Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8927
Peer-review started: October 20, 2014
First decision: December 11, 2014
Revised: January 6, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: August 7, 2015
Processing time: 292 Days and 5.7 Hours
AIM: To evaluate the nutritional status and its association with proinflammatory cytokines in children with chronic liver disease.
METHODS: We performed a cross-sectional study with 43 children and adolescents, aged 0 to 17 years, diagnosed with chronic liver disease. All patients regularly attended the Pediatric Hepatology Unit and were under nutritional follow up. The exclusion criteria were fever from any etiology at the time of enrollment, inborn errors of the metabolism and any chronic illness. The severity of liver disease was assessed by Child-Pugh, Model for End-stage Liver Disease (MELD) and Pediatric End Stage Liver Disease (PELD) scores. Anthropometric parameters were height/age, body mass index/age and triceps skinfold/age according to World Health Organization standards. The cutoff points for nutritional status were risk of malnutrition (Z-score < -1.00) and malnutrition (Z-score < -2.00). Interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α levels were assessed by commercial ELISA kits. For multivariate analysis, linear regression was applied to assess the association between cytokine levels, disease severity and nutritional status.
RESULTS: The median (25th-75th centile) age of the study population was 60 (17-116)-mo-old, and 53.5% were female. Biliary atresia was the main cause of chronic liver disease (72%). With respect to Child-Pugh score, cirrhotic patients were distributed as follows: 57.1% Child-Pugh A, a mild presentation of the disease, 34.3% Child-Pugh B, a moderate stage of cirrhosis and 8.6% Child-Pugh C, were considered severe cases. PELD and MELD scores were only above the cutoff point in 5 cases. IL-6 values were increased in patients at nutritional risk (34.9%) compared with those who were well-nourished [7.12 (0.58-34.23) pg/mL vs 1.63 (0.53-3.43) pg/mL; P = 0.02], correlating inversely with triceps skinfold-for-age z-score (rs = -0.61; P < 0.001). IL-6 levels were associated with liver disease severity assessed by Child-Pugh score (P = 0.001). This association remained significant after adjusting for nutritional status in a linear regression model.
CONCLUSION: High IL-6 levels were found in children with chronic liver disease at nutritional risk. Inflammatory activity may be related to nutritional status deterioration in these patients.
Core tip: Inflammatory activity has been suggested as a component of the pathogenesis of illness-related malnutrition. Several studies have evaluated proinflammatory cytokines in pediatric chronic liver disease, but none have addressed the possible association between these biomarkers and nutritional status. This study showed that the interleukin-6 levels were significantly increased in children and adolescents at nutritional risk. To the best of our knowledge, this is the first study to analyze the relationship between the cytokine levels and nutritional status in children and adolescents with chronic liver disease.
- Citation: Santetti D, de Albuquerque Wilasco MI, Dornelles CTL, Werlang ICR, Fontella FU, Kieling CO, dos Santos JL, Vieira SMG, Goldani HAS. Serum proinflammatory cytokines and nutritional status in pediatric chronic liver disease. World J Gastroenterol 2015; 21(29): 8927-8934
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8927.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8927
Biliary atresia (BA) is an obstructive cholangiopathy that is present at birth or developed during the first two weeks of life[1]. It is the most common cause of chronic liver disease in children and can progress to cirrhosis[2]. The etiology of this disease is still unknown[3]. Late referral to specialized centers is still a problem that can affect patient survival[4]. Alpha-1 antitrypsin deficiency, Alagille syndrome and progressive familial intrahepatic cholestasis (PFIC) are other causes of liver disease in the pediatric population[5]. Complications related to chronic liver disease and cirrhosis include portal hypertension, variceal bleeding, ascites and failure to thrive[6-8].
It is well established that nutritional status is an important factor in the prognosis of chronic liver disease[9]. Growth deficits may influence pre- and post-transplantation mortality[10,11]. The presence of pre-transplant stunting seems to be associated with longer hospital stays and increased costs with hospitalization at the time of transplantation[12]. Early identification of nutritional risk is important so that a multidisciplinary team can implement individualized dietary interventions[13].
Malnutrition in liver disease is related to several conditions, such as reduced caloric intake, anorexia, early satiety, abnormalities in the metabolism of macronutrients, hypermetabolism and increased proinflammatory cytokines[14-16]. In cholestatic patients, there is a significant decrease in the bile acids concentrations in the intestine with consequent malabsorption of lipids and fat-soluble vitamins[17]. This clinical issue remains common, especially in end stage liver disease[18,19].
Recently, inflammatory activity is suggested as a component of the pathogenesis of illness-related malnutrition[20,21]. Moreover, it is possible that proinflammatory cytokines could impair growth and muscle breakdown through several pathways[20,22]. The inclusion of the assessment of proinflammatory cytokines, such as the concentrations of interleukin-1 beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP), is currently recommended in routine clinical care[20,23,24].
Many studies have evaluated the cytokine profiles of children with chronic liver disease[25,26], but none have explored the possible association between these biomarkers of inflammation and nutritional status. Given the lack of studies on this relationship, the present study aimed to evaluate the nutritional status of children and adolescents with chronic liver disease, and its association with inflammatory activity, by measuring the proinflammatory cytokines IL-1β, IL-6 and TNF-α.
The present cross-sectional study evaluated a total of 43 children and adolescents from 3 mo to 17 years of age with a clinical diagnosis of chronic liver disease. All patients regularly attended the Pediatric Hepatology Unit, Hospital de Clinicas de Porto Alegre, a tertiary reference center for pediatric liver disease and liver transplantation in Southern Brazil. All patients were under regular nutritional follow up and their dietary intake was evaluated as a systematic approach of the multidisciplinary team at our institution.
In our study, cirrhosis was diagnosed by histological and/or standard ultrasonographic and clinical criteria in patients with chronic liver disease. The histological criteria were the presence of nodular formation and fibrosis on liver biopsy. Ultrasonographic findings were the presence of esophageal varices on endoscopy and/or ultrasound, showing heterogeneous echogenicity of the liver and signs of portal hypertension. The clinical criteria were hepatosplenomegaly, ascites, hypoalbuminemia and coagulopathy[27].
The exclusion criteria were as follows: fever of any etiology at the time of enrollment, inborn errors of the metabolism and any chronic illness besides cirrhosis.
The severity of liver disease was assessed according to Child-Pugh score[28]; model for end-stage liver disease (MELD) score[29] for adolescents older than 12 years of age and pediatric end stage liver disease (PELD) score[30] for participants younger than 12 years of age. Scores higher than 15 were considered the cut-off point for liver disease severity.
The anthropometric parameters used in this study included the following: body mass index-for-age (BMI/A), height-for-age (H/A), mid upper arm circumference-for-age (MUAC/A) and triceps skinfold-for-age (TSF/A), according to World Health Organization (WHO) reference techniques[31,32]. The same trained researcher performed all measurements. The variables were presented as Z-scores. For children under 5 years of age, the WHO Anthro (version 3.2.2) software was applied. Children older than 5 years of age were evaluated using WHO Anthro Plus and anthropometric standards proposed by Frisancho[33] to calculate the MUAC/A and TSF/A. The risk of malnutrition was defined based on Z-score < -1.00 for BMI/A or TSF/A and malnutrition as Z-score < -2.00. For statistical purposes, the two categories, risk of malnutrition and malnutrition, were assembled in a single group called nutritional risk. In patients with ascites, we did not consider values of BMI/A, because this parameter may underestimate the diagnosis of malnutrition due to fluid retention.
Blood samples were collected from all patients during the performance of routine tests such as: serum albumin, creatinine, conjugated bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), prothrombin time, international normalized ratio (INR) and CRP. All laboratory tests were executed according to standard operating protocols from the Biochemistry Laboratory of the local institution.
For cytokine assessment (IL-1β, IL-6 and TNF-α), serum (2.0 mL) was immediately separated by centrifugation for 15 min at 3000 rpm and stored at -80 °C, until analysis. The cytokine concentrations were measured in duplicate using commercial ELISA kits (RD Systems, Inc., Minneapolis, MN, United States) according to the manufacturer’s protocol. The minimum detectable levels for cytokines were as follows: 1.0 pg/mL (IL-1β), 0.7 pg/mL (IL-6) and 5.5 pg/mL (TNF-α).
Continuous variables were expressed as mean ± SD or median and interquartile range (25th-75th centile). To evaluate the association between categorical variables chi-square test was used. Regarding the correlation between quantitative variables Spearman correlation rank was applied. To compare two groups of quantitative variables we used Mann-Whitney U test, and to compare more than two groups, we used Kruskal-Wallis test. The linearity was tested and logarithm adjustment was performed. For multivariate analysis, linear regression was applied to assess the association between the cytokine levels, disease severity and nutritional status. Data were considered statistically significant at P≤ 0.05.
The study population’s median (25th-75th centile) age was 60 (17-116) mo. BA was the main cause of chronic liver disease (72%). Eight patients had no cirrhosis criteria at the time of enrollment. Of all 35 cirrhotic patients, 24 were diagnosed by liver biopsy. From the remaining 11 without liver biopsy, all had ultrasonographic alterations that were compatible with cirrhosis and portal hypertension (splenomegaly and/or esophageal varices) without portal vein thrombosis.
Regarding Child-Pugh score, cirrhotic patients were distributed as follows: 57.1% Child-Pugh A with a mild presentation of the disease, 34.3% Child-Pugh with a moderate stage of cirrhosis and 8.6% Child-Pugh C were considered severe cases. PELD and MELD scores were only higher than the cutoff point of 15 in 5 cases. Complete demographic and clinical data are shown in Table 1.
| Variables | Patients (n = 43) |
| Age (yr) | |
| < 2 | 13 (30.2) |
| 2-5 | 9 (20.9) |
| 5-10 | 11 (25.6) |
| > 10 | 10 (23.3) |
| Female | 23 (53.5) |
| Nutritional status1 | |
| Well-nourished | 28 (65.1) |
| Risk of malnutrition | 10 (23.3) |
| Malnutrition | 5 (11.6) |
| Causes of chronic liver disease | |
| Biliary atresia | 31 (72) |
| Alpha-1 antitrypsin deficiency | 6 (14) |
| Sinusoidal obstruction syndrome | 1 (2.3) |
| Idiopathic chronic liver disease | 1 (2.3) |
| Cirrhosis by cytomegalovirus | 1 (2.3) |
| Cryptogenic cirrhosis | 3 (7) |
| Cholestasis (CB ≥ 2.0 mg/dL) | 12 (27.9) |
| Albumin (< 3.5 g/L) | 12 (27.9) |
| Portal hypertension | 24 (55.8) |
| Ascites | 3 (7) |
| Hepatomegaly | 25 (58.1) |
| Splenomegaly | 32 (74.4) |
| Cirrhosis | 35 (81.4) |
| Child-Pugh score | |
| A | 20/35 (57.1) |
| B | 12/35 (34.3) |
| C | 3/35 (8.6) |
| PELD score (> 15) | 4/27 (14.8) |
| MELD score (> 15) | 1/8 (12.5) |
Malnutrition was detected among 11.6% of the children and adolescents with chronic liver disease and 23.3% were considered at risk of malnutrition. The frequency of nutritional risk (risk of malnutrition plus malnutrition) was higher in children younger than 2 years of age, corresponding to 61.5% in this age group (P = 0.037). With respect to linear growth, a frequency of 23.3% of low height-for-age was found.
The overall median (25th-75th centile) levels detected for IL-1β, IL-6 and TNF-α were, respectively, 0.07 (0-0.30) pg/mL, 2.2 (0.58-6.8) pg/mL and 8.3 (4.6-11.9) pg/mL. Both IL-6 and TNF-α levels were different between age groups (P = 0.004; P = 0.003).
We found an inverse correlation between IL-6 and TSF/A (rs = -0.61; P < 0.001), IL-6 and MUAC/A (rs = -0.51; P = 0.001), and IL-6 and H/A (rs = -0.34; P = 0.023). IL-1β only correlated inversely with TSF/A (rs = -0.41; P = 0.006). There was no correlation between the serum TNF-α and TSF/A.
The IL-6 values were significantly increased in patients at nutritional risk (P = 0.02). The cytokine levels in well-nourished children with chronic liver disease compared with those at nutritional risk are presented in Table 2.
| Cytokine | Nutritional risk | Well-nourished | P value1 |
| Interleukin-1β (pg/mL) | 0.10 (0-0.66) | 0.05 (0-0.28) | 0.144 |
| Interleukin-6 (pg/mL) | 7.12 (0.58-34.23) | 1.63 (0.53-3.43) | 0.020 |
| TNF-α (pg/mL) | 10.74 (8.17-12.35) | 6.66 (4.28-11.26) | 0.880 |
A strong correlation was verified between IL-6 and PELD score (rs = 0.79; P < 0.001) as well as between TNF-α and MELD score (rs = 0.76; P = 0.017). The IL-6 levels and routine liver function test correlations are shown in Table 3. With respect to albumin, the serum levels correlated inversely with IL-6 levels (rs = -0.80; P < 0.001) as CRP had a strong positive correlation to this cytokine (rs = 0.76; P < 0.001).
| Liver function tests | Median (25th-75th) | rs | P value |
| Aspartate aminotransferase (U/L) | 73.50 (49.25-177.00) | 0.70 | < 0.001 |
| Alanine aminotransferase (U/L) | 60.00 (33.75-115.75) | 0.49 | 0.001 |
| Conjugated Bilirubin (mg/dL) | 0.7 (0.2-2. 57) | 0.72 | < 0.001 |
The IL-1β levels were not associated with liver disease severity assessed through Child-Pugh, PELD and MELD scores in our study population. The TNF-α levels were associated with PELD (P = 0.026) and Child-Pugh scores (P = 0.050).
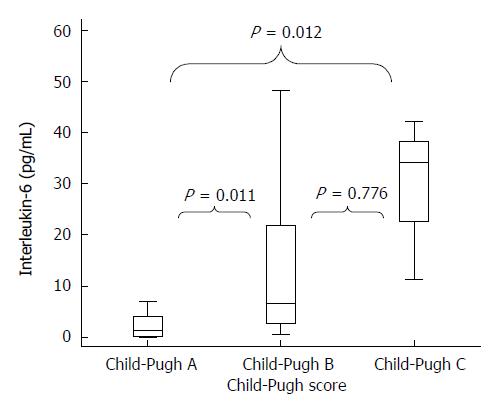
The IL-6 levels also had a significant association with liver disease severity, which was evaluated by both PELD score (P = 0.014) and Child-Pugh score (P = 0.001). The distribution of this cytokine among Child-Pugh’s categories A, B and C is shown in Figure 1. In the multivariate model, IL-6 remained associated with the disease severity that was measured by Child-Pugh score and PELD/MELD scores after adjusting for nutritional status (Table 4).
This study assessed the relationship between the serum proinflammatory cytokines and nutritional status in children and adolescents with chronic liver disease. We demonstrated that the IL-6 levels were significantly increased in children and adolescents at nutritional risk enrolled in our study. To the best of our knowledge, this is the first study to analyze the relationship between the cytokine levels and nutritional status in children and adolescents with chronic liver disease.
Our results agreed with previous findings that suggested that the serum concentrations of IL-6 could be used to identify patients at risk of nutritional deterioration[34]. Illness-related malnutrition appears to display an association with inflammatory components through increased resting energy expenditure, decreased calorie consumption, anorexia, nutrient loss, altered nutrient utilization and malabsorption[20]. This concept seems to be appropriate for children and adolescents with chronic liver disease who have an intense protein catabolism, changes in body composition and muscle loss.
Increased resting energy expenditure could be related to weight and growth impairment in pediatric chronic liver disease, which may be approximately 30% higher compared with a healthy child[35]. This hypermetabolic state could be due to inflammation by the presence of ascending cholangitis, ascites and disordered substrate and energy uptake[15]. Additionally, altered intestinal permeability with consequent endotoxemia is directly linked to increased cytokine production observed in cirrhosis. It is suggested that malnourished cirrhotic patients have increased intestinal permeability compared with those who are well-nourished[36], which could explain the higher levels of IL-6 found in our patients at nutritional risk. However, in cirrhotic adults, increased systemic levels of IL-6 did not correlate with body mass index[37]. We can assume that there are still conflicting data related to the possible relationship between malnutrition and proinflammatory cytokines production, especially in chronic liver disease.
The pathophysiology of malnutrition is thought to be the combined influence of undernutrition and inflammatory activity in the body composition[38]. It is well established that the presence of inflammatory activity induces peripheral loss of lean body mass[24]. In our study, we found a strong inverse correlation between the IL-6 levels and TSF/A Z-score, a parameter of body composition. This finding agrees with a possible link between the loss of lean body mass and inflammatory activity. However, these associations were not confirmed for IL-1β and TNF-α, which is in agreement with a study on cystic fibrosis patients[39]. Anthropometric parameters, such as TSF/A, are important tools for the nutritional assessment of cirrhotic patients, especially at advanced stages of the disease. It has been reported that there is a correlation between the liver disease severity, estimated by liver function tests, and impaired nutritional status, measured by anthropometric markers[40].
Concerning nutritional assessment tools, it is well known that serum albumin shows low sensitivity and specificity as a marker of nutritional status, especially in patients with liver disease. Furthermore, it is assumed that albumin could be indicative of the presence of inflammation[41]. In agreement with this theoretical assumption, we found a strong inverse correlation between the IL-6 levels and albumin. With respect to the relationship between CRP and IL-6, there was a positive correlation in our study once this cytokine stimulates the release of CRP by the liver during the acute phase of inflammation. A recent study reported that this acute-phase protein could also be used as a tool to predict short-term mortality in severely hospitalized cirrhotic patients[42].
Recent studies have suggested that IL-6 plays an important role in the pathogenesis of several diseases[43,44]. Focusing on liver disease patients, there seems to be an imbalance between proinflammatory and anti-inflammatory cytokines in cirrhosis. The activated Kupffer cells of the liver are involved in cytokine secretion. A study comparing children with BA and intra-hepatic cholestatic diseases have reported higher values of IL-6 in the BA group, indicating the presence of chronic inflammation. Nevertheless, there was no mention of the patient’s nutritional status. The authors concluded that IL-6 could contribute to determining the disease severity[45]. As for these studies, we found an association between the liver disease severity, assessed by Child-Pugh score, and increased IL-6 levels. Moreover, we also found a positive correlation between IL-6 and routine liver function tests, such as AST, ALT and CB.
Increased lipid oxidation and decreased glucose uptake were demonstrated by indirect calorimetry in cirrhotic patients, and this metabolic abnormality is correlated with the disease severity and circulating levels of TNF-α[46]. In our study, the TNF-α levels were also associated with liver disease severity, but they were not associated with the nutritional status assessed by anthropometric parameters. The IL-1β levels were not significantly related to the outcomes in our study. However, it is well established that proinflammatory cytokines can act through stimulating one another’s production[47]. We can speculate that this may have occurred in our study, because IL-1β could be somehow stimulating IL-6 and TNF-α production.
The limitation of the study may be due to the small number of severe liver disease patients (Child-Pugh C) because all patients were enrolled in the outpatient clinic. Moreover, the study design (cross sectional) did not allow for medium or long-term follow up to assess the nutritional and clinical outcomes. The strength of this study is that we found the relationship between high IL-6 levels in children and adolescents with chronic liver disease at nutritional risk. However, we could not determine the exact cause of increased IL-6 levels, which could be from either malnutrition or chronic liver disease or both.
We reported a relationship between high IL-6 levels and nutritional status in children and adolescents with chronic liver disease. Our findings suggest that inflammatory activity appears to be part of the evolution of pediatric chronic liver disease. Extensive knowledge of this inflammatory panorama is of main importance in our field, enabling the creation of new approaches to nutritional support. Further research is required to evaluate the effect of dietary intervention on inflammatory response, in children and adolescents with chronic liver disease.
We thank the Unit of Biostatistics, Grupo de Pesquisa e Pós Graduação, Hospital de Clinicas de Porto Alegre, Brazil.
Malnutrition in liver disease is frequently related to decreased caloric intake, early satiety, and abnormalities in the metabolism of macronutrients. Additionally, it has also been hypothesized that malnutrition might be related to inflammatory activity in patients with chronic diseases.
This is essentially a clinical study that assessed the relationship between the cytokine levels and nutritional status in children and adolescents with chronic liver disease.
Several studies evaluated the cytokine profiles of children with chronic liver diseases, but none addressed the possible association between these biomarkers of inflammation and nutritional status. This is the first study to analyze the relationship between the cytokine levels and nutritional status in children and adolescents with chronic liver disease.
Understanding the role of proinflammatory cytokines, such as IL-6, in pediatric chronic liver disease, might be a helpful tool for assessing the illness-related malnutrition presented by these patients.
Cytokines are inflammatory mediators produced by T cells. The circulating levels of these biomarkers are identified in the presence of overproduction with consequent impact on homeostasis. Proinflammatory cytokines, such as Interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α, may be associated with illness-related malnutrition.
This manuscript highlights the association between the IL-6 levels and nutritional status deterioration. Their findings suggest that inflammatory activity appears to be part of the evolution of pediatric chronic liver disease. Extensive knowledge of this inflammatory panorama is of primary importance in their field, enabling the creation of new approaches to nutritional support.
P- Reviewer: Ampuero J, Carrillo MC, Ramos S, Sazci A S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 638] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 2. | Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013;56:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Santos JL, Carvalho E, Bezerra JA. Advances in biliary atresia: from patient care to research. Braz J Med Biol Res. 2010;43:522-527. [PubMed] |
| 4. | Carvalho Ed, Santos JL, Silveira TR, Kieling CO, Silva LR, Porta G, Miura IK, De Tommaso AM, Brandão MÂ, Ferreira AR. Biliary atresia: the Brazilian experience. J Pediatr (Rio J). 2010;86:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Suchy FJ. Neonatal cholestasis. Pediatr Rev. 2004;25:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Davenport M. Biliary atresia: clinical aspects. Semin Pediatr Surg. 2012;21:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Kelly DA, Davenport M. Current management of biliary atresia. Arch Dis Child. 2007;92:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Shneider BL, Abel B, Haber B, Karpen SJ, Magee JC, Romero R, Schwarz K, Bass LM, Kerkar N, Miethke AG. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Tsouka A, McLin VA. Complications of chronic liver disease. Clin Res Hepatol Gastroenterol. 2012;36:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, Anand R. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. J Pediatr. 2005;147:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | DeRusso PA, Ye W, Shepherd R, Haber BA, Shneider BL, Whitington PF, Schwarz KB, Bezerra JA, Rosenthal P, Karpen S. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology. 2007;46:1632-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Barshes NR, Chang IF, Karpen SJ, Carter BA, Goss JA. Impact of pretransplant growth retardation in pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2006;43:89-94. [PubMed] |
| 13. | Hartman C, Shamir R, Hecht C, Koletzko B. Malnutrition screening tools for hospitalized children. Curr Opin Clin Nutr Metab Care. 2012;15:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Nightingale S, Ng VL. Optimizing nutritional management in children with chronic liver disease. Pediatr Clin North Am. 2009;56:1161-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Sanchez AJ, Aranda-Michel J. Nutrition for the liver transplant patient. Liver Transpl. 2006;12:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | McKiernan P. Neonatal jaundice. Clin Res Hepatol Gastroenterol. 2012;36:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Dornelles CT, Goldani HA, Wilasco MI, Maurer RL, Kieling CO, Porowski M, Ferreira CT, Santos JL, Vieira SM, Silveira TR. Ghrelin, leptin and insulin in cirrhotic children and adolescents: relationship with cirrhosis severity and nutritional status. Regul Pept. 2013;180:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Neto JS, Pugliese R, Fonseca EA, Vincenzi R, Pugliese V, Candido H, Stein AB, Benavides M, Ketzer B, Teng H. Four hundred thirty consecutive pediatric living donor liver transplants: variables associated with posttransplant patient and graft survival. Liver Transpl. 2012;18:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Mehta NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney LN, Monczka JL, Plogsted SW, Schwenk WF. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 21. | White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 835] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 22. | Zoico E, Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev. 2002;60:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Soeters PB, Reijven PL, van Bokhorst-de van der Schueren MA, Schols JM, Halfens RJ, Meijers JM, van Gemert WG. A rational approach to nutritional assessment. Clin Nutr. 2008;27:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Soeters PB, Schols AM. Advances in understanding and assessing malnutrition. Curr Opin Clin Nutr Metab Care. 2009;12:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Kobayashi H, Yamataka A, Lane GJ, Miyano T. Levels of circulating antiinflammatory cytokine interleukin-1 receptor antagonist and proinflammatory cytokines at different stages of biliary atresia. J Pediatr Surg. 2002;37:1038-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Narayanaswamy B, Gonde C, Tredger JM, Hussain M, Vergani D, Davenport M. Serial circulating markers of inflammation in biliary atresia--evolution of the post-operative inflammatory process. Hepatology. 2007;46:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1564] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 28. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5726] [Article Influence: 110.1] [Reference Citation Analysis (2)] |
| 29. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3674] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 30. | McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 274] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | World Health Organization. WHO Child Growth Standards: Methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: World Health Organization 2006; . |
| 32. | World Health Organization. WHO Child Growth Standards: Methods and development: Head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age. Geneva: World Health Organization 2007; . |
| 33. | Frisancho A. Anthropometric Standards An Interactive Nutritional Reference of Body Size and Body Composition for Children and Adults. 2nd ed. Michigan: University of Michigan Press 2008; . |
| 34. | Mehta NM, Duggan CP. Nutritional deficiencies during critical illness. Pediatr Clin North Am. 2009;56:1143-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Greer R, Lehnert M, Lewindon P, Cleghorn GJ, Shepherd RW. Body composition and components of energy expenditure in children with end-stage liver disease. J Pediatr Gastroenterol Nutr. 2003;36:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Norman K, Pirlich M. Gastrointestinal tract in liver disease: which organ is sick? Curr Opin Clin Nutr Metab Care. 2008;11:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Wiest R, Weigert J, Wanninger J, Neumeier M, Bauer S, Schmidhofer S, Farkas S, Scherer MN, Schäffler A, Schölmerich J. Impaired hepatic removal of interleukin-6 in patients with liver cirrhosis. Cytokine. 2011;53:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Meijers JM, van Bokhorst-de van der Schueren MA, Schols JM, Soeters PB, Halfens RJ. Defining malnutrition: mission or mission impossible? Nutrition. 2010;26:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | King SJ, Nyulasi IB, Bailey M, Kotsimbos T, Wilson JW. Loss of fat-free mass over four years in adult cystic fibrosis is associated with high serum interleukin-6 levels but not tumour necrosis factor-alpha. Clin Nutr. 2014;33:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Hurtado-López EF, Larrosa-Haro A, Vásquez-Garibay EM, Macías-Rosales R, Troyo-Sanromán R, Bojórquez-Ramos MC. Liver function test results predict nutritional status evaluated by arm anthropometric indicators. J Pediatr Gastroenterol Nutr. 2007;45:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Jensen GL. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr. 2006;30:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 43. | Beberashvili I, Sinuani I, Azar A, Yasur H, Shapiro G, Feldman L, Averbukh Z, Weissgarten J. IL-6 levels, nutritional status, and mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2253-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, Jung HS. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | El-Faramawy AA, El-Shazly LB, Abbass AA, Ismail HA. Serum IL-6 and IL-8 in infants with biliary atresia in comparison to intrahepatic cholestasis. Trop Gastroenterol. 2011;32:50-55. [PubMed] |
| 46. | Shiraki M, Terakura Y, Iwasa J, Shimizu M, Miwa Y, Murakami N, Nagaki M, Moriwaki H. Elevated serum tumor necrosis factor-alpha and soluble tumor necrosis factor receptors correlate with aberrant energy metabolism in liver cirrhosis. Nutrition. 2010;26:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |