Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8203
Peer-review started: November 17, 2014
First decision: December 29, 2014
Revised: March 1, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: July 14, 2015
Processing time: 239 Days and 20.8 Hours
Fulminant hepatic failure (FHF) is a critical illness that can be comorbid to primary liver damage. FHF shows a high mortality rate, and patients with FHF require intensive therapy, including plasma apheresis. However, intensive care at the present is not enough to restore the severe liver damage or promote hepatocellular reproduction, and a standard therapy for the treatment of FHF has not been established. An 86-year-old female with FHF was admitted to our hospital. Her manifestation demonstrated a clinical situation of systemic inflammatory response syndrome (SIRS) and disseminated intravascular coagulation. A diagnosis of fulminant hepatitis was made according to the definition given in the position paper of the American Association for the Study of Liver Diseases. Her serum hepatocyte growth factor (HGF) level had increased to 11.84 ng/mL. The HGF level indicated massive liver damage as seen in FHF. Recombinant thrombomodulin (rTM) was administered daily from the admission day for 1 wk at 380 U/kg. The patient’s white blood cells and C-reactive protein responded to the rTM treatment within a few days. The HGF level and PT recovered to the normal range. The levels of proinflammatory cytokines (tumor necrosis factor-α and interleukin-1β) were suppressed by the administration of rTM. The patient’s hepatic function (e.g., PT and albumin) completely recovered without plasma exchange. rTM may modulate the over-response of SIRS with the improvement of proinflammatory cytokines. The underlying mechanism is thought to be the inhibitory effect of rTM on high-mobility group box 1 (HMBG1). The pathogenesis of HMBG1 protein in fulminant hepatic failure has been already known. A novel favorable effect of rTM for SIRS would be promising for FHF, and the wide application of rTM for SIRS should be considered.
Core tip: Fulminant hepatic failure (FHF) is a critical illness that can be comorbid to primary liver damage. However, no standard therapy for the treatment of FHF has been established. We experienced a fatal FHF case with systemic inflammatory response syndrome followed by disseminated intravascular coagulopathy (DIC). We administered recombinant thrombomodulin (rTM) for the treatment of DIC, which ameliorated all lethal conditions (coagulopathy and inflammation). Monitoring of proinflammatory cytokines, hepatocyte growth factor, and prothrombin time revealed the response of FHF to rTM. We hypothesized an anti-inflammatory effect of rTM-enhanced hepatocyte regeneration through inactivation of high-mobility group box 1.
- Citation: Kurokohchi K, Imataki O, Kubo F. Anti-inflammatory effect of recombinant thrombomodulin for fulminant hepatic failure. World J Gastroenterol 2015; 21(26): 8203-8207
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8203
Fulminant hepatic failure (FHF) is a critical illness that can be comorbid to primary liver damage[1]. FHF shows a high mortality rate, and patients with FHF require intensive therapy[2]. However, intensive care at the present is not enough to restore the severe liver damage or promote hepatocellular reproduction, and a standard therapy for the treatment of FHF has not been established[1]. FHF is produced from any background of liver injury, such as those due to drugs, autoimmune disorders, and acute or chronic viral infections[3]. Severe FHF is fatal, and the survival rate of FHF patients in the Japanese population has been estimated as 11.5%-24.4%[4]. Almost all patients with FHF are at risk of multi-organ failure. The only effective therapy for FHF is liver transplantation, but the indications for this are limited[2]. To rescue FHF patients, non-transplantation therapy, corticosteroids, and plasma apheresis can be applied, but clinical evidence of the efficacy of these non-transplantation therapies is scarce. Several risk factors for fulminant hepatitis are known. FHF patients with systemic inflammatory response syndrome (SIRS) also show poor prognoses. The concomitance of any complication also significantly decreases the survival rate of FHF patients[2,4].
Thrombomodulin (TM) is a physiological anticoagulation factor that acts as a direct inactivator of thrombin and a suppressor of coagulation factors Va and VIIIa via activated protein C (APC)[5]. Recombinant thrombomodulin (rTM) was developed as a therapeutic agent for disseminated intravascular coagulation (DIC) syndrome, and it is widely used in a variety of clinical situations[6]. A loss or lack of TM disrupts the protein C anticoagulant pathway and causes thrombosis[7]. Some clinical situations associated with SIRS (including severe infection, sepsis, trauma, and organ inflammation) may cause a decrease of TM, which is a substantial pathogenesis of DIC syndrome[8]. Additionally, a novel anti-inflammatory effect of TM and the APC pathway has been gradually unveiled[5], and aggregated knowledge has established the dual mechanisms of TM in DIC and SIRS by the modulation of aberrant coagulopathy and the attenuation of inflammatory milieu. However, the anti-inflammatory effect of rTM in FHF has not been elucidated, and the greater involvement of rTM in SIRS, a non-specific inflammatory disease, is not yet understood.
An 86-year-old female visited an outpatient clinic of our hospital due to increased fever, diarrhea, and decreased blood pressure. Her laboratory data indicated intensely impaired liver function, bilirubinemia, and renal dysfunction: aspartate transaminase 8929 U/L, alanine transaminase 5449 U/L, lactate dehydrogenase (LDH) 7248 U/L, total bilirubin (Tbil) 3.1 mg/dL, blood urea nitrogen (BUN) 34.8 mg/dL, Cr 1.9 mg/dL, and PT 42%. She was diagnosed as having concomitant DIC based on the coagulation test: PT 63.5%, PT-INR 1.23, APTT 26.0 s, fibrinogen 273 mg/dL, FDP > 80 μg/dL, and D-dimer > 5.00 μg/mL. She did not have a history of viral hepatitis or autoimmune liver disease, such as primary biliary cirrhosis (PBC) or autoimmune hepatitis (AIH). Radiological findings on admission and on previous examinations revealed no fatty liver or splenomegaly. A diagnosis of fulminant hepatitis was made according to the definition given in the position paper of the American Association for the Study of Liver Diseases (AASLD)[2]. The cause of the acute hepatic failure was not specified, but we suspected that it was associated with her treatment with a non-steroidal anti-inflammatory drug (NSAID), diclofenac suppository 25 mg/d, that was administered for lumbago starting 2 mo prior[3].
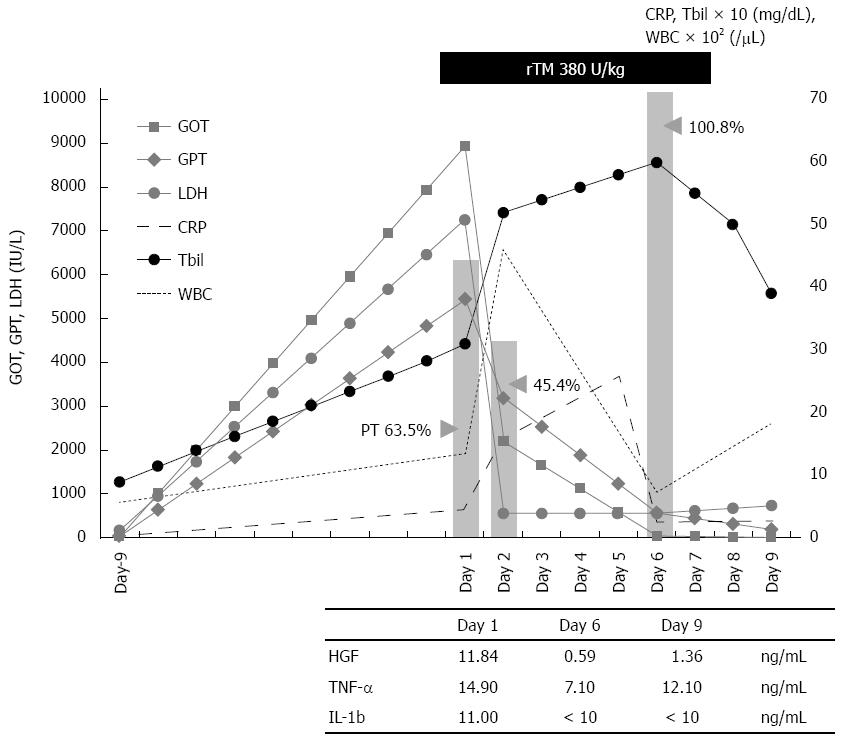
On the day of the patient’s admission to our hospital, corticosteroid pulse therapy was initiated (500 mg/d, × 3 d). At the same time, we started treatment with biological agents derived from human blood in order to conserve the patient’s liver function. We transfused fresh frozen plasma (FFP) 2 units/d every day until the end of treatment due to the patient’s death on the ninth day after her admission. We also administered rTM 380 U/kg per day for 6 d. Empirical treatment for infection was concomitantly driven by ceftriaxone (1 g × 1/d), cefozopran (1 g × 2/d), and cefoperazone/sulbactam (1 g × 2/d) appropriately dose-adjusted to the patient’s organ function (Figure 1). The clinical and laboratory alternation of the patient’s clinical course is illustrated in Figure 1, demonstrating a rapid decrease of liver function test values (GOT, GPT, LDH, ALP, and Tbil) immediately after the initiation of the treatment with rTM. The patient’s white blood cells and inflammatory reactive protein [C-reactive protein (CRP)] gradually improved over the next few days. We also found her hepatocyte growth factor (HGF) and inflammatory or proinflammatory cytokines [tumor necrosis factor (TNF)-α and interleukin (IL)-1β] levels were all diminished after the initiation of treatment with rTM (Figure 1).
Proteins reflecting the reproduction of liver cells such as PT and albumin were improved during the patient’s treatment, apparently responding to the rTM injection. Her hepatic function completely recovered without plasma exchange or any interventional apheresis. The patient did not exhibit the development of hepatic encephalopathy (e.g., confusion, stupor, and coma). Unfortunately, respiratory failure caused her death on day 9 after the initiation of treatment, but her hepatic failure was not a factor. An autopsy was not performed.
Severe FHF is fatal, with the only effective therapy being liver transplantation, but the indications for this are limited[2]. HGF was purified as a hepatocyte proliferation agent from patients with fulminant hepatitis and promotes hepatocyte mitosis[9]. Substantial elevations of HGF in FHF patients have been observed in clinical settings[9]. It was hypothesized that external supplementations of an overdose of HGF may promote the regeneration of hepatocytes and modulate hepatic function[10], but recombinant HGF (rhHGF) administration failed to show efficacy as a treatment for FHF in a Phase I/II study setting[10]. These observations may indicate that an elevated plasma HGF level corresponds to hepatocyte breakage, explaining why no obvious efficacy of additional HGF treatment has been observed to date. In fact, it is known that the large-scale production of HGF occurs in the onset of fulminant hepatitis irrespective of causes of hepatitis, such as drugs, viral infection, and autoimmune-mediated causes[11].
HGF has also been investigated as a hepatocyte protective agent for the therapy of hepatitis[12]. However, many clinical observations showed that additional external HGF supplementation was not hepatotropic and did not induce the regeneration of hepatocytes or produce better outcomes in humans[10]. The up-regulation of HGF expression in injured liver is mediated by the pro-inflammatory cytokines IL-1, IL-6, IFN-γ, and TNF-α[12]. We thus suggest that an increase in plasma HGF levels is a reflection of the degree of liver injury[12]. The dramatic improvement of our patient’s HGF level suggests that rTM treatment attenuated her liver cell damage. This is substantiated based on the patient’s profile of pro-inflammatory cytokines TNF-α and IL-1β, and during her rTM therapy.
The anti-inflammatory role of rTM has recently been highlighted in the field of clinical management for DIC[13]. rTM corrects coagulopathy through the inhibition of thrombin and the activation of protein C. The major pharmacological effect of rTM was demonstrated to be an enhanced physiological effect on APC[5]. Several research groups reported the anti-inflammatory effect of TM itself in the lectin-like domain, which can inactivate high-mobility group box 1 (HMGB1)[14], interfere with complement activation[15], and neutralize endotoxin[16]. HMGB1 is an inflammatory mediator that acts as a nuclear factor, and is secreted by activated monocytes and macrophages[17]. Some clinical articles support the pathogenesis of HMGB1 protein in FHF[18]. We did not define an elevation of HMGB1 in our patient’s case, however rTM treatment may have suppressed the activation of HMGB1. Corticosteroid therapy can also modulate the inflammatory response[19]. An inhibitory effect of glucocorticoids on HMGB1-induced TNF-α production has been observed[19]. However, the effect is only for the reduction of extracellular HMGB1 expression. The effect of systemic corticosteroid treatment was confined to a reduction in extracellular HMGB1 expression, but not intracellular expression[20]. The inflammatory cellular responses downstream from HMGB1 are less well understood[19]. Glucocorticoids inhibit HMGB1-induced TNF-α production in the pathway downstream from HMGB1. Moreover, HMGB1 is secreted mainly by monocytes and corticosteroid, and cannot adequately suppress activated monocytes[21]. Thus, other pathways from HMGB1 are assumed. This mechanism can be specifically targeted to therapeutic advantage in sterile SIRS associated with HMGB1 elevation, refractory to corticosteroids[19,21].
Because our patient’s clinical status before her admission to our hospital was not fully evaluated, we categorized her case as fulminant hepatitis of the subacute type[9] according to her medication history. Coagulopathy will occur generally in patients with FHF[2]. Beyond the anti-coagulant mechanism, however, the administration of rTM would be preferable to curing inflammatory status, especially SIRS, possibly by inactivating HMBG1. rTM as a novel treatment for FHF could become promising when the inflammatory profile in FHF is identified, but the categorization of inflammation in FHF is not yet underway, to the best of our knowledge.
In the present case, both a variety of biomarkers reflecting hepatocellular damage (including GOT, GPT, LDH, and ALP) and inflammatory cytokines decreased very rapidly immediately after the administration of rTM. In cases of FHF, most liver cells are often necrotized at once by the intense immunological reaction, resulting in the decrease of GOT and GPT levels. In those cases, liver cells are not reproduced, and the levels of PT and hepaplastin test do not recover. However, surprisingly, our patient’s decreased PT level was rapidly recovered (to over 100%) immediately after the administration of rTM. We have never experienced a case like this one in which the PT level was so remarkably recovered simply by the administration of FFP or a blood transfusion without plasma apheresis. Thus, rTM may play a critical role and become a new and effective therapeutic agent for the treatment of FHF and other disorders. Other organ failures with systemic or local inflammation may also be treatable with rTM[22]. We propose the necessity of confirming the effectiveness of rTM for the treatment of FHF in further studies.
An 86-year-old female came to an ambulatory care facility with fever, diarrhea, and hypotension.
The patient showed dehydration, which was diagnosed based on fever, tachycardia, and decreased blood pressure.
The cause of dehydration was initially thought to be an infection.
The patient’s laboratory tests showed severe liver and renal dysfunction with coagulopathy: aspartate transaminase 8929 U/L, alanine transaminase 5449 U/L, lactate dehydrogenase 7248 U/L, total bilirubin 3.1 mg/dL, BUN 34.8 mg/dL, Cr 1.9 mg/dL, and PT 42%.
Computed tomography imaging did not reveal hepatomegaly or splenomegaly, and did not detect an infection focus.
The authors administered recombinant thrombomodulin (rTM), which ameliorated the coagulopathy and hepatic function.
The potential role of rTM, the anti-inflammatory effect of which has been the focus in the clinical management of disseminated intravascular coagulopathy (DIC), is now being examined for the pathology of systemic inflammatory response syndrome (SIRS), including fulminant hepatic failure (FHF).
rTM is a novel product of human soluble thrombomodulin fragments that acts as a direct inactivator of thrombin and exerts a powerful inhibitory effect on activated Factors V and VIII by activating protein C.
This case suggested that rTM could be a promising agent to treat not only DIC, but also any inflammatory pathogenesis including SIRS, as shown in this case of FHF. The authors hypothesized that the underlying mechanism is an inhibition of the high-mobility group box 1 (HMGB1) pathway, which is known to be elevated in the sera of patients with FHF.
The anti-inflammatory pharmacological effect of rTM has been attracting more attention for further clinical application to other pathological conditions. In the present case, the clinical effect of rTM in FHF with SIRS was observed, revealing the kinetics of growth factors (hepatocyte growth factor) and inflammatory/proinflammatory cytokines (tumor necrosis factor-α and interleukin-1β).
P- Reviewer: Nagasawa M, Tsoulfas G S- Editor: Yu J L- Editor: Rutherford A E- Editor: Ma S
| 1. | Hoofnagle JH, Carithers RL, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240-252. [PubMed] |
| 2. | Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197. [PubMed] |
| 3. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1562] [Cited by in RCA: 1460] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 4. | Fujiwara K, Mochida S, Matsui A, Nakayama N, Nagoshi S, Toda G. Fulminant hepatitis and late onset hepatic failure in Japan. Hepatol Res. 2008;38:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 591] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 7. | Isermann B, Hendrickson SB, Zogg M, Wing M, Cummiskey M, Kisanuki YY, Yanagisawa M, Weiler H. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J Clin Invest. 2001;108:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Yamaguchi M, Gabazza EC, Taguchi O, Yano Y, Ikoma J, Kaito M, Kojima Y, Imoto I, Satomi A, D’Alessandro-Gabazza CN. Decreased protein C activation in patients with fulminant hepatic failure. Scand J Gastroenterol. 2006;41:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Gohda E, Tsubouchi H, Nakayama H, Hirono S, Sakiyama O, Takahashi K, Miyazaki H, Hashimoto S, Daikuhara Y. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J Clin Invest. 1988;81:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 487] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Ido A, Moriuchi A, Numata M, Murayama T, Teramukai S, Marusawa H, Yamaji N, Setoyama H, Kim ID, Chiba T. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following preclinical studies to ensure safety. J Transl Med. 2011;9:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Tsubouchi H, Hirono S, Gohda E, Nakayama H, Takahashi K, Sakiyama O, Miyazaki H, Sugihara J, Tomita E, Muto Y. Clinical significance of human hepatocyte growth factor in blood from patients with fulminant hepatic failure. Hepatology. 1989;9:875-881. [PubMed] |
| 12. | Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 13. | Di Nisio M, Baudo F, Cosmi B, D’Angelo A, De Gasperi A, Malato A, Schiavoni M, Squizzato A. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2012;129:e177-e184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Van de Wouwer M, Plaisance S, De Vriese A, Waelkens E, Collen D, Persson J, Daha MR, Conway EM. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4:1813-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Shi CS, Shi GY, Hsiao HM, Kao YC, Kuo KL, Ma CY, Kuo CH, Chang BI, Chang CF, Lin CH. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008;112:3661-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3283] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 18. | Majumdar M, Ratho R, Chawla Y, Singh MP. High levels of circulating HMGB1 as a biomarker of acute liver failure in patients with viral hepatitis E. Liver Int. 2013;33:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 1155] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 20. | Ulfgren AK, Grundtman C, Borg K, Alexanderson H, Andersson U, Harris HE, Lundberg IE. Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum. 2004;50:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Schierbeck H, Wähämaa H, Andersson U, Harris HE. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol Med. 2010;16:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Marumo S, Shirata M, Sakuramoto M, Fukui M. Severe drug-induced interstitial lung disease successfully treated with corticosteroid plus recombinant human soluble thrombomodulin. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |