vaccination setting: Cy/GVAX followed by CRS-207 (arm A) compared with Cy/GVAX alone (arm B)[137]. Overall survival was 6.1 mo in arm A and 3.9 mo in arm B (P = 0.02). Patients who received 3 or more doses of vaccine survived longer (9.7 and 4.6 mo for arms A and B, respectively). Higher mesothelin-specific CD8+ T-cell responses were associated with longer overall survival.
G17DT is an immunogen that induces neutralizing antibodies against the hormone gastrin-17. It was first tested in a randomized, double-blind, placebo controlled multicenter trial in 154 patients with advanced PDAC unsuitable for or unwilling to take chemotherapy[138]. The patients received five doses of the vaccine. The primary end point was survival. Patients who developed anti-G17DT survived longer (median survival 151 d) than non-responders (63 d) or those on placebo (83 d) (P = 0.03). The studies with this vaccine in PDAC patients were discontinued, although a phase III trial was registered (NCT02118077), and a recent phase II study in colorectal cancer patients has been published[139].
Peptide vaccines, although well tolerated, appear to be less promising. A peptide vaccine against mutant KRAS (codon 12) was tested in 24 positive of 62 patients with resected PDAC analyzed for mutations in codon 12 of KRAS[140]. Only 9 patients were evaluable for immunologic responses, and of these only one showed specific response to the patient’s KRAS mutation (assessed by delayed-type hypersensitivity). Median recurrence free survival time was 8.6 mo. No relationship was observed between immune response and clinical outcome. A personalized peptide vaccine administered to advanced PDAC patients in combination with standard gemcitabine[141] induced IgG responses in 14 of 36 patients. Median overall survival was 7.9 mo and 1-year overall survival rate was 26.8%. These results show a low benefit of the vaccine.
Adoptive T cell therapies: Adoptive cell transfer (ACT) therapies are designed to provide the patient with a highly amplified number of autologous tumor-specific cytotoxic T cells. To this end, tumor-specific T lymphocytes are isolated from the patient, expanded ex vivo and infused back into the patient’s bloodstream. There are several forms of anti-tumor ACT. One is based on the isolation and culture of tumor infiltrating lymphocytes (TIL) followed by selection of tumor-specific T cell clones and their expansion to obtain large numbers of cells that are infused back to the patient together with interleukin-2 (IL-2) (Aldesleukin) to stimulate their proliferation. Because the immune system and the tumor microenvironment contain regulatory CD4+ T cells that may prevent the transferred cells from functioning effectively, the patients are treated with chemotherapy agents, frequently with cyclophosphamide. The method can be improved by isolating T cells from the blood of patients that have received previously a cancer vaccine, which facilitates the expansion of tumor-reacting T cells.
However, in general the number of tumor-specific T cells in the TIL population results insufficient for therapeutic purposes. One strategy to overcome this problem involves transducing TIL isolated from a patient’s tumor with a retroviral vector to express a tumor-targeting T cell receptor (TCR) and expanding them in culture before re-infusing the cells back to the patient. This strategy has been shown to shrink tumors in patients with melanoma and synovial cell sarcoma in patients refractory to standard treatment[142]. The pre-condition for the treatment is that the TCR must be genetically matched to the patient’s immune type.
Another approach is based on chimeric antigen receptors (CAR)[143]. A CAR is an artificial molecule engineered to contain three pieces: an extracellular antibody-derived domain that binds a tumor surface antigen, the intracellular domain of the CD3 ζ chain (signal transmitter of endogenous TCR) and, linked to it, one or more stimulatory domains. The main advantage of this approach is the high affinity interaction of the CAR with the tumor antigen, which is independent of the MHC[144].
Table 3 shows a representative list of ongoing clinical studies applying ACT therapies using TIL and CAR approaches designed for advanced solid tumors including PDAC. In most cases, ACT is combined with chemotherapy, except in the RWH111-32 study, which uses only a bispecific antibody (anti-CD-3 and anti-EGFR).
In spite of the large number of preclinical and clinical studies focused on the improvement of existing therapies and the development of new therapeutic strategies, pancreatic adenocarcinoma remains an incurable lethal disease. After two decades of gemcitabine as standard reference, recent improvements in the chemotherapy of advanced disease have set a new standard, which is being evaluated also in patients with resectable and locally advanced tumors. In parallel, new developments in the immunotherapy field, in particular those based on antibodies or adoptive cell transfer, are already showing promising results in early phase clinical trials, in general in combination with chemotherapy. Furthermore, improvement in the understanding of the genetic and epigenetic changes taking place in tumor cells and stroma during progression to advanced PDAC, and their effects on tumor metabolism and immunoediting, may be of great value to identify better biomarkers that help in earlier detection of tumors and also in therapeutic decisions, in particular in adjuvant and neoadjuvant treatments. New developments in the fields of inhibitors, monoclonal antibodies and adoptive T cell transfer are expected to have a high impact in the treatment of pancreatic adenocarcinoma in coming years.
We thank R. Eritja (PhD) and A. Aviño (PhD) for stimulating discussions.
P- Reviewer: Hill NJ, Peracaula R S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Surveillance Research Program, NCI. SEER Stat Fact Sheets: Pancreas Cancer. Available from: http://seer.cancer.gov/statfacts/html/pancreas.html 2015. |
| 3. | Bockman DE. Anatomy of the Pancreas. The Pancreas: Biology, Pathobiology, and Disease, 2nd Ed. New York: Raven Press Ltd 1993; 1-8. |
| 4. | Longnecker DS. Anatomy and Histology of the Pancreas. The Pancreapedia. Exocrine Pancreas Knowledge Base. Longnecker: Daniel 2014; . |
| 5. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1030] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 6. | Rasheed ZA, Matsui W, Maitra A. Pathology of pancreatic stroma in PDAC. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India): Transworld Research Network 2012; . |
| 7. | Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci USA. 2013;110:12325-12330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA. 2014;111:E3091-E3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 9. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 1912] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 10. | Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1643] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 11. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3027] [Article Influence: 178.1] [Reference Citation Analysis (0)] |
| 12. | Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730-733.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 547] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 13. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 14. | Fernández-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 637] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 15. | Kim ST, Lim do H, Jang KT, Lim T, Lee J, Choi YL, Jang HL, Yi JH, Baek KK, Park SH. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol Cancer Ther. 2011;10:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111:817-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 394] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 17. | Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 430] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 18. | Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, Greninger P, Brown RD, Godfrey JT, Cohoon TJ. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 19. | Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 422] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 20. | Navas C, Hernández-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 21. | Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1573] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 22. | Chiche J, Brahimi-Horn MC, Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14:771-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 489] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 23. | Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezène P, Dusetti NJ, Loncle C, Calvo E, Turrini O. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2013;110:3919-3924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1227] [Cited by in RCA: 1526] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 25. | Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1442] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 26. | Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011;108:19611-19616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 27. | Hustinx SR, Leoni LM, Yeo CJ, Brown PN, Goggins M, Kern SE, Hruban RH, Maitra A. Concordant loss of MTAP and p16/CDKN2A expression in pancreatic intraepithelial neoplasia: evidence of homozygous deletion in a noninvasive precursor lesion. Mod Pathol. 2005;18:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293-311. [PubMed] |
| 29. | Jia J, Bosley AD, Thompson A, Hoskins JW, Cheuk A, Collins I, Parikh H, Xiao Z, Ylaya K, Dzyadyk M. CLPTM1L promotes growth and enhances aneuploidy in pancreatic cancer cells. Cancer Res. 2014;74:2785-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Wolpin BM, Rizzato C, Kraft P, Kooperberg C, Petersen GM, Wang Z, Arslan AA, Beane-Freeman L, Bracci PM, Buring J. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet. 2014;46:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 31. | Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1139] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 33. | Rebours V, Boutron-Ruault MC, Schnee M, Férec C, Maire F, Hammel P, Ruszniewski P, Lévy P. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 35. | De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 554] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 36. | Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e91551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 37. | Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020-5029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 39. | Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 689] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 40. | Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 902] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 41. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6290] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 42. | Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212-20217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1556] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 43. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. [PubMed] |
| 44. | Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, Kim BR. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Montgomery RC, Hoffman JP, Riley LB, Rogatko A, Ridge JA, Eisenberg BL. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997;4:551-556. [PubMed] |
| 46. | Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, Büchler MW, Werner J. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20:2188-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 47. | Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 48. | Heestand GM, Murphy J, Moughan J, Regine W, Luo J, Graber M, Kunz P, Fisher G, Guha C, Lin B. A novel biomarker panel examining response to adjuvant pancreatic cancer therapy in RTOG 9704. J Clin Oncol. 2014;32:abstr 176. |
| 49. | Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115-4121. [PubMed] |
| 50. | Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 853] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 51. | Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, Haque W, Hobbs BD, Krishnan S, Fleming JB. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 52. | Bachet JB, Maréchal R, Demetter P, Bonnetain F, Couvelard A, Svrcek M, Bardier-Dupas A, Hammel P, Sauvanet A, Louvet C. Contribution of CXCR4 and SMAD4 in predicting disease progression pattern and benefit from adjuvant chemotherapy in resected pancreatic adenocarcinoma. Ann Oncol. 2012;23:2327-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Poplin E, Wasan H, Rolfe L, Raponi M, Ikdahl T, Bondarenko I, Davidenko I, Bondar V, Garin A, Boeck S. Randomized, multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol. 2013;31:4453-4461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 54. | Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 55. | Zhou YF, Xu LX, Huang LY, Guo F, Zhang F, He XY, Yuan YZ, Yao WY. Combined detection of serum UL16-binding protein 2 and macrophage inhibitory cytokine-1 improves early diagnosis and prognostic prediction of pancreatic cancer. Oncol Lett. 2014;8:2096-2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Takahashi N, Fukushima T, Yorita K, Tanaka H, Chijiiwa K, Kataoka H. Dickkopf-1 is overexpressed in human pancreatic ductal adenocarcinoma cells and is involved in invasive growth. Int J Cancer. 2010;126:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 58. | Cascinu S, Falconi M, Valentini V, Jelic S. Pancreatic cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v55-v58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Martin RC, Scoggins CR, Egnatashvili V, Staley CA, McMasters KM, Kooby DA. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg. 2009;144:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Speer AG, Thursfield VJ, Torn-Broers Y, Jefford M. Pancreatic cancer: surgical management and outcomes after 6 years of follow-up. Med J Aust. 2012;196:511-515. [PubMed] |
| 62. | Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 64. | Fukutomi A, Uesaka K, Boku N, Kanemoto H, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Nakamura T, Kanai M, Hirano S, Yoshikawa Y, Ohashi Y. JASPAC 01: Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer. J Clin Oncol. 2013;31 (suppl):abstr 4008. |
| 65. | Sudo K, Nakamura K, Yamaguchi T. S-1 in the treatment of pancreatic cancer. World J Gastroenterol. 2014;20:15110-15118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Shimada T, Yamazaki H, Guengerich FP. Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica. 1996;26:395-403. [PubMed] |
| 67. | Chuah B, Goh BC, Lee SC, Soong R, Lau F, Mulay M, Dinolfo M, Lim SE, Soo R, Furuie T. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 2011;102:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | ESPAC-4. European Study Group for Pancreatic Ccancer Trial 4. Combination versus single agent chemotherapy in resectable pancreatic ductal and peri-ampullary cancers. UK Clinical Research Network Study Portfolio. Available from: http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=4307.2015. |
| 69. | Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275. [PubMed] |
| 70. | Boeck S, Hoehler T, Seipelt G, Mahlberg R, Wein A, Hochhaus A, Boeck HP, Schmid B, Kettner E, Stauch M. Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol. 2008;19:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-5518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 578] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 72. | Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-782; discussion 782-784. [PubMed] |
| 73. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1909] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 74. | Morganti AG, Falconi M, van Stiphout RG, Mattiucci GC, Alfieri S, Calvo FA, Dubois JB, Fastner G, Herman JM, Maidment BW. Multi-institutional pooled analysis on adjuvant chemoradiation in pancreatic cancer. Int J Radiat Oncol Biol Phys. 2014;90:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, Maisonneuve P, Sebastiano PD. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 77. | Tsvetkova EV, Asmis TR. Role of neoadjuvant therapy in the management of pancreatic cancer: is the era of biomarker-directed therapy here? Curr Oncol. 2014;21:e650-e657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Alamo JM, Marín LM, Suarez G, Bernal C, Serrano J, Barrera L, Gómez MA, Muntané J, Padillo FJ. Improving outcomes in pancreatic cancer: key points in perioperative management. World J Gastroenterol. 2014;20:14237-14245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol. 2013;24:2484-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 80. | Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846; discussion 846-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 605] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 81. | Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen JN, Dias LE. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 82. | Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 535] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 83. | Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 84. | Hammel P, Huguet F, Van Laethem J-L, Goldstein D, Glimelius B, Artru P, Borbath I, Bouche O, Shannon J, André T. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. J Clin Onco. 2013;l31:abstr LBA4003. |
| 85. | Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, Narayanan G, Ribeiro A, Portelance L, Merchan JR. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 87. | Rocha Lima CM, Green MR, Rotche R, Miller WH, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776-3783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 424] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 88. | Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 89. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 675] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 90. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2778] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 91. | Rauchwerger DR, Firby PS, Hedley DW, Moore MJ. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000;60:6075-6079. [PubMed] |
| 92. | Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 870] [Article Influence: 62.1] [Reference Citation Analysis (2)] |
| 93. | Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548-557. [PubMed] |
| 94. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 95. | Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol. 2010;11:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 96. | Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 97. | Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 98. | O’Reilly EM. Evolving panorama of treatment for metastatic pancreas adenocarcinoma. J Clin Oncol. 2013;31:1621-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794-7803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1422] [Cited by in RCA: 1488] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 100. | Lobo C, Lopes G, Silva O, Gluck S. Paclitaxel albumin-bound particles (abraxane) in combination with bevacizumab with or without gemcitabine: early experience at the University of Miami/Braman Family Breast Cancer Institute. Biomed Pharmacother. 2007;61:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Hersh EM, O’Day SJ, Ribas A, Samlowski WE, Gordon MS, Shechter DE, Clawson AA, Gonzalez R. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer. 2010;116:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Socinski MA, Manikhas GM, Stroyakovsky DL, Makhson AN, Cheporov SV, Orlov SV, Yablonsky PK, Bhar P, Iglesias J. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:852-861. [PubMed] |
| 103. | Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 104. | Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, Ellenrieder V, Jodrell DI, Tuveson DA. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63:974-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 105. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5640] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 106. | Peddi PF, Lubner S, McWilliams R, Tan BR, Picus J, Sorscher SM, Suresh R, Lockhart AC, Wang J, Menias C. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP. 2012;13:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 107. | Gunturu KS, Yao X, Cong X, Thumar JR, Hochster HS, Stein SM, Lacy J. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol. 2013;30:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 108. | Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, Kooby DA, Maithel SK, Landry J, El-Rayes BF. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 109. | Gill S, Ho MI, Kennecke HF, Renouf DJ, Cheung WI, Lim HJ. Defining eligibility of FOLFIRINOX for first-line metastatic pancreatic adenocarcinoma (MPC) in the province of British Columbia: A population-based retrospective study. Proceedings of the ASCO: Annual Meeting 2012; . |
| 110. | Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3368] [Cited by in RCA: 3845] [Article Influence: 192.3] [Reference Citation Analysis (0)] |
| 111. | Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1328] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 112. | Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA; Marimastat Pancreatic Cancer Study Group. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447-3455. [PubMed] |
| 113. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 397] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 114. | Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122-129. [PubMed] |
| 115. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. [PubMed] |
| 116. | Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Létourneau R, Bajetta E, Pithavala Y, Bycott P. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 117. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 118. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2543] [Article Influence: 158.9] [Reference Citation Analysis (0)] |
| 119. | Madden JI. Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer. Available from: http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle&ID=1653550&highlight=.2012. |
| 120. | Catenacci DVT, Bahary N, Nattam SR, Marsh RW, Wallace JA, Rajdev L, Cohen DJ, Sleckman BG, Lenz HJ, Stiff PJ. M. S, Kindler HL. Final analysis of a phase IB/randomized phase II study of gemcitabine (G) plus placebo (P) or vismodegib (V), a hedgehog (Hh) pathway inhibitor, in patients (pts) with metastatic pancreatic cancer (PC): A University of Chicago phase II consortium study. J Clin Oncol. 2013;31:abstr 4012. |
| 121. | Mitry E, Hammel P, Deplanque G, Mornex F, Levy P, Seitz JF, Moussy A, Kinet JP, Hermine O, Rougier P. Safety and activity of masitinib in combination with gemcitabine in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1769] [Article Influence: 136.1] [Reference Citation Analysis (0)] |
| 123. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2279] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 124. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10353] [Article Influence: 796.4] [Reference Citation Analysis (34)] |
| 125. | Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2095] [Article Influence: 209.5] [Reference Citation Analysis (0)] |
| 126. | Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 127. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1915] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 128. | Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4180] [Cited by in RCA: 5240] [Article Influence: 524.0] [Reference Citation Analysis (0)] |
| 129. | Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012;11:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 130. | Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 131. | Fensterer H, Schade-Brittinger C, Müller HH, Tebbe S, Fass J, Lindig U, Settmacher U, Schmidt WE, Märten A, Ebert MP. Multicenter phase II trial to investigate safety and efficacy of gemcitabine combined with cetuximab as adjuvant therapy in pancreatic cancer (ATIP). Ann Oncol. 2013;24:2576-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 132. | Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033-8040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 341] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 133. | Hoshi H, Sawada T, Uchida M, Iijima H, Kimura K, Hirakawa K, Wanibuchi H. MUC5AC protects pancreatic cancer cells from TRAIL-induced death pathways. Int J Oncol. 2013;42:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Nath S, Daneshvar K, Roy LD, Grover P, Kidiyoor A, Mosley L, Sahraei M, Mukherjee P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 135. | Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94-100; discussion 100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 136. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 137. | Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 463] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 138. | Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, Whitehead A, Takhar A, Rowlands BJ, Beckingham IJ. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas. 2012;41:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 139. | Rocha-Lima CM, de Queiroz Marques Junior E, Bayraktar S, Broome P, Weissman C, Nowacki M, Leslie M, Susnerwala S. A multicenter phase II study of G17DT immunogen plus irinotecan in pretreated metastatic colorectal cancer progressing on irinotecan. Cancer Chemother Pharmacol. 2014;74:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 140. | Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. 2011;34:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 141. | Yutani S, Komatsu N, Yoshitomi M, Matsueda S, Yonemoto K, Mine T, Noguchi M, Ishihara Y, Yamada A, Itoh K. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol Rep. 2013;30:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 142. | Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 632] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 143. | Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 144. | Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74:7168-7174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 299] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 145. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 146. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 147. | Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |